
Synthesis and microwave dielectric properties of Si/C/B powder
LI Zhi-min(李智敏), DU Hong-liang(杜红亮), LIU Xiao-kui(刘晓魁), LUO Fa(罗 发), ZHOU Wan-cheng(周万城)
State Key Laboratory of Solidification Processing, Northwestern Polytechnical University, Xi’an 710072, China
Received 10 April 2006; accepted 25 April 2006
Abstract:
Si/C/B powders were synthesized by carbothermal reduction of xerogels containing boride. Colorless, transparent and monolithic gel was obtained by using tetraethoxysilane, saccharose, tributyl borate, ethanol and distilled water as starting materials. When the xerogel was fired at 1 600 ℃ in the static argon atmosphere, the XRD pattern commences to show the crystallite peak corresponding with C. β-SiC was synthesized at 1 700 ℃, but amorphous remainders could not be eliminated completely. The XRD results show that the boron possibly enters into the silica network, leading to the formation of borosiloxane. Microstructure of β-SiC powders consists of agglomerated particles with diameters ranging from 30 to 100 nm. Though the samples prepared at 1 600 ℃ and 1 700 ℃ have better dielectric loss tangent than the sample at 1 500 ℃ due to exiting crystalline material, the dielectric constant and dielectric loss tangent of three samples reveal lower values.
Key words:
Si/C/B powder; β-SiC; carbothermal reduction; sol–gel; dielectric constant; dielectric loss tangent;
1 Introduction
Silicon carbide is often considered the most important carbide. Its many attractive properties, such as great hardness, high heat resistance, creep resisance, good oxidation resistance and low coefficient of thermal expansion, favor it for many structural applications[1-3]. On the other hand, SiC is a semiconducting material of great technological interest for devices designed to operate at high temperature, high power, high frequency and harsh environments[4]. As advanced ceramic materials based on SiC, which require extremely fine starting powders in general, nanopowder of silicon carbide has been developed extensively in recent years, and a number of methods have been reported on its synthesis, which include microwave, chemical vapor deposition(CVD), laser- or plasma-driven chemical vapor deposition, sol-gel and carbothermal reduction methods[5-7].
However, little information is available on its dopant for SiC powder and dielectric properties, especially at high frequencies for isolators and electromagnetic wave absorbing materials. ZHAO et al[8] compared the dielectric properties of nano Si/C/N composite powder with nano SiC powder, and showed good wave absorbing properties of Si/C/N composite powder due to doping of N. ZHANG et al[9] also obtained nano-sized solid solution SiC powders with Al and N by carbothermal reduction process of the xerogels of SiO2-Al2O3. But till now, little effort has been made in the synthesis of nano SiC powder doped by B. In this study, SiC powders doped by B are desired to synthesize via carbothermal reduction of xerogels containing boride, and the dielectric properties of the powder are measured and discussed.
2 ExperimentalThe mixture sol was prepared by using tetraethoxysilane (TEOS) ((C2H5)4SiO4), saccharose (C12H22O11), tributyl borate (C12H27BO3), ethanol and distilled water as starting materials. The tetraethoxysilane and ethanol, saccharose and water were mixed, respectively, and then mixed with tributyl borate together. During the process of stirring for homogeneity, the pH value of solution was kept at 3-4 with the catalyst of HCl. The sol fabricated was placed into the drying oven to gel at 60 ℃. Carbothermal reduction of silica was carried out in a tube furnace using graphite crucibles. Dried and ground gels were heated at 15 ℃/min in a static argon atmosphere, up to 1 500 ℃,1 600 ℃ and 1 700 ℃, respectively, with 1 h holding.
XRD(X’Pert PRO MPD, Cu Kα) was used to analyze the crystalline phase structures of samples. The conglomeration and morphology of powders were investigated with SEM(JSM-6360). The samples for dielectric parameter measurements were prepared by blending the synthesized powders with paraffin wax in a mass ratio of 1∶4, and then molded into 10.16 mm×22.83 mm×2.00 mm flange ring. Commercially available paraffin wax was used. The dielectric parameters were carried out with a network analyzer (HP 8510B) in the frequency ranges of 8.2-12.4 GHz.
3 Results and discussion3.1 Preparation of sol-gel
The process of hydrolysis and condensation for TEOS of mixture is complicated. In the present study, under the prerequisite of 50 mL TEOS, the quantity of saccharose is a nominal molar ratio of C/Si=4 and C12H27BO3 is a molar ratio of B/Si=0.10. It is very important to control the pH value and amounts of water in the sol process. If a little acid or ammonia is dripped into the TEOS-H2O system, the speed of hydrolysis and condensation will be increased significantly. Accordingly, the pH value of solution was kept at 3-4 with the catalyst of HCl during the stirring process.On the other hand, the amount of water must be appropriate to make the speed of hydrolysis and condensation equal with dissolving saccharose absolutely. A molar ratio of 20 for water and saccharose is employed. It is worth mentioning that ethanol could both dissolve TEOS and tributyl borate, and make the evolution of hydrolyzing of TEOS available to drinking in environmental water. After the sol was generated, drying was performed at 60 ℃ for 2 d. Colorless, transparent and monolithic gel was obtained, and the boride was distributed into the network of gel evenly as well.
3.2 Synthesis of powders
3.2.1 Phase structure of powders
The XRD patterns of the powders obtained by carbothermal reduction of xerogel are shown in Fig.1. From Fig.1(a), the powder fired at 1 500 ℃ indicates that there only exits an amorphous peak of 2θ=15-30?, which corresponds to the noncrystalline SiO2 and C possibly. When heated to 1 600 ℃, the pattern of powder commences to show a peak about 28? identical to crystallite C. It can be seen from the pattern at 1 700 ℃ that it causes beneficial change that there exits crystallite peaks, in agreement with the characteristic peak of β-SiC, whose results are similar to that of Ref.[4], but amorphous remainders could not be eliminated completely. All of the patterns do not indicate that any boride is present. GIAN et al[10] suggest that from 1 000 ℃ to 1 500 ℃ no boron-containing species are lost during such process due to higher temperature. So the boron would progressively enter the silica network, leading to the formation of borosiloxane in the process of carbothermal reduction, corresponding with the amorphous peak of XRD. Namely, in the process of synthesizing Si/C/B powder, the probability that the boride is volatilized is little due to higher temperature.
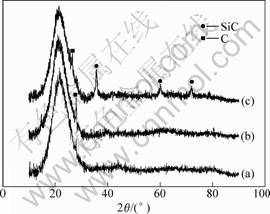
Fig.1 XRD patterns of powders synthesized at different temperatures: (a) 1 500 ℃; (b) 1 600 ℃; (c) 1 700 ℃
3.2.2 Characterization by SEM
SEM images of the powders shown in Fig.2 reveal that the powders are quite heterogeneous microstructures derived at 1 500 ℃, 1 600 ℃ and 1 700 ℃, respectively. The XRD patterns show that large amount of noncrystal exits in powders fired at 1 500 ℃ and
1 600 ℃. In Figs.2(a) and 2(b), no regular morphology with amorphous body testifies this result, and it is obvious that the morphology of powder at 1 500 ℃ is worse than the powder at 1 600 ℃. But at 1 700 ℃, because of the presence of β-SiC, the powder mostly composes of agglomerated particles with diameters ranging from 30 nm to 100 nm. Moreover, the powder also exits a combination of SiC powder and whiskers in Fig.3, whose component known from XRD is β-SiC. Additionally, CEROVIC et al[11] claim 99.8% SiC (mass fraction) to be light green. But the color of powder synthesized at 1 700 ℃ is dark grey SiC, which is caused by carbon residue known from XRD. In this study, the color of powder/whiskers combination is white, which is in agreement with the result of Ref.[12].
3.3 Dielectric properties
Fig.4 shows the real part of dielectric constant (ε′) and dielectric loss tangent (tanδ) of the samples in the microwave frequency range from 8.2 GHz to 12.4 GHz at room temperature. It is seen that the sample at 1 700 ℃, compared with the sample at 1 500 ℃, has better

Fig. 2 SEM images of powders at different temperatures:(a) 1 500 ℃; (b) 1 600 ℃; (c) 1 700 ℃

Fig.3 SEM image of powder/whiskers mixture at 1 700 ℃
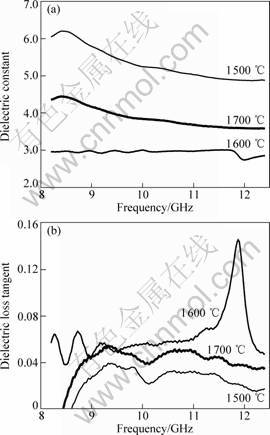
Fig.4 Dielectric constant (a) and dielectric loss tangent (b) of samples
dielectric loss tangent due to exiting β-SiC. The value of loss tangent at 1 600 ℃ approximates to the value at
1 700 ℃, attributing to the presence of crystalline C. On the contrary, the sample at 1 500 ℃ shows the highest value of dielectric constant ε′. But the dielectric constant ε′ and dielectric loss tanδ of three samples are low, which is undesired. Though β-SiC is generated at 1 700 ℃, it is well-known that pure β-SiC powder has inferior dielectric loss property. So the sample at 1 700 ℃ does not exhibit desired performance. If the boron could enter into the lattice of β-SiC during synthesized reaction, especially substitute the lattice site of C, the dielectric parameters should have desired values, or higher dielectric constant ε′ and loss tangent because of the electrical point defects caused by dopant of B. But it is pity that the boron enters the silica network to turn into borosiloxane, which would be crystallized out possibly at higher temperature than 1 700 ℃.
4 ConclusionsThe gel containing tributyl borate is generated for the aim of synthesis of Si/C/B. Though β-SiC is synthesized at 1 700 ℃ by carbothermal reduction of the xerogel in static argon atmosphere, amorphous remainders of the powder could not be eliminated completely. In the process of carbothermal reduction, the boron possibly enters the silica network leading to the formation of borosiloxane. Microstructure of β-SiC powder consists of agglomerated particles with diameters ranging from 30 nm to 100 nm, including β-SiC whiskers. The samples prepared at 1 600 ℃ and 1 700 ℃ have better dielectric loss tangent than the sample at 1 500 ℃ due to exiting crystalline material, but the dielectric constant ε′ and dielectric loss tan δ of three samples reveal lower values.
References
[1] SONG Yong-cai, LI Bin. Preparation of silicon carbide nanometer powders by organic-inorganic blended precursor[J]. Journal of Materials Science and Engineering, 2004, 22(3): 341-343.(in Chinese)
[2] MARTIN H P, ECKE R, MULLER E. Synthesis of nanocrystalline silicon carbide powder by carbothermal reduction[J]. Journal of European Ceramic Society, 1998, 18: 1737-1742.
[3] KLEIN S, WINTERER M, HAHN H. Reduced-pressure chemical vapor synthesis of nanacrystalline silicon carbide powders[J]. Chem. Vap. Deposition, 1998, 4: 143-149.
[4] MENG G W, CUI Z, ZHANG L D, et al. Growth and characterization of nanostructured β-SiC via carbothermal reduction of SiO2 xerogels containing carbon nanoparticles[J]. Journal of Crystal Growth, 2000, 209: 801-806.
[5] WANG Xiang-dong, QIAO Guan-jun, JIN Zhi-hao. Preparation of SiC/BN nanocomposite powders by chemical processing[J]. Materials Letters, 2004, 58: 1419-1423.
[6] DAI Xue-gang, ZHEN Guo-liang, RONG Jing-long, et al. Preparation of SiC ultrafine powder by plasma technique[J].Engineering Chemistry & Metallurgy, 1996, 17(4): 310-315. (in Chinese)
[7] TIAN Jie-mo, LI Jin-wang, DONG Li-min. Synthesis of SiC precursor by sol-gel process[J]. Journal of Inorganic Materials, 1999, 14(2): 297-301. (in Chinese)
[8] ZHAO Dong-lin, ZHOU Wan-cheng. Preparation and microwave permittivity of nana Si/C/N composite powders suspended in different materials[J]. Journal of Inorganic Materials, 2001, 16(5): 909-914. (in Chinese)
[9] ZHANG Bo, LI Jian-bao, SUN Jing-jing. Solid solution of Al and N in nano-sized α-SiC powder by carbothermal reduction of the xerogels of SiO2–Al2O3[J]. Materials Letters, 2001, 51:219-224.
[10] SORARU G D, BABONNEAU F, MAURINA S, et al. Sol-gel synthesis of SiBOC glasses[J]. Journal of Non-Crystalline Solids, 1998, 224: 173-183.
[11] CEROVIC L J, MILONJIC S K, ZEC S P. A comparison of sol-gel derived silicon carbide powders from saccharose and activated carbon[J]. Ceramics International, 1995, 21: 27l-276.
[12] JULBE A, LARNOT A, GUIZARD C, et al. Effect of boric acid addition in colloidal sol-gel derived SiC precursors[J]. Mat Res Bull, 1990, 25: 601-605.
Foundation item: Project(50572090) supported by the National Natural Science Foundation of China
Corresponding author: LI Zhi-min; Tel:+86-29-88488007; Fax:+86-29-88494574 ; E-mail: zmli@mail.xidian.edu.cn


