J. Cent. South Univ. Technol. (2008) 15: 757-762
DOI: 10.1007/s11771-008-0140-6![]()
Fabrication and anodic polarization behavior of lead-based porous anodes in zinc electrowinning
ZHONG Shui-ping(衷水平), LAI Yan-qing(赖延清), JIANG Liang-xing(蒋良兴),
L? Xiao-jun(吕晓军), CHEN Pei-ru(陈佩如), LI Jie(李 劼), LIU Ye-xiang(刘业翔)
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract:
A new type of lead-based porous anode in zinc electrowinning was prepared by negative pressure infiltration. The anodic polarization potential and corrosion rate were studied and compared with those of traditional flat anodes (Pb-0.8%Ag) used in industry. The anode corrosion rate was determined by anode actual current density and microstructure. The results show that the anodic oxygen evolution potential decreases first and then increases with the decrease of pore diameter. The anodic potential decreases to the lowest value of 1.729 V at the pore diameter of 1.25-1.60 mm. The porous anode can decrease its actual current density and thus decrease the anodic corrosion rate. When the pore diameter is 1.60-2.00 mm, the anodic relative corrosion rate reaches the lowest value of 52.1%.
Key words:
zinc electrowinning; negative pressure infiltration; porous anode; anode potential; corrosion rate;
1 Introduction
Insoluble anodes used in zinc electrowinning are mainly made of Pb-Ag alloys[1], which meet the basic needs of industrial production. The major disadvantage of this type of anode is the high oxygen evolution overvoltage (about 860 mV). Other problems are the high corrosion rate of lead and subsequent incorporation of lead corrosion products in the cathode, which decreases the purity of zinc product. Due to these problems there is always interest in finding ways to improve anode conductivity, corrosion resistance, electrochemical activity, mechanical strength and processing performance. A series of studies on various electrode materials, particularly Pb-alloy anode and Ti-based electrocatalytic coating anode were conducted. Detailed investigations have already been carried out for the binary alloys (Pb-Ca, Pb-Co), the ternary alloys (Pb-Ag-Ca, Pb-Ag-Ti, Pb-Ag-Sn, Pb-Sr-Sn, Pb-Ca-Sn) and the quaternary alloys (Pb-Ag-Ca-Sr, Pb-Ag-Ca-Ce, Pb-Ag-Sn-Co)[2-4]. Only the Pb-Co and Pb-Ag-Sn-Co anodes show certain electrocatalytic effects on the reaction of the oxygen evolution, the rest of the listed alloys only reduce silver content. But the manufacturing conditions of Pb-Co and Pb-Ag-Sn-Co alloys are difficult to control and the process is complicated, which restricts their commercial use. Inspired by the successful use of Ti-based electrode in the chlor-alkali industry, different kinds of Ti-based electrode (Ti/IrO2, Ti/RuO2, Ti/PbO2, Ti/TiO2/PbO2, Ti/SnO2+Sb2O3+ MnO2/PbO2) were investigated[5-7]. But these kinds of electrode are costly and cannot ultimately solve the passivation problem of Ti-base. Thus its application is confined.
Lead-based anode is still the anode that can be used industrially now and in the future[8]. According to this truth, our group proposed the lead-based porous anode based on the Tafel equation η=a+blg I in Ref.[9]. The equation shows that under the premise of without changing the composition of the anode, reducing the oxygen evolution overpotential can only resort to reduce the anode current density, which is bound to affect the cathodic zinc output and the current efficiency. The lead-based porous anode can meet the requirements of the reduction of the anode actual anode current density without affecting other process conditions.
Porous metal offers an interesting combination of structural and functional properties[10], such as a large specific surface area, a low density, an open or closed porosity, and good damping properties. They can be produced in various ways[11-12], for example, by powder metallurgy, chemical or physical deposition of metal on plastic foams, or by releasing gas into a melt prior to solidification. Cells existing in these porous metallic materials prepared by the above methods are smaller, and sometimes partial cells are closed[13-14]; cellular metallic structures can be cast as well, either by precision casting or by casting around a filler material. The main advantages of casting over the other manufacturing processes are that it is suitable for a wide range of alloys, its good pore diameter control and its capability of creating foams with a defined change in porosity[15-18].
In this work, the lead-based porous anodes were prepared by negative pressure infiltration. The polarization potential and corrosion rate of the anodes with different pore diameters were studied and the microstructures of them were also analyzed.
2 Experimental
2.1 Raw materials and major equipment
The main raw materials were Pb-0.8%Ag (mass fraction) alloy, MSO4 filler particles and release agent. The main experiment equipment includes two furnaces, a self-made device for negative pressure infiltration and seal gasket. Negative pressure infiltration casting process was used to prepare the porous anode. The principle of negative pressure infiltration casting is shown in Fig.1.
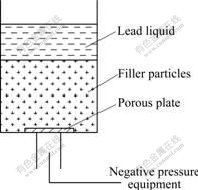
Fig.1 Schematic diagram of negative pressure infiltration casting
2.2 Preparation of porous anode
The negative pressure infiltration casting process steps are as follows: 1) pretreating and classifying filler particles; 2) preheating the mold filled with particles to a certain temperature; 3) pouring Pb-0.8%Ag alloy melt into the infiltration mold, and under a certain degree of vacuum, making Pb-0.8%Ag penetrate into the mold, determining time needed to experiment by the specific circumstances, usually in 3-9 s; 4) removing samples after solidification and cooling, then removing filler particles; 5) inspecting the situation of macro-defect quality of infiltration in the test centre. The process is shown in Fig.2.
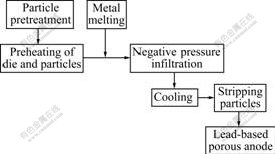
Fig.2 Process of negative pressure infiltration casting
2.3 Testing and characterization
2.3.1 Porosity testing
The porosity of the porous lead is an important parameter, which directly influences the true surface area of lead-based porous anode and the actual anode current density. All samples were cut through wire cutting to obtain appropriate size and shape. So it is convenient to get the porosity of lead-based porous anodes.
2.3.2 Oxygen evolution potential test
Oxygen potential was tested by chrono- potentiometry (CP). The porous electrode studied was cut into dimensions of 10 mm×10 mm×5 mm, welded with a plastic isolated copper wire and sealed with epoxy resin left 1 cm2 to be exposed to air. Before electrochemical testing and corrosion testing, the working electrodes were washed with water, degreased with alkali and ethanol, and then washed with deionized water.
The CP tests were carried out in a glass three- electrode cell. A platinum plate of 4 cm2 was served as counter electrode. The potential was measured with respect to saturated calomel electrode (SCE). As shown in this work, if not particularly demonstrated, electrode potential was relative to the SCE. The temperature was kept constant ((37±0.5) ℃) by means of an HH-1 thermostat.
The electrolyte was prepared by analytically pure grade chemicals and deionized water. The composition of the basic electrolyte (BE) for zinc electrowinning was as follows: 60 g/L Zn2+ (0.92 mol/L ZnSO4·7H2O) and 160 g/L (1.63 mol/L) H2SO4. The volume of electrolyte used in each test was 400 mL. Galvanostatic measurements were performed at an anodic current density of 50 mA/cm2 for 120 h. A multimeter (UNI- T70D) with a high ohmic input of 5×1012 Ω indicated the potential during the prolonged polarization and the data were collected every 1 h by PentiumⅣ personal computer.
2.3.3 Corrosion rate test
Atomic absorption spectrophotometer (Hitachi, Z-5000) was used to test the Pb2+ concentration in anodic polarization after 120 h in the solution. The concentration of Pb2+ was used to compare changes in corrosion rate calculated by
![]() (1)
(1)
where vk is the corrosion rate, c1 is the Pb2+ concentration after anodic polarization in the solution, c2 is the Pb2 + concentration before anodic polarization in the solution, V is the electrolyte volume, and t is the time of anodic polarization.
2.3.4 Microstructure analysis
Due to the particularity of porous anode surface, it is not easy to observe the anode surface after the polarization. Therefore, the surface morphology under the same component plate lead alloy at the anode current density of 5, 10 and 50 mA/cm2 and constant anode polarization for 72 h was used to describe the influence of the current density on the microstructure. After the polarization, the sample of anode was removed immediately, and washed in deionized water with carefully cleaning and drying, and then scanned under electron microscope (JEOL Japan, JSM-6360LV type) to observe the morphologies of anodic polarized samples.
3 Results and discussion
3.1 Morphology and structure
Fig.3(a) shows the photo of the sample fabricated under the following conditions: casting temperature of lead alloy liquid 450 ℃, filler particles preheating tem- perature 280 ℃, vacuum degree 0.03 MPa, size of filler particles 1.60-2.00 mm. Fig.3(b) shows a section of Fig.3(a). It can be seen the pores on radial orientation are evenly arranged and the pore structures are mostly determined by the filler particle size. Therefore, the negative pressure infiltration technique can be used to prepare lead-based porous anode with uniform pore structure and controlled pore sizes.

Fig.3 Photos of porous Pb-0.8%Ag alloy with pore diameter of 1.60-2.00 mm: (a) Sample diagram; (b) Section diagram
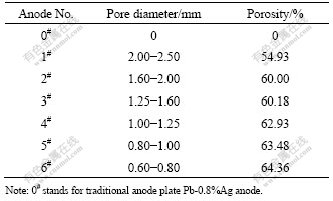
From Table 1 it can be seen that the porosity increases with the size of the filler particles. According to the principle of the negative pressure infiltration, the space occupied by the particles is the pore structure of porous lead alloy. Therefore, the larger the size of particles, the greater the gap among particles and the smaller the resistance to the flow of liquid lead. The gap is easy to fill up, so the porosity decreases.
Table 1 Relationship between pore diameter and porosity of lead-based porous anode
3.2 Analysis of oxygen evolution potential
Fig.4 shows that the oxygen evolution potential of porous anode is obviously lower than that of flat anode, and it decreases to different degrees with the decrease of anode pore diameter. For anodes 1#-3#, when the anode pore diameter changes from 2.00-2.50 to 1.25-1.60 mm, the oxygen evolution potentials decreases 69, 102 and 106 mV respectively compared with those of the flat anodes (1.835 V). However, for anodes 4# and 6# further decrease of anode pore diameter will increase the oxygen evolution potential. When the anode pore diameter changes from 1.00-1.25 to 0.60-0.80 mm, the oxygen evolution potentials increase from 1.735 to 1.767 V, reducing 100 and 68 mV respectively, compared with those of the flat anodes. The reason is that with the decrease of anode pore diameter, the specific surface area of anode increases and the actual current density reduces, and thus the oxygen evolution potential decreases. But when the pore diameter is further reduced, it will be difficult for the anode bubble to escape, which can increase the oxygen evolution potential.
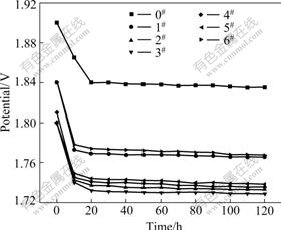
Fig.4 Polarization curves of anodes 0#-6#
From Fig.4 it can also be found that the anode
potential changes rapidly in the start phase and then stabilizes gradually for anode with different pore diameters, but the time is different. For anode 0#, the potential is around 1.910 V at the beginning of polarization and it reduces to about 1.840 V after polarization for 20 h. After polarization for 120 h the potential stabilizes at about 1.835 V. While for porous anode the potential can stabilize just after polarization for 10 h, taking anode 1# as example, the potential is 1.841 V at the beginning and decreases to 1.776 V after polarization for 10 h and stabilizes at about 1.766 V after polarization for 120 h. This is because a nonconductive PbSO4 layer is first generated on the fresh anode surface when the current density and potential of anode surface without covering with PbSO4 are increased. Due to the high oxygen evolution potential on the surface of Pb, the generated PbSO4 and the uncovered Pb will transform into PbO2 covering the anode surface and then the reaction of oxygen evolution will take place on the surface. When the reaction reaches balance, the anode potential will perform as steady state at the microscopic level.
For porous anode, due to its large specific surface area, the actual current density will be reduced when it works, which is beneficial for the generation of PbSO4, PbO2 and O2 to reach balance, that is, the anode potential stabilizes fast at the microscopic level.
3.3 Corrosion rate and microstructure change of anodic polarization
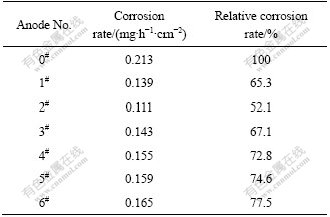
As can be seen from Table 2, the corrosion rates of porous anodes are lower than those of flat anodes, which are 52.1%-77.5% of those of the flat anodes, and anode 2# has the best corrosive resistance. In the polarized state, the major factors that influence the anodic corrosion rate include the actual current density, the microstructure of anodic material and the electrolyte composition. In this work the same electrolyte composition was used, therefore, the major factors that influence anodic corrosion rate are the former two.
Table 2 Corrosion rates of different anodes
3.3.1 Surface morphology after anode polarization
Fig.5 shows the surface morphologies of flat anodes with the same composition (Pb-0.8%Ag) after polarization for 72 h at different current densities. It can be seen that the anode has dense surface morphology and combines with the base body well when the current density is 5 mA/cm2. When the current density is 50 mA/cm2, the surface morphology of anode is loose after polarization for 72 h. So it is predictable that small current density can produce dense PbO2 coating on the anode surface, which is beneficial for protecting the anode against corrosion, while large current density will produce loose oxidized layer and then increase the corrosion rate.
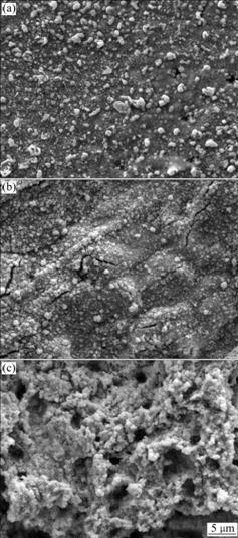
Fig.5 SEM images of Pb-0.8%Ag anode after anodic polarization for 72 h at different current densities: (a) 5 mA/cm2; (b) 10 mA/cm2; (c) 50 mA/cm2
Therefore, from the effect of current density on corrosion rate, anode 6# should have the smallest corrosion rate due to its largest specific surface area, but it is inconsistent with the tested results. This is because the corrosion rate depends not only on the anode pore diameter but also on the structure of the porous anode.
3.3.2 Effect of anode microstructure on corrosion rate
Figs.6(a) and (b) represent the microstructures of porous anode with the pore diameter of 0.80-1.00 and 0.60-0.80 mm, respectively. It can be seen that Fig.6(b) has thinner pore diameter wall and more ruptures than Fig.6(a), and the smaller the anode pore diameter, the greater the possibility of producing structural defects. It is because the smaller the filler particles are, the greater the resistance of lead flow will be produced. Meanwhile, the wettability between the filler particles and the lead is poor. All of these make more defects and greater corro- sion rate in porous anodes with smaller pore diameter. The greater the anode pore diameter is, the larger the filler particles and crevices are, the smaller the resistance of lead flow is. So it is easier for the crevice to be filled and more difficult to produce structural defects.
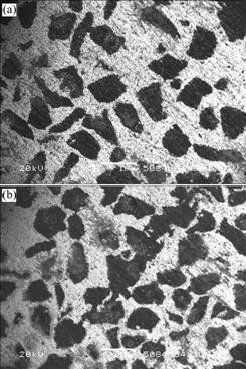
Fig.6 Microstructures of porous anode with different pore diameters: (a) 0.80-1.00 mm; (b) 0.60-0.80 mm
4 Conclusions
1) The lead-based porous anode with homogeneous pore structure and controllable pore diameter can be prepared using negative pressure infiltration when the casting temperature is 450 ℃, the preheating temperature of filler particles is 280 ℃ and the vacuum degree is 0.03 MPa.
2) The porous anodes display lower anodic polarization than traditional flat anodes. The anodic potential of porous anode decreases to the lowest value of 1.729 V at the pore diameter of 1.25-1.60 mm, which is about 106 mV lower than that of traditional flat anode (1.835 V).
3) It is easy to form dense PbO2 passive layer and reduce anodic corrosion rate when the current density is decreased. The porous anode can decrease its actual current density. When the anode pore diameters are 1.60-2.00 and 1.25-1.60 mm, the relative corrosion rates are 52.1% and 67.1% of those of traditional flat anodes. The anode corrosion rate decreases to the lowest value of 0.111 mg/(h·cm2) at the pore diameter of 1.60-2.00 mm.
4) The novel anode deserves further thorough investigations, especially the influence of Co2+ and Mn2+ ions on the kinetics of anodes and the quality of the cathodic zinc deposits.
References[1] PETROVAM, STEFANOVY, NONCHEVAZ, DOBREVT, RASHKOVS. Electrochemical behaviour of lead alloys as anodes in zinc electrowinning [J]. British Corrosion Journal, 1999, 34(3): 198-200.
[2] STEFANOVY, DOBREVT. Potentiodynamic and electron- microscopy investigations of lead-cobalt alloy coated lead composite anodes for zinc electrowinning [J]. Transactions of the Institute of Metal Finishing, 2005, 83(6): 296-299.
[3] IVANOVI, STEFANOVY, NONCHEVAZ, PETROVAM, DOBREVT, MIRKOVAL, VERMEERSCHR, DEMAERELJ P. Insoluble anodes used in hydrometallurgy (Part I): Corrosion resistance of lead and lead alloy anodes [J]. Hydrometallurgy, 2000, 57(2): 109-124.
[4] RASHKOVS, DOBREVT, NONCHEVAZ, STEFANOVY, RASHKOVAB, PETROVAM. Lead-cobalt anodes for electro- winning of zinc from sulphate electrolytes [J]. Hydrometallurgy, 1999, 52(3): 223-230.
[5] LI Bao-song, LIN An, GAN Fu-xing. Preparation and electro- catalytic properties of Ti/IrO2-Ta2O5 anodes for oxygen evolution [J]. Trans Nonferrous Met Soc China, 2006, 16(5): 1193-1199.
[6] HU Ji-ming, ZHANG Jian-qing, CAO Chu-nan. Oxygen evolution reaction on IrO2-based DSA type electrodes: Kinetics analysis of Tafel lines and EIS [J]. International Journal of Hydrogen Energy, 2004, 29(8): 791-797.
[7] STEFANOVY, DOBREVT. Developing and studying the properties of Pb-TiO2 alloy coated lead composite anodes for zinc electrowinning [J]. Transactions of the Institute of Metal Finishing, 2005, 83(6): 291-295.
[8] FELDER A, PRENGAMAN R D. Lead alloys for permanent anode in the nonferrous metals industry [J]. JOM, 2006, 58(10): 28-31.
[9] PENG Rong-qiu, REN Hong-jiu, ZHANG Xun-peng. Metallurgy of lead and zinc [M]. Beijing: Science Press, 2003: 413. (in Chinese)
[10] IRRETIERA, BANHARTJ. Lead and lead alloy foams [J]. Acta Materialia, 2005, 53(18): 4903-4917.
[11] ZHOU Xiang-yang, LI Shan-ni, LI Jie, LIU Ye-xiang. Preparation of precursor for stainless steel foam [J]. Journal of Central South University of Technology, 2008, 15(2): 209-213.
[12] DAI Chang-song, ZHANG Bin, WANG Dian-long, YI Ting-feng, HU Xin-guo. Preparation and performance of lead foam grid for negative electrode of VRLA battery [J]. Materials Chemistry and Physics, 2006, 99(2/3): 431-436.
[13] ZHANG Yong. Low-pressure infiltrating technology of foam aluminum and its familiar defects [J]. Foundry Technology, 2004, 8(8): 596-599. (in Chinese)
[14] RUSSELL G, JEAN-FRANCOIS D, ARIANE M, LUC S, ANDREAS M. The effect of preform processing on replicated aluminium foam structure and mechanical properties [J]. Scripta Materialia, 2006, 54(12): 2069-2073.
[15] BANHART J. Manufacture, characterization and application of cellular metals and metal foams [J]. Progress in Materials Science, 2001, 46(6): 559-632.
[16] BERCHEM K, MOHR U, BLECK W. Controlling the degree of pore opening of metal sponges, prepared by the infiltration preparation method [J]. Mater Sci Eng A, 2002, A323(1/2): 52-57.
[17] MA Li-qun, SONG Zhen-lun, HE De-ping. Cellular structure controllable aluminium foams produced by high pressure infiltration process [J]. Scripta Materialia, 1999, 41(7): 785-789.
[18] PALMER R A, GAO K, DOAN T M, GREEN L, CAVALLARO G. Pressure infiltrated syntactic foams—Process development and mechanical properties [J]. Mater Sci Eng A, 2007, A464(1/2): 85-92.
Foundation item: Project(2007SK2009) supported by the Science and Technology Research Project of Hunan Province, China
Received date: 2008-08-20; Accepted date: 2008-09-26
Corresponding author: LAI Yan-qing, Professor; Tel: +86-731-8876454; E-mail: 13975808172@126.com
(Edited by CHEN Wei-ping)
Abstract: A new type of lead-based porous anode in zinc electrowinning was prepared by negative pressure infiltration. The anodic polarization potential and corrosion rate were studied and compared with those of traditional flat anodes (Pb-0.8%Ag) used in industry. The anode corrosion rate was determined by anode actual current density and microstructure. The results show that the anodic oxygen evolution potential decreases first and then increases with the decrease of pore diameter. The anodic potential decreases to the lowest value of 1.729 V at the pore diameter of 1.25-1.60 mm. The porous anode can decrease its actual current density and thus decrease the anodic corrosion rate. When the pore diameter is 1.60-2.00 mm, the anodic relative corrosion rate reaches the lowest value of 52.1%.


