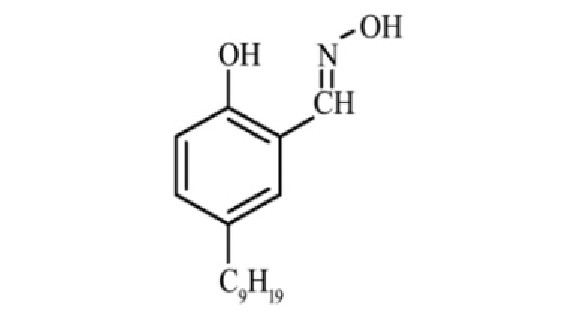
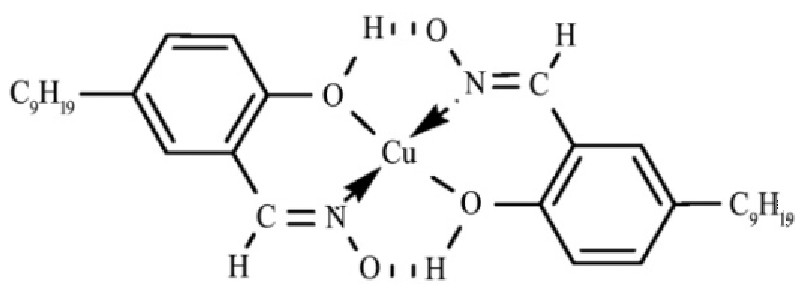
网络首发时间: 2019-04-02 13:16
稀有金属2020年第5期
湿法冶金提取分离铜镍技术研究进展
段亨攀 刘红盼 杨项军
重庆文理学院材料与化工学院
云南大学化学科学与工程学院
摘 要:
随着低品位铜镍伴生矿和二次资源逐渐成为铜镍冶金原料的重要组成,使得冶金过程中金属与杂质、金属与金属之间的纯化分离更加复杂。目前,湿法冶金技术以相对经济和环保优势,在铜镍提取、分离领域一直备受关注,本文从“浸出和萃取”两个过程阐述了国内外湿法冶金提取分离铜镍技术的研究进展。首先介绍了酸浸、氨浸和生物浸出等不同浸出介质对铜镍浸出效率的作用情况,并对各浸出方法的优缺点进行了总结和对比。指出氨浸出可利用氨与原料中铜镍离子络合,实现高选择性浸出、杂质少、便于后续的纯化富集,是未来绿色铜镍冶金的发展方向。同时,综述了不同浸出体系下混合铜镍之间萃取分离的作用机制以及对应的研究现状,着重分析了氨浸体系中铜镍二者高效萃取分离所面临的挑战和未来研究方向,尤其是开发高选择性萃取分离技术,以促进铜镍湿法冶金的研究和工业应用。
关键词:
湿法冶金 ;浸出 ;萃取 ;铜镍分离 ;
中图分类号: TF803.2
作者简介: 段亨攀(1991-),男,云南保山人,硕士,研究方向:化工分离,E-mail:duanhengpan@163.com; *杨项军,教授,电话:0871-65036626,E-mail:ilabgo@163.com;
收稿日期: 2019-01-22
基金: 国家自然科学基金项目(51464044); 重庆文理学院项目(2017YCH37,2017RCH07)资助;
Research Progress of Extraction and Separation of Copper and Nickel in Hydrometallurgy
Duan hengpan Liu Hongpan Yang Xiangjun
School of Material and Chemical Engineering,Chongqing University of Arts and Sciences
School of Chemical Science and Technology,Yunnan University
Abstract:
With the low grade associated ore and secondary resources gradually becoming the important materials composition of copper and nickel metallurgy,separation of the metal from impurities became more complicated. Hydrometallurgy of extraction-separation of copper and nickel was paid wide attention due to the economical and environmental benefits. In this paper,the research progress of copper-nickel extraction and separation was reviewed through the process of leaching and extraction. Firstly,the effects of different leaching media such as acid leaching,ammonia leaching and biological leaching on the leaching efficiency of copper and nickel were introduced,and the advantages and disadvantages of these leaching methods were summarized and compared. It was pointed out that ammonia leaching was the potential direction for the future copper-nickel extraction owing to forming complexation of ammonia with copper and nickel to achieve high selectivity leaching with few impurities and facilitate subsequent purification and enrichment. Furthermore,the mechanism of separation of copper and nickel under the different leaching system as well as the corresponding research situation were reviewed. The paper focused on the present challenges and future research directions of high efficiency separation of copper and nickel in ammonia leaching system. It could be expected to promote research and industrial applications of copper-nickel separation by developing the high selectivity separation technology.
Keyword:
hydrometallurgy; leaching; extraction; separation of copper and nickel;
Received: 2019-01-22
随着单一性高品位铜、镍矿的枯竭,资源需求与供给、冶炼成本与效益之间的矛盾日益突显。铜镍的冶炼原料越来越多依赖于成分复杂的铜镍低品位伴生铜镍矿和二次资源回收,使得金属与杂质、金属与金属之间的纯化分离更加复杂,低成本冶金途径和复杂组分分离研究急需得到迫切关注。
传统的火法冶炼铜镍大约占全球产量的四分之三,铜镍伴生矿在前期处理过程中可通过浮选法将铜和镍分开,如选矿过程中添加相应药剂实现“抑铜浮镍”或“抑镍浮铜”
[1 ,2 ,3 ]
。然而,面对低品位伴生矿或二次铜镍共存资源时,浮选法分离法变得相对局限;在复杂的体系下,更为高效的铜镍分离新技术研究将成为重要的发展方向。
湿法冶金较火法冶炼成本低、污染小,几乎可以用于除钢铁以外所有金属的冶炼,特别适用于低含量成分富集、复杂组分纯化分离
[4 ]
。在当前环保建设和资源匮乏的背景下,“浸出-萃取-电积”的湿法冶炼工艺以其相对绿色的处理过程正在冶金行业崛起。截至目前,湿法冶金从低品位铜镍硫化矿、电子废料、铜镍合金和电镀污泥等二次资源中提纯分离铜镍已经获得了广泛的研究和应用。然而,为了解决酸液的无序浸出、氨浸体系的铜镍共萃等典型问题,铜镍与杂质之间、铜镍二者之间更高选择性的分离手段一直被孜孜不倦的研究探索。本文从湿法冶金中“浸出和萃取”两个角度概述了铜镍提取、分离的研究进展,重点介绍了氨浸介质中铜、镍萃取分离的挑战。
1 浸出分离铜镍现状
浸出伴随着复杂的物理、化学和生物溶解行为,是湿法冶炼的首要相变过程。浸出将固态铜镍金属成分溶解为溶液离子态,根据浸出液的不同,可分为酸浸、氨浸和微生物浸出等。
1.1 酸浸
硫酸、硝酸等无机酸液作为铜镍湿法冶金最传统的浸出介质,该种浸出法选择性较差,铜镍成分及钙镁铁盐杂质通常能同时溶出。Huang等
[5 ]
在低品位硫化浮选矿中回收铜镍钴的研究中,以H2 SO4 作为浸出液加压浸出,铜镍钴溶解的同时,大量铁钙镁也共同进入溶液。谢燕婷等
[6 ]
利用混酸法对铜镍硫化尾矿中铜镍钴等有价金属进行了浸出实验,在H2 SO4 体系中添加HNO3 作为氧化剂,常温常压下,铜镍钴浸出率分别为91.5%,85.0%和54.6%;随之浸出的铁通过硫化沉淀法去除,去除率超过98%,但铜和镍在除铁过程中损失也分别达到了10%和6%。
电镀污泥中包含铜镍等大量重金属成分,Li等
[7 ]
利用酸浸提取和电积还原从电镀污泥回收铜镍,酸浸过程采用10%H2 SO4 作为浸出液,铜浸出率可达95%,镍铁铬等浸出率均超过80%。全桂香等
[8 ]
使用H2 SO4 ,HCl和HNO3 3种浸出体系处理电镀污泥,污泥中铜和镍含量分别为84.6和9.1 g·kg-1 ,对比发现1.5 mol·L-1 的H2 SO4 浸出效果最佳,浸出固液比为1∶15,45℃下2 h铜镍浸出率可分别达到97.59%和91.60%。
朱福良等
[9 ]
对铜镍合金二次资源进行了铜镍选择性浸出研究,发现HNO3 浸出体系能将铜镍快速浸出,铜和镍无法选择性分离;而6.0 mol·L-1 的HCl体系下,铜镍浸出率差异较大,在70℃下3 h铜和镍浸出率分别为3%和90%,可实现二者的选择性分离。然而,在浸出阶段直接完成铜镍二者之间选择性分离的报道并不多,运用范围相对局限。
酸浸出效率高,几乎适用于任何原料处理,工业化也较为成熟。但由于酸的无选择性浸出,特别在提取低含量铜镍资源时,酸的有效利用率极低;提纯过程中,大量共同酸解溶出的廉价金属盐需要辅助复杂的除杂工艺,而且除杂过程容易造成铜镍成分损失。
1.2 氨浸出
与酸浸相比,氨浸行为相对温和,氨与铜镍形成氨合络离子将铜镍从固体矿中溶出,由于能与氨形成配合离子的金属元素不多,氨浸具有较高的浸出选择性。绝大多数的钙镁铁盐不能被溶解留在固相中,浸出液成分在浸出阶段得到了初步净化,为后续的再分离纯化提供便利
[10 ]
。
Muzawazi等
[11 ]
在混合铜镍浮选精矿的浸出研究中,使用4 mol·L-1 的NH4 OH和(NH4 )2 CO3 溶液作为浸出液,最佳条件下,铜镍浸出率接近100%。王猛等
[12 ]
在氨浸回收废线路板中的铜镍锌的研究中,以4 mol·L-1 NH4 OH和1 mol·L-1 (NH4 )2 CO3 作为浸出液,0.2 MPa O2 为氧化剂,55℃下2.5 h铜镍浸出率分别超过了99%和94%,而锡、铅和铁基本难以溶解进入浸出液。刘建华等
[13 ]
使用氨浸体系对铜镍含量均小于1%的电镀废渣进行了浸出研究,最佳条件下,3 h铜、镍浸出率分别为95%和82%,并且浸出液基本没有溶入钙、铁等杂质。
Muzenda等
[14 ]
对铜镍锍的氨浸过程影响因素做了系统性研究,发现铜和镍最大浸出的氨浓度分别为2和3 mol·L-1 ,最佳浸出时间为270 min;而且温度提高有利于铜镍的浸出,但超过70℃后氨的挥发会造成浸出率下降;此外,在pH 11.2~10.4之间,镍的浸出率比铜高,在pH 10.4~9.3下却得到相反的结果。
氨溶液对不能形成络氨离子的金属盐溶解能力很低,与酸液的无序浸出相比,氨浸可实现选择性浸出。这种具有选择性和目的性的浸出手段符合了当前经济、环保的发展要求,但由于氨易挥发,氨浸系统要求浸出设备气密性较高。此外,氨浸和酸浸一样,混合铜镍的同时浸出使得铜镍二者之间在浸出阶段基本无法分离。
1.3 其他浸出
除了常用的酸浸和氨浸之外,铜镍的微生物浸出也备受关注。微生物浸出通过微生物自身及代谢过程与矿物元素作用,可实现对矿石中的特定元素选择性浸出
[15 ,16 ]
。Mehta等
[17 ]
在使用曲霉菌浸出锰结核金属元素的研究中,发现曲霉菌可以释放草酸和柠檬酸等有机酸,有助于金属的浸出溶解,最佳条件下,30 d铜、镍、浸出率可由对照组的4.9%,8.2%分别提高至97%,98%。Madrigalarias等
[18 ]
同样利用曲霉菌生物浸出从废旧手机和和电脑主板中提取铜镍等金属,发现不同菌株对铜和镍浸出效率差异较大,总体上铜浸出效果优于镍。
Li等
[19 ]
研究了4种混合嗜热菌对低品位硫化镍铜矿的生物浸出,发现添加L-半胱氨酸有助于降低体系pH值、加快微生物生长、提高氧化还原电位,从而提高铜和镍浸出率,16 d镍和铜的浸出率可由未加L-半胱氨酸之前的80.4%和68.2%分别提高至83.7%和81.4%。
Pourhossein等
[20 ]
在微生物浸出回收发光二极管废料中铜镍等金属研究中,发现过高的浆料密度会引起中毒反应从而降低细菌活性,在LED粉末的浆料密度为20 g·L-1 时,氧化亚铁酸二硫杆菌的可适应浸出环境,铜和镍最佳浸出率可分别达到84%和96%。
微生物浸出时间较长,总体浸出效率不高,但以其污染小、投资少的优点用于回收贫矿或低含量铜镍资源有一定的价值。目前微生物浸出离工业化还有很大差距,但微生物作为辅助浸出是一个不错的发展方向,崔兴兰等
[21 ]
在酸浸前使用氧化亚铁硫杆菌对铜镍硫化矿进行预处理,研究发现这种微生物预处理可以将铜镍的浸出率由原来的70%左右提高到90%以上。此外,利用微生物对铜镍的浸出差异,驯化特异性菌种在特定环境中实现单一性金属元素浸出,可能为铜镍的选择性浸出分离提供新的研究思路。
总之,浸出作为铜镍湿法冶金的开始,浸出介质的选择关系到后续铜镍离子之间的纯化分离行为,如酸介质虽然无选择性浸出、杂质多,但铜镍二者之间的萃取分离比较容易;而氨浸可除去部分杂质干扰,但氨浸体系下铜镍容易被萃取剂共萃,铜镍之间分离难度增加。
2 萃取分离铜镍研究进展
浸出后,铜镍成分进入水溶液成为离子态,浸出液经萃取分离可以实现离子的纯化和富集。萃取通过有机萃取剂与金属离子萃取的配位、氢键等作用将离子从水相带入有机相,伴随着有机化学的发展,国内外对铜镍萃取剂的研究不断推进,产品也推陈出新。如铜萃取剂中,德国汉高公司的Lix984、科宁公司的M5640以及国内的N902等萃取效率高、性能稳定,目前已经成熟地运用于工业化。图1和2分别表示M5640萃取剂的主要分子成分及对铜离子的螯合萃取作用
[22 ]
。
图1 M5640萃取剂主要成分
Fig.1 Major constituent of M5640
[22]
图2 M5640分子与Cu离子螯合萃取
Fig.2 Chelate extraction of M5640 and Cu2+
[22]
然而具有高度专一萃取能力的萃取剂并不多,因为萃取过程的配位、螯合效应等作用对同类分子依然适用,铜镍两种元素理化性质相似,萃取剂在萃铜的同时,往往也能萃取镍。目前铜镍之间的萃取分离基本可以通过3种手段实现:第一,酸浸体系中,调节体系pH使萃取剂依次选择性萃取铜、镍;第二,氨性介质中,先共萃铜镍,再使用不同浓度的H2 SO4 溶液依次选择性反萃镍、铜;第三,向萃取剂中添加改性剂,以“反协同萃取”的方式提高铜镍分离效率。
2.1 酸浸体系中选择性萃取分离铜镍
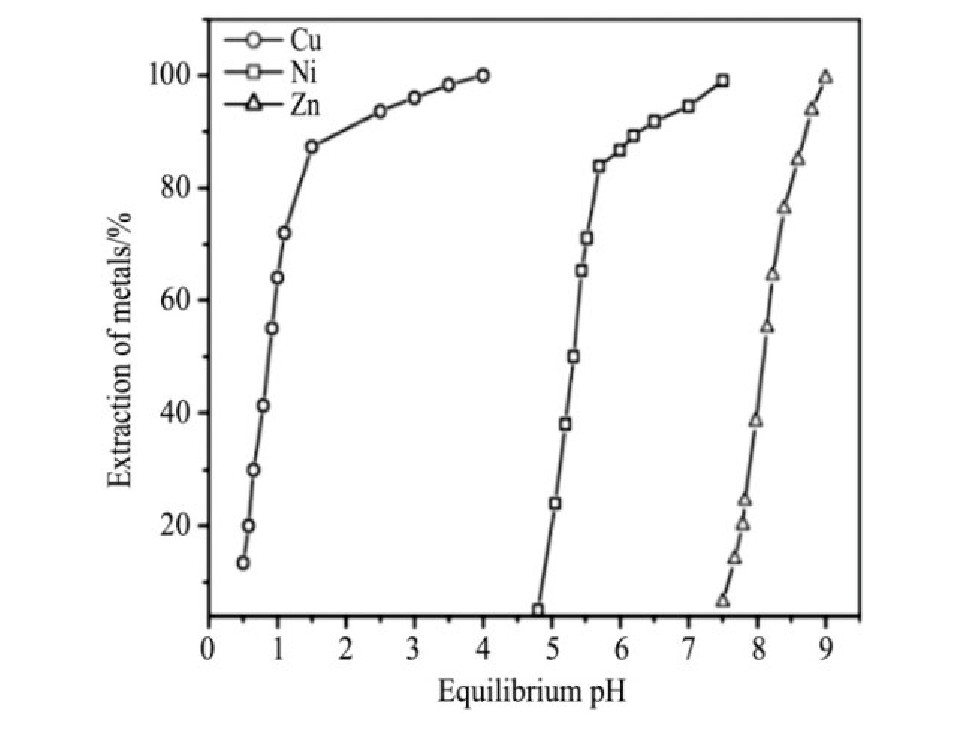
酸浸后的酸性介质中,萃取效率对溶液pH变化较为敏感,Reddy等
[23 ]
通过调节溶液pH从铜镍锌混合溶液中萃取分离铜镍锌,如图3,铜和镍依次在pH为4.0和7.5条件下被Lix84i萃取分离,经两级逆流萃取,铜镍萃取率均高达99.4%。
Li等
[24 ]
使用Lix984n分离H2 SO4 介质中铜和镍,在pH为4的溶液中,镍基本不被萃取,而铜的萃取率为92.3%;将溶液调节pH至10.5后,镍萃取率达到了93%。萃取后,铜镍有机相分别通过170和200 g·L-1 的H2 SO4 溶液反萃,二者回收率均超过99%。Lix984和Lix984n成分相似,Li等
[25 ]
使用Lix984萃取剂分离铜镍的研究也取得了相似的结果。
类似的萃取剂包括Lix84,Cyanex272
[26 ]
,Lix63
[27 ]
,Cyphos IL101
[28 ]
和Versatic 10等
[29 ]
,都有相同的萃取规律,即在低pH酸性介质中“萃铜留镍”,而镍通过调高溶液pH被再萃取,完成铜和镍之间的萃取分离。当然,在实际的酸浸体系中,酸浓度一般较高,调高pH萃取回收镍需消耗大量碱试剂、不利于酸的重复利用;此外,由于酸浸介质的无序浸出使得浸出液成分复杂,萃取分离前需要对浸出液除杂净化。
图3 pH对金属离子萃取率的影响
Fig.3 Effect of equilibrium pH on the percentage extraction of metals
[23]
2.2 氨浸体系中“共萃-选择性反萃”分离铜镍
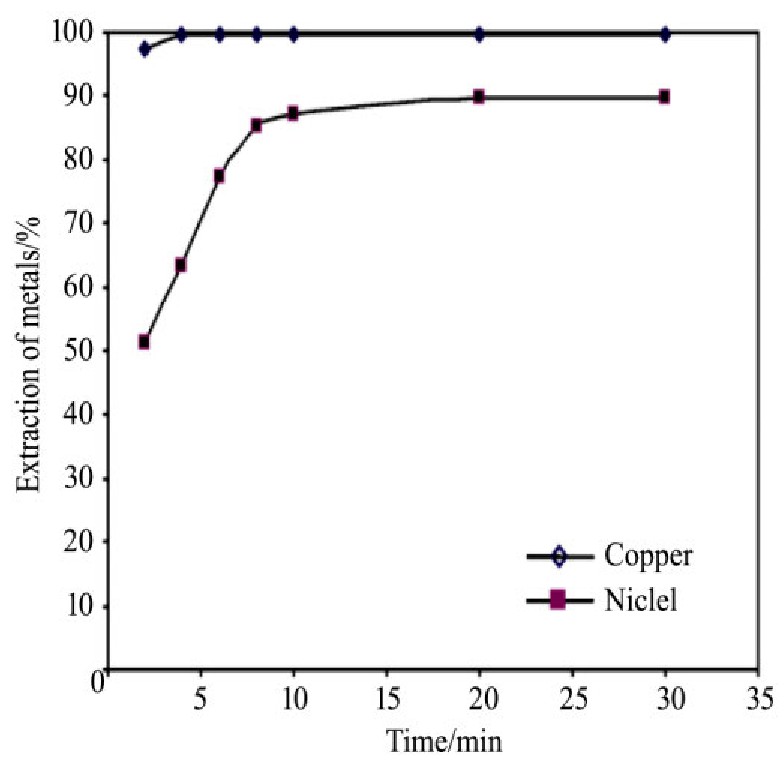
与酸浸不同,氨浸介质产生的氨浸体系中,溶液呈碱性,加之氨与铜镍离子配位的影响,萃取剂对铜镍的萃取行为表现截然不同,此时,铜和镍会被共同萃取。Sridhar等
[30 ]
在使用Lix984n萃取剂从氨/碳酸铵介质中分离铜镍发现,铜和镍均能被萃取剂共萃,如图4所示,达到萃取平衡时,铜几乎被完全萃取,镍萃取率也接近90%;而且在pH 7~9范围内,pH变化对铜和镍萃取的影响不明显。铜镍被共萃入有机相后,镍可经9.8 g·L-1 H2 SO4 溶液三级反萃,回收接近100%,此时铜几乎不被反萃;剩余的铜使用180 g·L-1 H2 SO4 二级反萃,回收率超过99%,共萃的铜镍通过不同浓度的H2 SO4 溶液反萃得以分离。
同样地,萃取剂M5640
[31 ]
,Lix984
[32 ]
以及早期Lix64n
[33 ]
和Lix84
[34 ]
等都可在氨介质中先共萃铜镍,然后使用低浓度H2 SO4 反萃镍,再由高浓H2 SO4 溶液反萃回收铜,实现铜和镍的选择性反萃分离。铜和镍的萃取和反萃过程可认为是离子交换反应,如图5所示,M5640萃取剂通过螯合和解离作用完成对金属离子萃取和反萃。由于铜和镍被螯合萃取后形成的螯合物稳定性不同,铜螯合物稳定性较高,需要高浓度酸才能解络,而镍螯合物可优先被低浓度酸解络,选择性反萃分离铜镍正是利用这种动力学差异完成的。
图4 铜镍离子萃取的动力学
Fig.4 Kinetics of copper and nickel extraction
[30]
图5 M5640(M=Cu或Ni)萃取剂与金属离子的萃取与反萃过程
Fig.5 Extraction and reverse extraction of metal ions by M5640(M=Cu or Ni)
[22]
当然,在使用低浓度酸反萃镍时,反萃速率有限,反应时间较长;而且从“低镍富铜”原料中提取铜时,镍的共萃会降低萃取剂对铜的萃取能力,增加萃取和反萃次数,分离成本偏高。
2.3“反协同萃取”选择性萃取分离铜镍
协同萃取通过混合萃取剂“1+1>2”的萃取作用在湿法冶金分离中运用广泛,而“反协同萃取”则作用相反
[35 ,36 ]
。Hu等
[37 ]
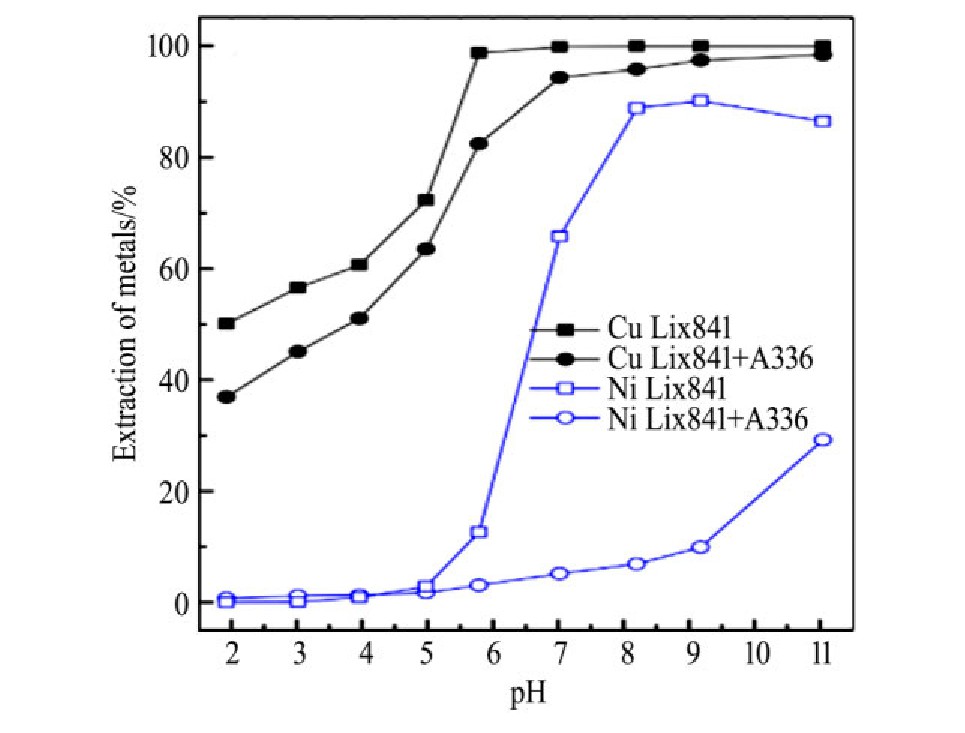
在分离氨溶液中铜镍的研究中,发现向Lix84i萃取剂中添加Aliquat 336后,铜和镍的萃取率均下降,而且二者下降趋势差异很大,如图6所示,在溶液pH为9.18时,混合前后镍萃取率从90.1%下降至9.9%,而铜萃取率下降不明显,铜镍分离系数显著提高。
本课题组
[38 ]
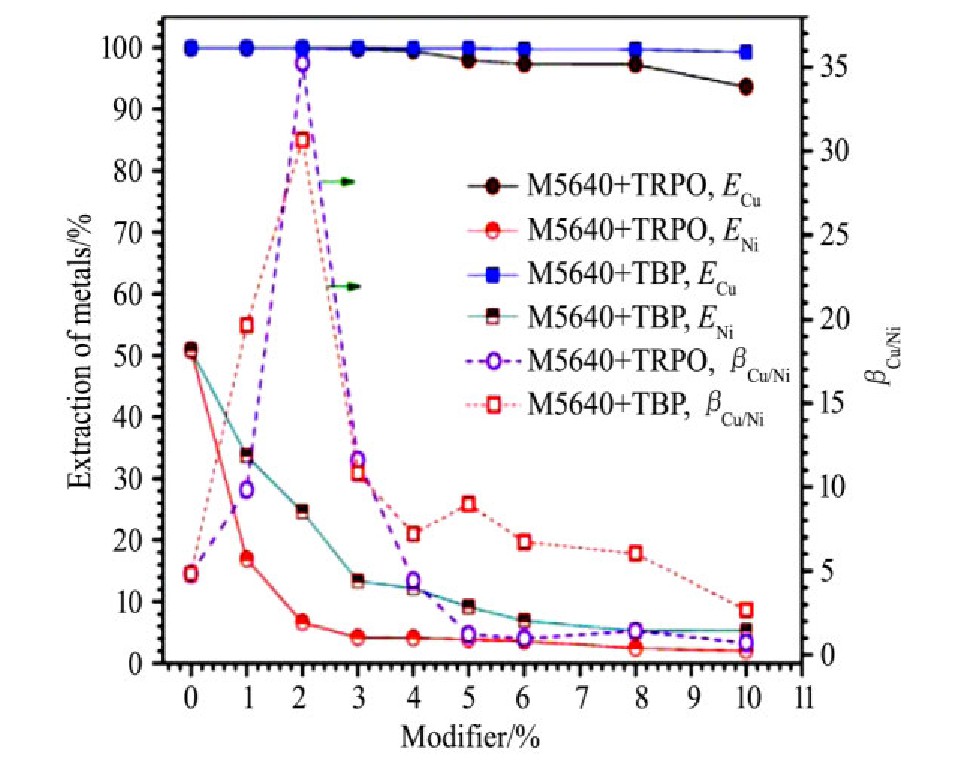
在研究使用TRPO和TBP作为修饰剂与M5640萃取剂混合,利用反协同效应分离氨性介质中铜镍也得到相似的结论,如图7所示。其中,以10%M5640和2%TRPO混合的煤油溶液作为萃取相,最佳条件下,铜萃取率可达99.96%,而镍萃取率由不加TRPO时的51.0%降低至6.6%。关于反协同萃取剂的机制,我们认为,TRPO等具有P=O键,容易与M5640分子中的-OH形成氢键等作用,如图8所示,这种作用降低了M5640对铜和镍的螯合效应,阻碍萃取反应的进行,即形成反协同效应,而且这种抑制萃取对镍的作用远远高于铜。
图6 A336存在对Lix84I萃取分离铜镍的影响
Fig.6 Extraction and separation behaviors of Cu(II)and Ni(II)with Lix84I in absence and presence of A336
[37]
图7 修饰剂对铜镍萃取分离的影响
Fig.7 Effect of modifier concentration on the extraction and separation of copper and nickel
[38]
膜萃取技术将萃取剂负载到多孔薄膜上,在膜的两侧,萃取和反萃可同时进行。利用反协同分离原理,本课题组
[39 ]
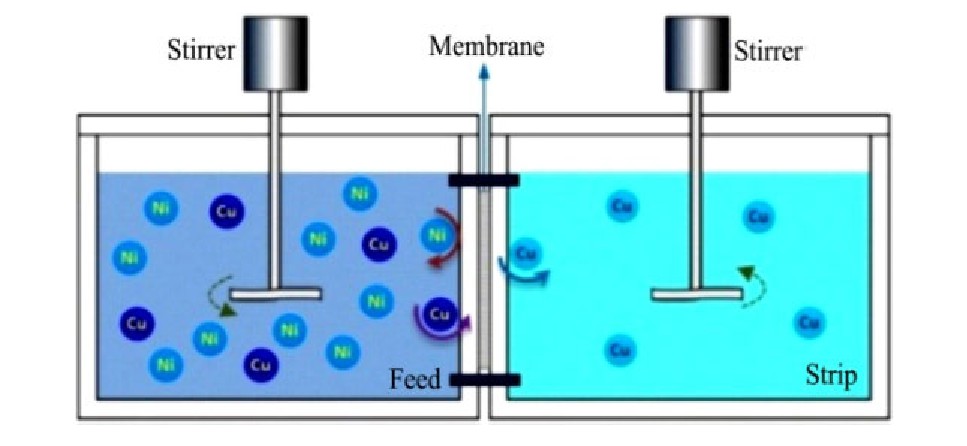
使用负载M5640-TRPO载体的支撑液膜分离氨性介质中铜镍,如图9所示,反协同作用使得料液相中的镍难以被膜载体萃取,难以进入反萃相;最佳条件下,12 h后几乎全部的铜离子由料液相进入反萃相,而镍的通过率只有6.1%。
在目前的研究中,反协同萃取分离铜镍的分离极限还未知,对反协同剂的优化筛选和作用机制探究是该研究方向的主要内容。而且,由于反协同效应对铜萃取也有一定的抑制作用,因此,在加强对“镍抑制和铜萃取”的铜镍分离矛盾之间需要探索最佳的实验条件。
图8 TRPO与M5640的氢键作用
Fig.8 Hydrogen-bond interaction of TRPO and M5640
图9 支撑液膜分离铜镍
Fig.9 separation copper and nickel by supported liquid mem-brane
[39]
此外,利用萃取剂在氨性和酸性介质中对铜镍的萃取差异,结合支撑液膜技术,本课题组
[40 ]
设计了一种Sandwich模型,如图10所示,3个迁移池中分别含有混合铜镍的氨/铵盐溶液(No.1),5 g·L-1 H2 SO4 溶液(No.2)和50 g·L-1 H2 SO4 溶液(No.3)。氨介质中的铜和镍被膜载体M5640(SLM1)萃取后,经5 g·L-1 H2 SO4 反萃共同进入中间的迁移池;低浓度酸介质中的铜继续可被支撑液膜载体(SLM2)萃取,同时被高浓度酸反萃进入第三个迁移池中,而镍不再被萃取迁移停留在中间的迁移池;最终,36 h后来自No.1迁移池的98.0%镍和98.9%铜可被分别分离至No.2和No.3两个迁移池中。Sandwich模型可以有效的将氨介质中铜镍分离,但由于目前支撑液膜技术本身膜稳定性难题还未得到突破,因此,该模型距工业化应用还有一定的距离。
图1 0 Sandwich模型分离铜镍
Fig.10 Separation copper and nickel by Sandwich Model
[40]
3 结语
从“浸出和萃取”两方面介绍了湿法冶金提取分离铜镍的研究现状,并着重总结了目前氨浸体系中萃取分离铜镍的几种手段。由于铜或镍的单一性浸出还难以实现,萃取作为重要的分离纯化手段,在实际问题中,需要权衡浸出体系和萃取分离方法的选择搭配。氨介质的选择性浸出将是未来发展的绿色处理工艺,但氨浸体系中铜镍的高效分离方法还有待提高和完善。一方面,反协同萃取作为分离铜镍的新思路,可进一步深入探究反协同萃取的分离机制,探寻理论分离极限,甚至推进“抑镍萃铜”向“抑镍促铜”的方向发展。其次,“抑铜萃镍”的研究还鲜见报道,如果萃取过程能够实现“抑铜萃镍”,可拓宽铜镍分离的应用场景。此外,有机萃取剂作为铜镍萃取分离的载体,开发具有高度专一性的铜或镍萃取剂将会是铜镍萃取分离的终极挑战。
参考文献
[1] Deng W,Wang C L,Zhao K L,Rao X Y.Experimental study on copper-nickel separation flotation with the combined depressant[J].Multipurpose Utilization of Mineral Resources,2011,(5):34.(邓伟,王昌良,赵开乐,饶系英.组合抑制剂用于铜镍分离浮选的试验研究[J].矿产综合利用,2011,(5):34.)
[2] Evdokimov S I,Evdokimov V S.Efficient flotation technology for natural copper-nickel and waste material[J].Gornyi Zhurnal,2016,2016(2):74.
[3] Ma P F,Han T K,Weng C J.Research status of flotation separation of copper nickel sulphide ores[J].Conservation and Utilization of Mineral Resources,2015,(5):68.(马鹏飞,韩统坤,翁存建.铜镍硫化矿浮选分离研究现状[J].矿产保护与利用,2015,(5):68.)
[4] Zhang,G,Guan,W,Xiao,and ZhangL,Q.A novel process for tungsten hydrometallurgy based on direct solvent extraction in alkaline medium[J].Hydrometallurgy,2016,165:233.
[5] Huang K,Li Q,Chen J.Recovery of copper,nickel and cobalt from acidic pressure leaching solutions of lowgrade sulfide flotation concentrates[J].Minerals Engineering,2007,20(7):722.
[6] Xie Y T,Xu Y B,Yan L,Yang R D.Studies on the leaching of Ni,Cu and Co from Ni-Cu sulfide tailings[J].Nonferrous Metals(Extractive Metallurgy),2006,(4):14.(谢燕婷,徐彦宾,闫兰,杨汝栋.铜镍硫化矿尾矿中有价金属的湿法提取研究[J].有色金属(冶炼部分),2006,(4):14.)
[7] Li P P,Peng C S,Li F M,Song S X,Juan A O.Copper and nickel recovery from rlectroplating sludge by the process of acid-leaching and electro-depositing[J].International Journal of Environmental Research,2011,5(3):797.
[8] Quan G X,Yan J L.Optimal conditions for acid-leaching of heavy metals from electroplating sludge[J].Environmental Engineering,2013,31(2):92.(全桂香,严金龙.电镀污泥中重金属酸浸条件试验[J].环境工程,2013,31(2):92.)
[9] Zhu F L,Huang D,Yu Q Q,Xie J P.Selective leaching of copper and nickel from copper-nickel alloy[J].Hydrometallurgy of China,2008,27(4):227.(朱福良,黄达,于倩倩,谢建平.铜镍合金中Cu,Ni的选择性浸出研究[J].湿法冶金,2008,27(4):227.)
[10] Fu M,Hu J,Li Y,Song Y,Hu H,Chen Q.A novel strategy achieving enrichment of metal values from and into ammoniacal solutions[J].Separation&Purification Technology,2015,151:97.
[11] Muzawazi C,Petersen J.Heap and tank leaching of copper and nickel from a platreef flotation concentrate using ammoniacal solutions[J].Canadian Metallurgical Quarterly,2015,54(3):297.
[12] Wang M,Cao H B,Zhang Y.Selective recovery of copper,zinc and nickel from printed circuit boards by ammonia leaching under pressure[J].Environmental Science,2011,32(2):596.(王猛,曹宏斌,张懿.加压氨浸法选择性回收废线路板中的铜、锌和镍[J].环境科学,2011,32(2):596.)
[13] Liu J H,Zhang H R,Wang R X.Process of ammonium leaching of nickel and copper from electroplating residues[J].China Nonferrous Metallurgy,2011,40(5):73.(刘建华,张焕然,王瑞祥.氨法浸出电镀废渣中镍铜的工艺[J].中国有色冶金,2011,40(5):73.)
[14] Muzenda E,Ramatsa I M,Ntuli F,Abdulkareem A S,AfolabiA S.Parametric effects on leaching behavior of nickel-copper matte in ammonia[J].Particulate Science&Technology,2013,31(4):319.
[15] Bosecker K.Bioleaching:metal solubilization by microorganisms[J].Fems Microbiology Reviews,1997,20:591.
[16] Wu Z L,Kong W Z,Zhu Y G.Bioleaching of high-arsenic copper concentrate[J].Chinese Journal of Rare Metals,2015,39(12):1123.(伍赠玲,孔维长,朱永官.高砷硫化铜精矿细菌浸出实验研究[J].稀有金属,2015,39(12):1123.)
[17] Mehta K D,Das C,Pandey B D.Leaching of copper,nickel and cobalt from Indian Ocean manganese nodules by Aspergillus niger[J].Hydrometallurgy,2010,105(1):89.
[18] Madrigalarias J E,Argumedodelira R,Alarcon A.Bioleaching of gold,copper and nickel from waste cellular phone PCBs and computer goldfinger motherboards by two aspergillus nigerstrains[J].Brazilian Journal of Microbiology,2015,46(3):707.
[19] Li S,Zhong H,Hu Y.Bioleaching of a low-grade nickel-copper sulfide by mixture of four thermophiles[J].Bioresource Technology,2014,153:300.
[20] Pourhossein F,Mousavi S M.Enhancement of copper,nickel,and gallium recovery from LED waste by adaptation of acidithiobacillus ferrooxidans[J].Waste Management,2018,79:98.
[21] Cui X L,Liu Y Q,Li Y,Wang S H,Lu A H,Wang JH,Wang C Q,Zhu J N.The acceleration of the extraction of valuable metals from Jinchuancopper-nickel sulfide tailings by acidithiobacillus ferrooxidans[J].Acta Petrologica et Mineralogica,2013,32(6):797.(崔兴兰,刘玉强,李艳,王少华,鲁安怀,王纪华,王长秋,朱纪念.氧化亚铁硫杆菌促进金川铜镍硫化矿尾矿砂提取有价金属实验研究[J].岩石矿物学杂志,2013,32(6):797.)
[22] Wu F,LüJ.Study on separation of Cu2+ from nickle sulphate solution with extractant M5640[J].Journal of Wuyi University(Natural Science Edition),2004,18(1):25.(吴芳,吕军.铜萃取剂M5640从硫酸镍溶液中分离铜的应用研究[J].五邑大学学报(自然科学版),2004,18(1):25.)
[23] Reddy B R,Priya D N.Process development for the separation of copper(II),nickel(II)and zinc(II)from sulphate solutions by solvent extraction using LIX 84 I[J].Separation&Purification Technology,2005,45(2):163.
[24] Li L,Zhong H,Cao Z,Yuan L.Recovery of copper(II)and nickel(II)from plating wastewater by solvent extraction[J].Chinese Journal of Chemical Engineering,2011,19(6):926.
[25] Li L,Zhong H.Separation and recovery of copper(II),nickel(II)from simulated plating wastewater by solvent extraction using Lix984[J].Advanced Materials Research,2011,365:252.
[26] Agrawal A,Manoj M K,Kumari S,Bagchi D,Kumar V,Pandey B D.Extractive separation of copper and nickel from copper bleed stream by solvent extraction route[J].Minerals Engineering,2008,21(15):1126.
[27] Zhu Z,Zhang W,Pranolo Y,Cheng C Y.Separation and recovery of copper,nickel,cobalt and zinc in chloride solutions by synergistic solvent extraction[J].Hydrometallurgy,2012,127:1.
[28] Li Y,Fu M B,Ren Y.Behavior and mechanism of extraction and separation of copper/nickel from chloride solution by ionicliquid extracant cyphos IL101[J].Hydrometallurgy of China,2018,37(4):286.(李娅,付明波,任昀.离子液体萃取剂Cyphos IL101从氯化体系中萃取分离铜/镍行为及机制[J].湿法冶金,2018,37(4):286.)
[29] Qiu Y,Yang L,Huang S,Ji Z,Li Y.The separation and recovery of copper(II),nickel(II),cobalt(II),zinc(II),and cadmium(II)in a sulfate-based solution using a mixture of Versatic 10 acid and Mextral 984H[J].Chinese Journal of Chemical Engineering,2017,25(6):760.
[30] Sridhar V,Verma J K,Kumar S A.Selective separation of copper and nickel by solvent extraction using LIX984N[J].Hydrometallurgy,2009,99(1):124.
[31] Sridhar V,Verma J K,Shenoy N S.Separation of nickel from copper in ammoniacal/ammonium carbonate solution using ACORGA M5640 by selective stripping[J].Minerals Engineering,2010,23(5):454.
[32] Li J C.Experimental study on separation of nickel,copper and cobalt[J].China Nonferrous Metals,2013,(13):58.(李金春.镍铜钴分离试验研究[J].中国有色金属,2013,(13):58.)
[33] Pandey B D,Kumar V,Bagchi D,Akerkar D D.Extraction of nickel and copper from the ammoniacal leach solution of sea nodules by LIX 64N[J].Khirurgiia,1989,28(11):93.
[34] Parija C,Sarma P V R B.Separation of nickel and copper from ammoniacal solutions through co-extraction and selective stripping using LIX84 as the extractant[J].Hydrometallurgy,2000,54(2):195.
[35] Yang X J,Yuan X H,Duan H P,Huang R,Huang,ZJ,Guo H.Extraction of Au(I)from aurocyanide solution by using a synergistic system of primary amine N1923/bis(2-ethylhexyl)sulfoxide:amechanism study[J].Hydrometallurgy,2016,162:16.
[36] Sun Q,Yang L M,Huang S T,Xu Z,Li Y,Hu Y HN.Research and prospect on synergistic solvent extraction mechanism[J].Chinese Journal of Rare Metals,2016,40(11):1177.(孙启,杨丽梅,黄松涛,徐政,李岩,胡祎罕娜.协同萃取机制的研究现状及展望[J].稀有金属,2016,40(11):1177.)
[37] Hu J,Wang S,Hu H,LiH,Peng K,Liu D.Improvement of separation efficiency of Cu(II)and Ni(II)in ammoniacal solutions by antagonistic effect of Aliquat336 on LIX84I[J].Separation&Purification Technology,2013,118:828.
[38] Yang R S,Wang S X,Duan H P,Yuan X H,Huang ZJ,Guo H.Efficient separation of copper and nickel from ammonium chloride solutions through the antagonistic effect of TRPO on Acorga M5640[J].Hydrometallurgy,2016,163:18.
[39] Duan H P,Wang S X,Yang X J,Yuan X H,Zhang Q,Huang Z J,Guo H.Simultaneous separation of copper from nickel in ammoniacal solutions using supported liquid membrane containing synergistic mixture of M5640and TRPO[J].Chemical Engineering Research&Design,2017,117:460.
[40] Duan H P,Wang Z Y,Yuan X H,Wang S X,Guo H,Yang X J.A novel sandwich supported liquid membrane system for simultaneous separation of copper,nickel and cobalt in ammoniacal solution[J].Separation&Purification Technology,2017,173:323.