Article ID: 1003-6326(2005)05-1150-06
Marmatite bioleaching with moderately
thermoacidophilic bacterial strain and
mineral analyses of solid residues
SHI Shao-yuan(石绍渊)1, FANG Zhao-heng(方兆珩)2
(1. Institute of Environmental Engineering, Peking University, Beijing 100871, China;
2. Institute of Processing Engineering, Chinese Academy of Sciences, Beijing 100080, China)
Abstract:
The bioleaching of a marmatite flotation concentrate with a moderately thermoacidophilic iron-oxidizing bacterial strain (MLY) is influenced significantly by temperature, pH, particle size, pulp density of ores and bacterial strains. Under such leaching conditions as the initial pH value of 1.5, temperature of 50℃, pulp density of 5%, particle size less than 35.5μm (over 90%) and inoculating the adapted strains of MLY, the leached Zn is over 95% after 10d of bioleaching. SEM observations show the cell attachment and the surface features of solid residues under different leaching conditions. XRD and EDX analyses show that a mass of elemental sulfur form during the bioleaching process. The technological feasibility of a microbiological process using MLY for extracting zinc from the marmatite concentrate is demonstrated.
Key words:
marmatite; bioleaching; moderately thermoacidophilic bacteria; mineral analyses CLC number: TF111.3;
Document code: A
1 INTRODUCTION
Much research effort has been directed towards developing processes using Acidithiobacillus (formerly Thiobacillus) ferrooxidans for micro-biological leaching of heaps and dumps of low-grade minerals[1-3], which cannot be exploited economically by conventional techniques. It has been proposed that bacterial leaching can be also used to treat sulfide concentrates[4, 5]. Marmatite is an important resource of zinc ore in China, which is difficult to be processed effectively by traditional technologies due to its high content of iron. Recently, Shi and Fang[6, 7] carried out some researches on the marmatite flotation concentrate bioleaching with the microorganisms. Although the mesophilic bacteria have the potential to dissolve a number of mineral sulfides, its widespread commercial acceptance still remains some restriction because of its poor leaching kinetics compared with other hydrometallurgical alternatives. A number of mesophilic, moderate and extreme thermophilic organisms have been characterized by their ability to oxidize either sulfur or ferrous iron. Many of these organisms have been shown to enhance the oxidation rate of sulfide minerals manifold as compared with chemical oxidation system[8-10].
In the recent years, moderately thermophilic microorganisms have received increased attention since their optimal growth temperature presents a kinetic advantage over mesophilic microorganisms[11], less energy is required to reach the working temperature and they can grow in higher pulp densities than extreme thermophiles[12]. A moderately thermoacidophilic iron-oxidizing bacterium (designated as MLY) was isolated by Institute of Microbiology, Chinese Academy of Science, and it is able to oxidize ferrous iron, pyrite and elemental sulfur autophically and mixotrophically in the presence of yeast extract[13].
In this work, the bioleaching of the marmatite concentrate with MLY was investigated in batch experiments. The effects of some factors, such as pH, temperature, pulp density and particle size of ore on the marmatite bioleaching were examined. The leached Zn from marmatite flotation concentrate was compared among the sterile controls, original strain and adapted strain of MLY. The mineral sample and solid residues were analyzed by spectral method.
2 EXPERIMENTAL
The experiments were carried out by using the marmatite flotation concentrate from a lead-zinc mine in Yunnan Province of China, which was composed of marmatite and some pyrite as shown by XRD analysis. The elemental analysis result of the concentrate is listed in Table 1. The mineral sample was ground to activate mineral surface before experiments, its particle size was less than 35.5μm (over 90%), and it was preleached for 3d before inoculating the microorganisms.
Table 1 Chemical composition of marmatite flotation concentrate
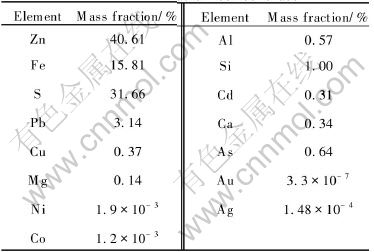
The strains of MLY were grown in the modified Leathen medium[14]. The original strain was adapted to the nutrient medium containing 5% marmatite concentrate replacing 1% Fe2+ ions as the sole energy source. The culture used in tests was adapted to the mineral through continuous subculture over one year.
In a number of 250mL Erlenmeyer flasks, 90mL Leathen medium (without iron) was added by the required content of marmatite concentrate and inoculated 10mL inoculum of a five-day-old MLY. The initial density of cells was approximately 1×107 cell/mL. To determine the contribution from chemical leaching of zinc, sterile controls were also run in each set of experiments with 1mL formaldehyde (A.R) added as bactericide.
Suitable amount of solution was withdrawn from each flask at the regular time intervals to estimate the concentration of zinc ions in the leaching solution, which was measured by titration with EDTA. The cell attachment and surface features of solid residues were observed by SEM and their reaction products and mineral composition changes were examined by XRD and EDX analyses.
3 RESULTS AND DISCUSSION
3.1 Effect of pH, temperature, particle size and pulp density on leaching
In a set of batch experiments, the pH was set respectively at 1.0, 1.5 and 2.0. Experimental results from Fig.1(a) indicate that the optimum of pH for the extraction of zinc is around 1.5, because the growth and activity of MLY are related closely to the pH value of the leaching solution[13]. The cell density is lower at pH ≤1.0, and a mass of jarosite form at the initial pH value of 2.0, which leads to decline in the leaching rate.
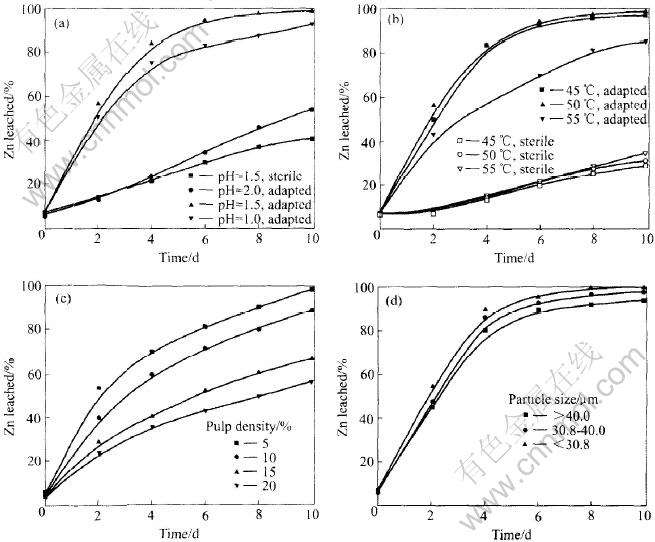
Fig.1 Effects of pH, temperature, particle size and pulp density on leaching ratio of zinc
Fig.1(b) indicates that the dissolution of marmatite is affected little by temperature under sterile controls. In contrast, the dissolution rate of marmatite is affected markedly at the different temperatures in the presence of the adapted strains of MLY and the optimal temperature is 50℃ for the marmatite bioleaching. The cell density decreases significantly at 55℃ for the adaptation culturing after a long period. The growth and activity of microorganism are influenced when the temperature is too high or low. Moreover, the lower temperature can also lead to the decrease of the leaching kinetics of marmatite.
The results from Fig.1(c) show that the leaching ratio of Zn decreases with the increase of pulp density, other than the concentration of Zn2+ ions in leach liquor. The decline in leaching rate at the higher pulp density might be attributed to interference of the solid with the mass transfer of O2 or CO2 to bacterial strains[15]. Moreover, the biological activity of microorganisms decreases with the increase of pulp density, which leads to the increase of shearing effect[16].
Data from Fig.1(d) show that the leaching rate of zinc increases with decreasing particle size. The specific surface area of large particles is low, so its dissolution rate could be limited by the availability of surface at lower specific surface and pulp density. In addition to decreasing the particle size of ores, the grinding can accelerate the marmatite leaching for renewing particle surface and removing the flotation agent adsorbed on the substrate surface.
3.2 Marmatite leaching with different strains
Shake flask leaching experiments are carried out to show the degradability of marmatite by MLY under such leaching conditions as the initial pH value of 1.5, temperature of 50℃, pulp density of 5%, particle size of 〈35.5μm (over 90%). Fig.2 shows the leached Zn from the marmatite flotation concentrate in the presence of adapted strains, original strains, and under the sterile controls, respectively. It is found that the dissolution rate of marmatite by the adapted strains of MLY is the highest among them, and the leaching ratio is over 95% after 10d of leaching and the concentration of Zn2+ ion is around 20g/L. Meanwhile the leaching ratio is less than 50% in the presence of the original strain and around 25% under the sterile control, respectively. It is obvious that the microorganisms play a key role in the dissolution of marmatite during the leaching process, and the adapted strains of MLY can provide more rapid and complete oxidation of marmatite than the original strain.
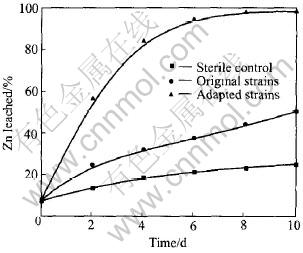
Fig.2 Leaching rate of marmatite with original strains, adapted strains and sterile control
The cell density of the MLY adapted strains increases with leaching time and arrives at the maximum, about 1.0×109 cell/mL, after inoculating for 5d, and remains roughly constant until the end of the leaching experiment. The cell density is lower in the system inoculated with the original strains of MLY and even less than the initial cell density. This means that the activity of the adapted strains is higher than that of the original strain. The adaptation of bacterial strains is an effective method to increase the dissolution rate of marmatite by improving the growth and activity of microorganisms.
3.3 SEM observation of solid residues
SEM images of the concentrate and solid residues in Fig.3 indicate that many small serrations and slots form on the surface (shown in Fig.3(b)) due to the acidic dissolution, compared with the mineral sample (shown in Fig.3(a)). Extracelluar polymeric substance (EPS) is formed in the presence of the MLY adapted strains (shown in Fig.3(c)). It is reported in Refs.[17, 18] that bacterial cells attach to sulfide mineral or sulfur through EPS. Hansford and Vargas[19] believed that the binding of Fe3+ ion compounds in the EPS is decisive for the interactions between bacterial cells and substrate. The bacteria attachment onto the surface of mineral particles is shown in Fig.3(d) in the leaching systems inoculated by the adapted strains of MLY, and the bacterial cells seem to preferentially attach to the locations with crystal defect.
3.4 XRD and EDX analyses of solid residues
XRD patterns in Fig.4 indicate that the miner al compositions of solid residues in the different leaching period are very similar, but the contents of the various components are different. The content of marmatite in the leached residues decreases drastically with the leaching time and disappears completely after 15d bioleaching. The spectral line density of elemental sulfur formed during the leaching process is strengthened gradually with time although the bacteria are able to oxidize elemental sulfur into SO2-4. The contents of pyrite and galena increase with time, which means that the mineral impurities are more resistant to bio-oxidation than marmatite.
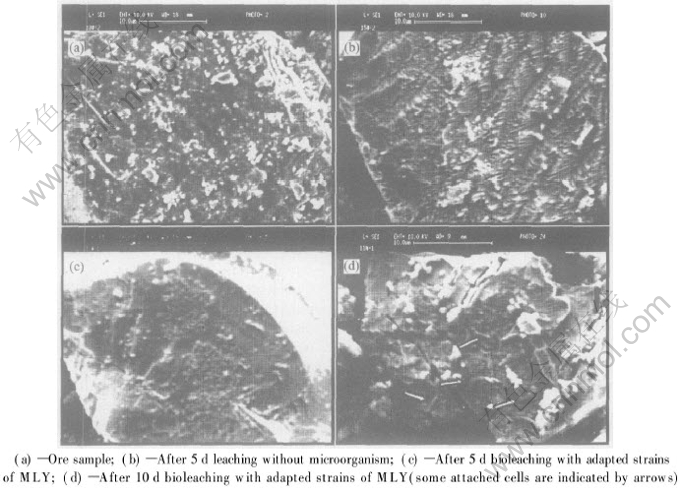
Fig.3 Typical SEM images of concentrate and solid residues of marmatite
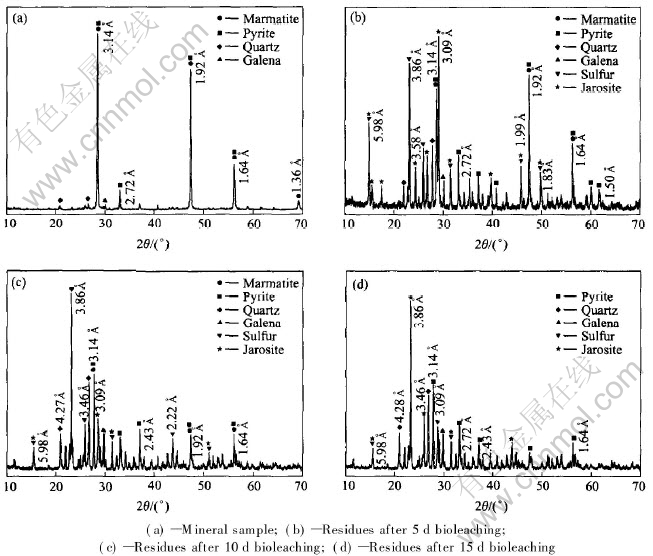
Fig.4 XRD patterns of concentrate and solid residues
EDX spectra of the concentrate and solid residues after 5, 10 and 15d bioleaching are shown in Fig.5. It is found that the peak density of sulfur increases with the leaching time, and the spectrum intensity of zinc decreases gradually and disappears completely after 15d bioleaching. The result of EDX analyses is consistent with that of XRD. Marmatite is preferentially dissolved as an anode in oxidation reaction during the bioleaching of complex sulfides for its low redox potential value in the sulfide series[20].
4 CONCLUSIONS
The bioleaching of the marmatite concentrate by a moderately thermoacidophilic iron-oxidizing bacterium shows that the dissolution of marmatite is affected signficantly by temperature, pH, particle size and pulp density of ores. The adapted strains of MLY can provide more rapid and complete oxidation of marmatite than its original strain and under sterile controls. Under such leaching conditions as the initial pH value of 1.5, temperature of 50℃, pulp density of 5%, particle size of 〈35.5μm (over 90%) and inoculating the adapted strains, the leached Zn is over 95% after 10d bioleaching. The adaptation of bacterial strains might accelerate the marmatite bioleaching by improving the growth and activity of microorganisms. SEM images indicate that the mineral sample and solid residues present the different surface features, and the attached bacterial cells and the EPS formed on the surface of mineral substrate are also observed. XRD and EDX analyses show that a mass of elemental sulfur form during the bioleaching process, and marmatite is dissolved preferentially in the bioleaching of mixed sulfides.
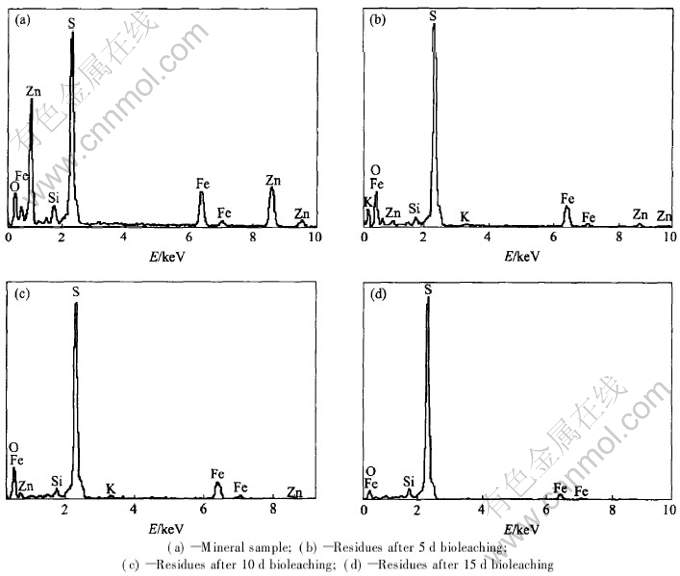
Fig.5 EDX patterns of concentrate and solid residues
REFERENCES
[1]Brierley C L. Bacterial succession in bioheap leaching [J]. Hydrometallurgy, 2001, 59: 249 - 255.
[2]Lizama H M, Harlamovs J R, McKay D J, et al. Heap leaching kinetics are proportional to the irrigation rate divided by heap height [J]. Minerals Engineering, 2005, 18(6): 623 - 630.
[3]YANG Song-rong, XIE Ji-yuan, QIU Guan-zhou, et al. Research and application of bioleaching and biooxidation technologies in China [J]. Minerals Engineering, 2002, 15(5): 361 - 363.
[4]Tipre D R, Dave S R. Bioleaching process for Cu-Pb-Zn bulk concentrate at high pulp density [J]. Hydrometallurgy, 2004, 75(1-4): 37 - 43.
[5]Mason L J, Rice N M. The adaptation of Thiobacillus ferrooxidans for the treatment of nickel-iron sulphide concentrates [J]. Minerals Engineering, 2002, 15(11): 795 - 808.
[6]Shi S, Fang Z. Bioleaching of marmatite flotation concentrate by Acidithiobacillus ferrooxidans[J]. Hydrometallurgy, 2004, 75(1-4): 1 - 10.
[7]Shi S, Fang Z. Bioleaching of marmatite flotation concentrate by Acidithiobacillus ferrooxidans and leptospirillum ferrooxidans [J]. Trans Nonferrous Met Soc China, 2004, 14 (3): 569 - 575.
[8]Lorenzo P, Gómez E, Silóniz M I, et al. Chalcopyrite bioleaching and thermotolerance of three acidophilic, ferrous-oxidising bacterial isolates[J]. Biotechnology Letters, 1997, 19(12): 1197 - 1200.
[9]Gomez E, Ballester A, Blazquez M L, et al. Silver-catalyzed bioleaching of a chalcopyrite concentrate with mixed cultures of moderately thermophilic microorganisms [J]. Hydrometallurgy, 1999, 51(1): 37-46.
[10]Rubio A, Garcia Frutos F J. Bioleaching capacity of an extremely thermophilic culture for chalcopyritic materials [J]. Minerals Engineering, 2002, 15(9): 689 - 694.
[11]Tuovinen O H, Bhatti T M, Bigham J M, et al. Oxidative dissolution of arsenopyrite by mesophilic and moderately thermophilic acidophiles [J]. Appl Environ Microbiol, 1994, 60(9): 3268 - 3274.
[12]Brierley C L. Mining biotechnology: research to commercial development and beyond [A]. Rawlings D E. Biomining: Theory, Microbes and Industrial Processes [C]. Springer-Verlag and Landes Bioscience, 1997. 81 - 101.
[13]LI Ya-qin, HE Zheng-guo. Studies on the characteristics of a moderately thermoacidophilic iron-oxidizing bacterium [J]. Microbiology, 2001, 28(6): 45 - 47.(in Chinese)
[14]Leathen W W, Mcintyre L D, Brady S A. A medium for the study of bacterial oxidation of ferrous iron [J]. Science, 1951, 114: 280 - 281.
[15]Torma A E, Walden C C, Branion R M R. Microbiological leaching of a zinc sulfide concentrate [J]. Biotechnology and Bioengineering, 1970, 7: 501 - 517.
[16]Boon M, Heijnen J J. Chemical oxidation kinetics of pyrite in bioleaching processes [J]. Hydrometallurgy, 1998, 48(1): 27 - 41.
[17]Gehrke T, Telegdi J, Thierry D, et al. Importance of extracellular polymeric substances from Thiobacillus ferrooxidans for bioleaching [J]. Appl Environ Microbiol, 1998, 64(7): 2743 - 2747.
[18]Kinzler K, Gehrke T, Telegdi J, et al. Bioleaching—a result of interfacial processes caused by extracellular polymeric substances (EPS) [J]. Hydrometallurgy, 2003, 71(1): 83 - 88.
[19]Hansford G S, Vargas T. Chemical and electrochemical basis of bioleaching processes [J]. Hydrometallurgy, 2001, 59(2-3): 135 - 145.
[20]Mehta A P, Murr L E. Fundamental studies of the contribution of galvanic interaction to acid-bacterial leaching of mixed metal sulfides [J]. Hydrometallurgy, 1983, 9: 235 - 256.
Received date: 2005-01-07; Accepted date:2005-05-11
Correspondence: SHI Shao-yuan, PhD; Tel: +86-10-62755914; E-mail: shishaoyuan@iee.pku.edu.cn


