
Preparation and electrocatalytic properties of Ti/IrO2-Ta2O5 anodes for
oxygen evolution
LI Bao-song(李保松), LIN An(林 安), GAN Fu-xing(甘复兴)
School of Resource and Environmental Science, Wuhan University, Wuhan 430079, China
Received 20 October 2005; accepted 7 March 2006
Abstract:
The preparation and electrocatalytic activity for oxygen evolution of the thermally prepared Ti anodes coated with IrO2 -Ta2O5 were studied. The structure and morphologies of the oxide films with different contents of IrO2 were determined by XRD and SEM respectively. Their electrochemical properties were studied by Linear Sweep Voltammetry, Tafel Plot and Cyclic Voltammetry. The results show that iridium and tantalum can form solid solution and the mutual solubility is affected by the ratio of Ir to Ta in coating solution. With increasing IrO2 content in the coatings, the amount of fine crystallites of IrO2 is increased and the electrocatalytic capability of oxygen evolution is strengthened. The coating adhesion and rigidity decrease, which affects electrochemical activity of the anode when the content of IrO2 is too high. The electrochemically active surface area is determined not only by the content of IrO2 but also the structure and morphology of the anode coatings. It is probably due to the existence of proper quantities of inert Ta2O5 which results in a typical morphology of cracks and solid solution structure.
Key words:
IrO2-Ta2O5; electrocatalytic activity; oxygen evolution; oxide coated anode;
1 Introduction
Oxygen and chlorine evolution reaction are the common and most important electrochemical reactions in electrolysis industry. As the Ti/RuO2-TiO2 anode has been successfully developed and widely employed in the electrochemical industry, the electrocatalytic anodes for chlorine evolution have attracted much attention in the past decade and display a huge economic and social value[1,2]. However, the performance of RuO2-TiO2 anodes which exhibit excellent capability for chlorine evolution deteriorate quickly in oxygen evolving conditions such as electrogalvanizing of steel, electro- winning of some nonferrous metals, electrometallurgy of sheet copper for printing circuitry and sulfate chromium electroplating[3]. Depending on the application, materials with low over potential for oxygen evolution reaction are required. Nevertheless, the study of electrocatalytic activity anode of oxygen evolution is more difficult than that of chlorine evolution due to the corrosive electrolytes of sulfate solution and the oxidative medium[4-6]. IrO2-Ta2O5 anodes thermally prepared on titanium substrates have received much attention as the most dominant catalyst for oxygen evolution in electrolysis industrial. This is because IrO2-Ta2O5 coating exhibits high corrosion-resistant properties and the best electrocatalytic activity for the O2 evolution reaction in sulfate system[7]. A lot of work has been done on the preparation and characterization of IrO2-Ta2O5 anode and this anode has been widely used for oxygen evolution in the electrochemical industry. Nevertheless, the mechanism of electrocatalytic activity for O2 evolution has not been sufficiently investigated in the previous literatures. The deep understanding of the relationship among composition, microstructure, morphology and electrocatalytic activity is still a challenge for electrochemists[8-10]. These problems such as unsatisfied stability, a very limited service life in oxygen evolution condition due to corrosive and oxidative environment, unclear mechanism of the electrocatalytic reaction of oxygen evolution, haven’t been solved completely yet, which limits their application largely.
In this investigation, the morphology, micro- structure and the electrocatalytic properties of the Ti/IrO2-Ta2O5 anodes with different mole ratios of IrO2 were investigated by XRD and SEM respectively.
Electrochemical properties were studied by Linear Sweep Voltammetry, Tafel Plot and Cyclic Voltammetry. Mechanism of electrocatalytic activity of O2 evolution and the internal relationship among composition, morphology, microstructure and electrocatalytic properties were analyzed.
2 Experimental
2.1 Electrode preparation
The anodes were prepared by thermal decomposition on titanium substrates at 500 ℃. The precursors H2IrCl6 and TaCl5 were dissolved in hydrochloric acid and ethanol, in which the total metal concentration was kept at around 0.2 mol/L. Solutions were prepared using deionized water and reagent grade chemicals. Titanium plates (TA1, 20 mm×20 mm×1.5 mm), which was used as the support for the oxide films, were initially sand-blasted or degreased in acetone and etched in 10% boiling oxalic acid solution for 2 h. After being rinsed with deionized water, the IrO2 layers were obtained by brushing the corresponding coating solution on Ti substrates. After being dried at 100 ℃ for the solvent being evaporated, the samples were heated at 500 ℃ for 8-15 min. The entire procedure was repeated about 10 to 20 times until the amount of iridium oxide formed on the titanium substrate was 15 g/m2, then the samples were heated at the same annealing temperature for 1 h.
2.2 Electrochemical measurement
All of the electrochemical measurements were carried out in a typical three electrodes electrochemical glass cell, taking platinum foil as a counter-electrode and saturated calomel electrode(SCE) as a reference electrode. These experiments were performed in 0.5 mol/L H2SO4 solution at 25 ℃ with a CHI660B potentiostat. Accelerated electrolysis life tests were carried out under the condition of 0.5 mol/L H2SO4 solution, 2 A/cm2 of anodic current density, Ti plate as a counter electrode and 40 ℃. As for electrochemical tests, the surface of the specimen was covered with epoxy resin except for the working area (1 cm2) on one side [11].
2.3 Microstructure analysis
The morphology and surface composition of the oxide electrodes were analyzed by S-650 scanning electronic microscope(SEM). The operating voltage of electronic beam was 25 keV for morphological observation. X-ray diffraction(XRD) was used to analyze the structure of coatings. The inspection was carried out on a D/MAX-R3 type diffractometer equipped with Cu Kα radiation, 40 keV and nickel filter. The scanning rate was 8 (?)/min.
3 Results and discussion
3.1 Structure and morphology
The morphologies of the IrO2-Ta2O5 coating have been observed by SEM. Fig.1 presents the scanning electron micrographs for freshly prepared IrO2-Ta2O5 oxide anodes with different fractions of IrO2 thermally prepared at 500℃. As can be seen from Fig.1, the surface morphologies are different with the IrO2 content varied in the coating though they are all composed of cracks and flat area on the whole. As for 10%IrO2+ 90%Ta2O5 coating, it is clear that the surface features are islands separated by cracks and there is no fine particles aggregated. The cracks are wide and deep compared with the others. As for 40%IrO2+60%Ta2O5 coating, the microstructure of the coating is heterogeneous in the whole range. The cracks are less and narrow in comparison with sample in Fig.1(a). The surface observation of Fig.1(b) by SEM at a large magnification reveals that the segregated IrO2 particles form though the amount is less than that of Fig.1(c). However, as for 70%IrO2+ 30%Ta2O5, the surface of the anode consists of dried-mud cracks surrounded by compact areas with much superficial agglomerates, which tends to connect to form networks. A large number of fine particles aggregate in the gap of the cracks, which is consistent with the morphology in Refs.[12,13]. But a large number of fine particles spread all over the surface of the 100% IrO2 coating and few cracks exist on the surface. This indicates that there is an internal relationship between the composition and morphology. With the increasing of IrO2 content in the coating, more finer crystallites of IrO2 aggregate on the surface, which has a significant influence on the electrocatalytic capability of oxygen evolution. However, the coating adhesion and rigidity will decrease, which affects electrocatalytic activity of the anode when the content of IrO2 is too high. The electrochemically active surface area is determined not only by the content of IrO2 but also the structure and morphology of the anode coating. It is probably due to the existence of proper quantities of inert Ta2O5, which results in a typical morphology of cracks and solid solution structure. The solid solution structure is helpful to improving the anode corrosion resistance. The morphology of cracks could improve anode active surface area and electrocatalytic activity of oxygen evolution. It can be concluded that the microstructure of the IrO2-Ta2O5 anodes can be controlled by adjusting the component content of coating solution.
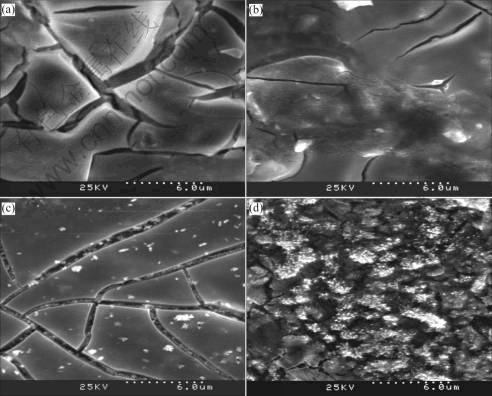
Fig.1 SEM images of IrO2-Ta2O5 anodes thermally prepared at 500 ℃: (a) 10% IrO2; (b) 40% IrO2; (c) 70% IrO2; (d) 100% IrO2
XRD patterns of the coatings are shown in Fig.2 and Fig.3. The resulting peak positions and intensities were compared with the JCPDS reference files for IrO2(No.15-870), Ta2O5 (No. 21-1198) and Ti (No.5- 682). As can be seen from the XRD patterns, the type and number of crystallite phase vary with the content ratio of Ir to Ta. The peaks of IrO2 are fairly narrow and strong. This suggests a good crystallinity of IrO2. So the segregated crystallites on coating surface can be considered the enriched IrO2 rutile[14]. Diffraction peaks corresponding to the Ti support are also observed but no TiO2 is detected. Ti may come from the penetration of X-ray to reach the substrate in some thin areas and/or the diffusion into the coating from substrate during the thermal preparation[15-17]. There are no obvious peaks of Ta2O5. The amorphous peaks of β-Ta2O5 only appear in 90% Ta2O5 coating and the intensities are not strong. These results suggest that the amount of β-Ta2O5 decreases quickly with the content of iridium increasing. As IrO2 content is up to 55%, the crystallite phase in the mixture exists entirely as rutile phase and none of amorphous peaks is detected. This result indicates that the crystallization of Ta2O5 is affected by IrO2 component. Apart from β-Ta2O5, Ti and IrO2 rutile, a certain amount of undetectable amorphous crystallite phases are also identified with IrO2 mole fraction of no more than 40%. The presence of the undetectable phases indicates an incomplete decomposition of the corresponding chloride mixture.

Fig.2 XRD patterns of Ti/IrO2-Ta2O5 anodes with different mole fractions of IrO2 thermally prepared at 500 ℃: (a) 10% IrO2; (b) 40% IrO2; (c) 70% IrO2; (d) 100% IrO2
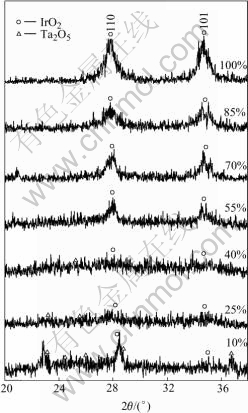
Fig.3 XRD patterns of IrO2-Ta2O5 anodes with different mole ratios of IrO2 in scanning range of 20° to 38°
Fig.3 presents XRD patterns of the IrO2-Ta2O5 anodes in the range of 20? to 38?. It indicates that there is a solid solution of Ta component in IrO2 rutile in IrO2-Ta2O5 coating and the solubility of Ta in rutile phase is affected by the ratio of Ir to Ta. MASATSUGU [18] and KRYSA et al[19] reported that since the ion radius of Ta3+, Ta5+ and Ir4+ are extremely close (0.74, 0.72 and 0.71 ?, respectively), Ir and Ta compounds tend to form a solid solution during the thermolysis formation of the mixed oxides. They also pointed out that the rutile lattice is deformed and the cell volume increases due to access of a larger ion of Ta component[20]. The finer the crystallites of IrO2, the more contributions of Ta2O5 modification to the mixed modified phase. The existence of proper quantities of inert Ta2O5 in the coating results in a typical morphology of cracks and solid solution structure. Proper ratio of Ir to Ta leads to an improvement of electrocatalytic activity of oxygen evolution and good electrolysis durability.
3.2 Polarization behaviors
It has been widely accepted that, on metals and/or oxides, the minimum required potential for oxygen evolution is determined by the metal/metal oxide or the lower metal oxide/higher metal oxide couple[21]. As for IrO2 based oxide anodes, IrO2/IrO3 couple is generally concerned as the oxygen evolution governing couple. Fig.4 shows that the current of oxygen evolution at 1.5 V on Ti/IrO2-Ta2O5 anodes as a function of IrO2 content. The current increases slowly from 10% to 40%. Then the current increases quickly and reaches the maximum at the content of 70% IrO2. At the content of 100% IrO2, the current of oxygen evolution is high. Nevertheless, the adhesion and rigidity of the coating are worse in comparison with the others. As shown in Fig.5(a), with the IrO2 content increasing, the maximum current of oxygen evolution increases quickly from 10% to 45%. Then the maximum current is nearly steady at 100% IrO2 content on the whole. On the contrary, the overpotential of oxygen evolution drops quickly from 10% to 60% and then it is nearly stable. The characterization of corrosion current and corrosion potential can be seen in Fig.5(b). Fig.6 shows the Tafel Plot and Linear Sweep Voltammetry of Ti/IrO2-Ta2O5 electrodes in 0.5 mol/L H2SO4 solution at 25 ℃. The reason of current vibration of 40% IrO2 on Tafel line in Fig.6(b) is that the anode reaction surface area is varied as the oxygen bubbles form and burst on the coating surface during the course of oxygen evolution.

Fig.4 Current of O2 evolution at φ=1.5 V (vs SCE) on Ti/IrO2- Ta2O5 anodes
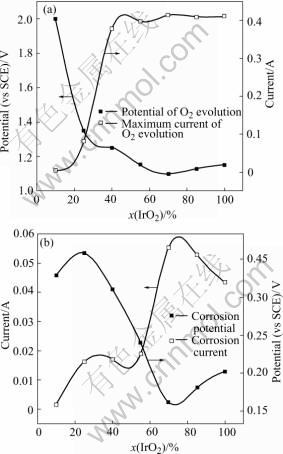
Fig.5 Overpotential and maximum current of oxygen evolution (a), corrosion current and corrosion potential (b) of Ti/IrO2-Ta2O5 electrodes
3.3 Cyclic voltammetry and electrocatalytic activity
The voltammetric charge capacity (Q) obtained by integration of the cyclic voltammograms indicates the amount of protons exchanged with the solution[22]. The peaks of the IrO2 electrode are due to the redox transitions of oxygen iridium group species on electrode surface, Ir3+/Ir4+ and Ir4+/Ir6+. Therefore, the value of Q is expected to be proportional to the electrochemically active surface area of the oxide electrode and thought to be able to represent the number of electrochemically active sites on the surface[23]. For the fresh anode sintered at 500 ℃, the oxygen evolution reaction and hydrogen evolution reaction are observed at about 1.2 and -0.6 V respectively. In Fig.7, the pair of peaks at 0.7 V are attributed to the surface redox transition of Ir3+/Ir4+ and this reaction has good reversibility, which is concluded from the symmetrical shape of the peaks. This indicates that the surface electrochemistry of the electrode is governed by the active component of IrO2.
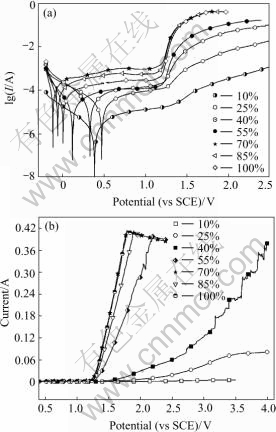
Fig.6 Tafel Plot (a) and Linear Sweep Voltammetry (b) of Ti/IrO2-Ta2O5 electrodes in 0.5 mol/L H2SO4 (v=10 mV/s)
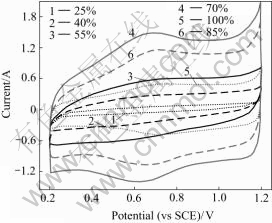
Fig.7 Cyclic voltammograms of Ti/IrO2-Ta2O5 electrodes with different contents of IrO2 (v=20 mV/s)
As can be seen from Fig.8, the voltammetric charges increase then decrease with the content of IrO2 increasing, with a maximum at 70% IrO2, which is similar to the results of current of O2 evolution. Then the function of Ta2O5 is displayed. The reason is probably that proper quantities of inert Ta2O5 with the active composition IrO2 form solid solution and this microstructure is useful to the improvement of the coating adhesion and corrosion-resistance ability in sulfate electrolysis. The typical morphology of cracks is strengthened and leads to an increase of surface active area and an improvement of electrocatalytic activity of oxygen evolution. So the electrode electrocatalytic activity depends on not only the composition of the active layer but also the structure and morphology of the IrO2-Ta2O5 coating.
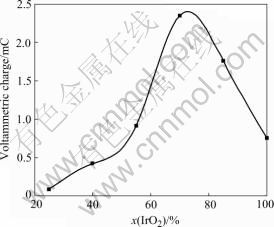
Fig.8 Voltammetric charge of Ti/IrO2-Ta2O5 electrodes with different contents of IrO2
3.4 Electrode stability and deactivation
It is well known that the IrO2-Ta2O5 anodes are deactivated with electrolysis[24]. As can be seen from Fig.9, the accelerated service life of Ti/70%IrO2-30% Ta2O5 anode is about 630 h. During the accelerated electrolysis, the potential decreases quickly in the first 50 h. This stage can be attributed to the wear and erosion of the loose part of the porous oxide coatings prepared by thermal decomposition under the action of intense oxygen evolution. Then the potential tends to stabilize for about 550 h, occupying the main portion of the whole electrolysis time. This stage is related to the electro- chemical dissolution of IrO2. At the end of the electrolysis, the potential starts to increase rapidly, and in a short time the anode is deactivated. This stage may be attributed to the detachment of the coating in some areas and the passivation of the substrate. This is in reasonable agreement with the Ir dissolution rate of IrO2 anode during the accelerated life test reported by XU et al[2] and JIN et al[25].
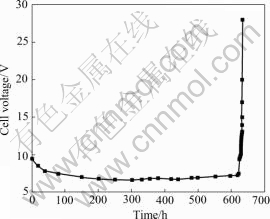
Fig.9 Change of cell voltage with operation time during accelerated electrolysis of Ti/70%IrO2-30%Ta2O5 anodes at 2 A/cm2 in 0.5 mol/L H2SO4 solution
4 Conclusions
1) With the increasing of IrO2 content in IrO2-Ta2O5 coatings, more fine crystallites of IrO2 are found on the coating surface, resulting in the active surface area improved and the electrocatalytic capability of oxygen evolution strengthened. However coating adhesion and rigidity decrease, which affects electrochemical activity of the anode when the content of IrO2 is too high. This shows that the electrochemically active surface area is determined not only by the active content of IrO2 but also by the structure and morphology of the anode coating. The proper ratio of Ir to Ta is necessary for the best electrocatalytic capability of oxygen evolution and electrolysis stability.
2) Iridium and tantalum can form solid solution and the mutual solubility is affected by the ratio of iridium to tantalum. The solid solution structure is helpful to improving the electrolysis stability and prolonging the anode service life. The addition of proper quantities of Ta2O5 results in a typical morphology of cracks, which leads to an increase of surface active area and an improvement of electrocatalytic activity of oxygen evolution.
3) The electrochemical stability of the IrO2-Ta2O5 anode is apt to deteriorate under the influence of the strong acid and oxygen evolution. In the corrosive and oxidative working medium, how to improve the anode electrolysis stability with high electrocatalytic activity of oxygen evolution is an important work which hasn’t been solved yet.
References[1] HU Ji-ming, WU Ji-xun, MENG Hui-min, SUN Dong-bai, ZHU Yan-rong, YANG De-jun. Degradation characteristics of Ti/(IrO2+Ta2O2) coating anodes in H2SO4 solution [J]. Trans Nonferrous Met Soc China, 2000, 10(4): 511-515.
[2] ALVES V A, DA SILVA L A, BOODTS J F C. Surface characterization of IrO2/TiO2/CeO2 oxide electrodes and Faradaic impedance investigation of the oxygen evolution reaction from alkaline solution [J]. Electrochimica Acta, 1998, 44: 1525-1534.
[3] DE OLIVEIRRA-SOUSA A, DA SILVA M A S, MACHADO S A S, AVACA K A, DE LIMA-NETO P. Influence of the preparation method on the morphological and electrochemical properties of Ti/IrO2 coated electrodes [J]. Electrochimica Acta, 2000, 45: 4467-4473.
[4] HU Ji-ming, ZHANG Jian-qing, CAO Chu-nan. Thermolytic formation and microstructure of IrO2+Ta2O5 mixed oxide anodes from chloride precursor [J]. Thermochimica Acta, 2003, 403: 257-266.
[5] OTOGAWA R, MORIMITSU M, MATSUNAGA M. Effects of microstructure of IrO2-based anodes on electrocatalytic properties [J]. Electrochimica Acta, 1998, 44: 1509-1513.
[6] PAUPORTE T H, ANDOLFATTO F, DURAND R. Some electrocatalytic properties of anodic iridium oxide nanoparticles in acidic solution [J]. Electrochimica Acta, 1999, 45: 431-439.
[7] DE PAULI C P, TRASATTI S. Composite materials for electrocatalysis of O2 evolution: IrO2+SnO2 in acid solution [J]. Journal of Electroanalytical Chemistry, 2002, 538-539: 145-151.
[8] HU Ji-ming, ZHANG Jian-qing, CAO Chu-nan. Oxygen evolution reaction on IrO2-based DSA type electrodes: kinetics analysis of Tafel lines and EIS [J]. International Journal of Hydrogen Energy, 2004, 29: 791-797.
[9] KIM K W, LEE E H, KIM J S. A study on performance improvement of Ir oxide-coated titanium electrode for organic destruction [J]. Electrochimica Acta, 2002, 47: 2525-2531.
[10] FOTI G, MOUSTY C, REID V, COMNINELLIS C H. Characterization of DSA type electrodes prepared by rapid thermal decomposition of the metal precursor [J]. Electrochimica Acta, 1998, 44: 813-818.
[11] KAMEGAYA Y, SASAKI K M. Improved durability of iridium oxide coated titanium anode with interlayers for oxygen evolution at high current densities [J]. Oguri Electrochimica Acta, 1995, 40: 889-895.
[12] SIMOND O, COMNINELLIS C H. Anodic oxidation of organics on Ti/IrO2 anodes using Nafion as electrolyte [J]. Electrochimica Acta, 1996, 42: 2013-2018.
[13] HU Ji-ming, ZHANG Jian-qing, MENG Hui-min. Microstructure, electrochemical surface and electrocatalytic properties of IrO2+Ta2O5 oxide electrodes [J]. Journal of Materials Sciences, 2003, 38: 705-712.
[14] CARDARELLI F, TAXIL P, SAVALL A, COMNINELLIS C, MANOLI G, LECLERC O. Preparation of oxygen evolving electrodes with long service life under extreme conditions [J]. Journal of Applied Electrochemistry, 1998, 28(3): 245-250.
[15] BOCK C, SPINNEY H, MACDOUGALL B. A study of the deactivation and service life of Ir oxide anodes supported on Al substrates [J]. Journal of Applied Electrochemistry, 2000, 30(5): 523-532.
[16] LIAO P C, CHEN C S, HO W S, HUANG Y S, TIONG K K. Characterization of IrO2 thin films by Raman spectroscopy [J]. Thin Solid Film, 1997, 301: 7-11.
[17] KRISTOF J, SZILAGYI T, HORVATH E, BATTISTI A D, FROST R L, REDEY A. Investigation of IrO2/Ta2O5 thin film evolution [J]. Thermochimica Acta, 2004, 413: 93-99.
[18] MORIMITSU M, OTOGAWA R, MATSUNAGA M. Effects of cathodizing on the morphology and composition of IrO2-Ta2O5/Ti anodes [J]. Electrochimica Acta, 2000, 46: 401-406.
[19] KRYSA J, MAIXNER J, MRAZ R, ROUSAR I. Effect of coating thickness on the properties of IrO2-Ta2O5 anodes [J]. Journal of Applied Electrochemistry, 1998, 28: 369-372.
[20] GORODETSKII V V, NEBURCHILOV V A. Titanium anodes with active coatings based on iridium oxides: a sublayer between the active coating and titanium [J]. Russian Journal of Electrochemistry, 2003, 39(10): 1111-1115
[21] ROSSI A, BOODTS J F C. Ir-based oxide electrodes oxygen evolution reaction from mixed solvents [J]. Journal of Applied Electrochemistry, 2002, 32: 735-741.
[22] XU L F, SCANTLEBURY J D. A study on the deactivation of an IrO2-Ta2O5 coated titanium anode [J]. Corrosion Science, 2003, 45: 2729-2740.
[23] HU J M, MENG H M, ZHANG J Q, CAO C N. Degradation mechanism of long service life Ti/IrO2-Ta2O5 oxide anodes in sulphuric acid [J]. Corrosion Science, 2002, 44: 1655-1668.
[24] MORIMITSU M, TAMURA H, MATSUNAGA M, OTOGAWA R. Polarization behaviour and lifetime of IrO2-Ta2O5-SnO2/Ti anodes in p-phenolsulfonic acid solutions for tin plating [J]. Journal of Applied Electrochemistry, 2000, 30(4): 511-514.
[25] JIN Shi-xiong, YE Si-yu. Oxygen evolution on titanium anodes coated with conductive metallic oxides: kinetics and mechanism in alkaline solution [J]. Electrochimica Acta, 1996, 41: 827-834.
Foundation item: Project(50499330) supported by the National Natural Science Foundation of China
Corresponding author: LI Bao-song; Tel: +86-27-68775799; E-mail: lbs79@126.com
(Edited by YANG Bing)
Abstract: The preparation and electrocatalytic activity for oxygen evolution of the thermally prepared Ti anodes coated with IrO2 -Ta2O5 were studied. The structure and morphologies of the oxide films with different contents of IrO2 were determined by XRD and SEM respectively. Their electrochemical properties were studied by Linear Sweep Voltammetry, Tafel Plot and Cyclic Voltammetry. The results show that iridium and tantalum can form solid solution and the mutual solubility is affected by the ratio of Ir to Ta in coating solution. With increasing IrO2 content in the coatings, the amount of fine crystallites of IrO2 is increased and the electrocatalytic capability of oxygen evolution is strengthened. The coating adhesion and rigidity decrease, which affects electrochemical activity of the anode when the content of IrO2 is too high. The electrochemically active surface area is determined not only by the content of IrO2 but also the structure and morphology of the anode coatings. It is probably due to the existence of proper quantities of inert Ta2O5 which results in a typical morphology of cracks and solid solution structure.


