Trans. Nonferrous Met. Soc. China 28(2018) 974-979
Green synthesis of Ag nanoparticles: Effect of algae life cycle on Ag nanoparticle production and long-term stability
Oksana VELGOSOVA1, Anna  1, Elena
1, Elena  2, Jaroslav
2, Jaroslav  2
2
1. Institute of Materials and Quality Engineering, Faculty of Materials, Metallurgy and Recycling, Technical University of  ,
,  9/A, 040 01
9/A, 040 01  , Slovakia Republic;
, Slovakia Republic;
2. Department of Materials Engineering, Faculty of Mechanical Engineering, Czech Technical University in Prague, Karlovo nam. 13, 121 32 Prague 2, Czech Republic
Received 22 February 2017; accepted 23 June 2017
Abstract:
Spherical Ag nanoparticles (AgNPs) were biologically synthesized using four different extracts prepared from Parachlorella kessleri algae cultivated for 1, 2, 3 and 4 weeks. The influence of algae life cycle on AgNPs formation and effect of different storage conditions on AgNPs long-term stability were investigated. The age of algae influenced the rate of AgNPs synthesis and amount of AgNPs in solution. The age of algae did not influence the AgNPs long-term stability. UV–vis and TEM observation revealed that long-term stability of AgNPs can be influenced by storage temperatures, and low temperature positively influences the AgNPs stability. AgNPs stored at dark and at temperature of ~5 °C showed the best long-term stability regardless of the culture age. Such AgNPs remained spherical, fine (5-20 nm) and stable (no agglomeration) even after 6 months.
Key words:
Ag nanoparticles; stability; Parachlorella kessleri;
1 Introduction
The possibility of silver nanoparticles (AgNPs) synthesized with different sizes, shapes and amounts puts this product in focus for the widest range of needs (water disinfection applications, medical implants, bandages, surgical coatings, biosensors, catalysis and antimicrobial surface coatings, optoelectronics, cosmetics, dietary supplements, food packaging, and textiles) [1-5]. For that reason, lots of physical, chemical and biological methods focused on AgNPs formation were developed [6-11]. Physical methods (chemical vapour deposition, molecular beam epitaxy etc.) usually require expensive technology, chemical methods belong to more affordable methods but they are not environment friendly. The green syntheses gain advantage over physical and chemical methods because overall material and energy consumptions are extremely lower, offering a low-cost green alternative [9]. Many different kinds of plant extracts, bacteria, fungi and algae have been used as biological materials for the synthesis of nanoparticles [12-17]. Reducing sugars, ketones/ aldehydes, amine groups, water soluble heterocyclic compounds and proteins are naturally present in such materials and play a key role not only as the silver ions reduction agents but also as AgNPs stabilizers [18,19].
The biochemical pathways responsible for the production of metal NPs using biological materials are well researched. However, the life cycle or stability of AgNPs is still the subject of research. There was some research in the field of environmental, health and safety risks aimed to better understand over the entire life cycle of chemically prepared AgNPs [20,21]. However, the long-term stability of AgNPs prepared by green synthesis is still not researched in detail, so lots of questions are not answered.
For the synthesis of fine AgNPs, the extract of Parachlorella kessleri algae (1, 2, 3 and 4 weeks old algae) was used. The aim of the study was to investigate the impact of culture age and storage conditions (exposure to dark at different temperatures) on long-term stability of AgNPs. Since knowledge in the field of AgNPs stability is not well known, the results reported in this study will shed light on entire AgNPs life cycle.
2 Experimental
Algae Parachlorella kessleri, (syn. Chlorella kessleri) strain LARG/1, supplied by CCALA, No. 253, supplied by Institute of Botany, Czech Academy of Sciences were used for the experiments. The algae were cultivated in Petri dishes for 1, 2, 3 and 4 weeks at the ambient temperature. Nutrient medium consisted of 2% agar and Millieu Bristol nutrient solution. After cultivation the algae of different age were collected separately and treated by boiling in water bath for 15 min and centrifuging at 3000 r/min for 15 min. The cells amount used for extract preparation was determined by automatic cell counter and was the same for all extracts. The liquid phases obtained after centrifugation (four different extracts, labelled as: 1wE – the algal extract derived from one week old algae; 2wE – the algal extract derived from algae cultivated for two weeks; 3wE – the algal extract derived from algae cultivated for 3 weeks; 4wE – the algal extract derived from algae cultivated for four weeks) were removed and transferred into four Erlenmeyer flasks containing 0.92 mmol/L AgNO3 solution (concentration of Ag 100 mg/L). The solutions were left for 4 days at room temperature. Subsequently two AgNPs solutions were chosen, divided into Erlenmeyer flasks and stored in dark at room temperature and at 5 °C (in refrigerator).
The AgNPs stability was monitored by measuring the UV-vis spectra of the solutions in 10 mm optical-path-length quartz semimicrocuvettes (UNICAM UV/vis Spectrometer UV4). The size and morphology of the nanoparticles were studied by a transmission electron microscope (JEOL model JEM-2000FX microscope operated at an accelerating voltage of 200 kV). EVETM automatic cell counter was used to obtain Parachlorella kessleri exact cell count.
3 Results and discussion
All organisms, from little one cell algae to moss, go during their life through a number of biological phases. Therefore, the composition and amount of trace elements, vitamins, bioflavonoids, lipoid acids, enzymes, amino acids, proteins, as well as plant substances and fatty acids in algae changed with time. The contribution and ability of culture to create the AgNPs logically changed during their life. The extracts prepared from algae with different age was used for comparing their ability to form AgNPs.
Addition of the green algae extracts to AgNO3 solutions led to changes of solution colour (Fig. 1). The colour change is the result of the radiation absorption in the visible region of the electromagnetic spectrum (380-450 nm) due to the localised surface plasmon of AgNPs [21-23].
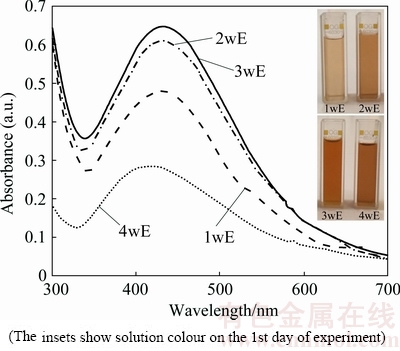
Fig. 1 UV-vis absorption spectra of AgNPs (after 24 h) (The insets show solution colour on the 1st day of experiment)
After 24 h the solution colours changed in dependence of the extract type from light brown (1wE) to dark brown (4wE) (Fig. 1), which confirmed the presence of AgNPs in all experimental solutions. UV-vis spectra in Fig. 1 show surface plasmon resonance (SPR) bands of AgNPs. It is clear that regardless of culture age is possible to prepare AgNPs, but using 2wE and 3wE extracts is the most efficient. This two extracts gave symmetric and narrow SPR bands with the highest absorbance. Depending on the SPR band shape it is possible to assume the AgNPs shape and size uniformity [15,24]. Small spherical nanoparticles exhibit a single, symmetrical SPR band, whereas large and/or different shaped particles reveal two or three peaks. Based on the SPR band shape and the absorbance (Fig. 1), it is possible to say that AgNPs prepared by 1wE, 2wE and 3wE extracts are symmetric with narrow interval of size distribution.
The weaker symmetry of SPR band of nanoparticles prepared by 4wE extract indicates larger particles with wider size distribution and in comparison to above mentioned SPR bands the absorbance is the lowest, which indicates the low concentration of AgNPs in solution.
For further analyses and comparison of algae ability to form nanoparticles, the best and the weakest results (Fig. 1) (prepared by 3wE and 4wE extract) were chosen. The SPR bands of these AgNPs measured on the 1st, 2nd and 4th day of experiment are shown in Fig. 2(a).
The UV-vis spectroscopy (Fig. 2(b)) shows symmetric SPR bands with a maximum wavelength at about 428 nm. The increasing of absorbance intensity with time indicates that Ag+ ions are still presented in solution [25]. The TEM image of AgNPs formed on the 4th day (Fig. 2(b)), clearly confirmed the formation of fine, spherical AgNPs surrounded by a thin layer of organic material which is characteristic of AgNPs prepared in plant extracts. Particle size histogram revealed that more than 90% of AgNPs are down to 20 nm in diameter.

Fig. 2 UV-vis absorption spectra of AgNPs (1st day) prepared by 3wE and 4wE extracts (a); and SPR bands of AgNPs, size distribution and TEM images of AgNPs on 4th day of experiment for 3wE (b) and for 4wE (c)
The SPR bands measured on the 1st, 2nd and 4th day of AgNPs prepared by 4wE extract, (Fig. 2(c)), show SPR bands with weaker symmetry, maximum absorbances were lower, 0.477, 0.607 and 0.646 for the 1st, 2nd and 4th day of experiment respectively, and slight red shifts of wavelength are evident, from 414 to 425 nm for the 1st and 4th day respectively. Nanoparticle size histogram (on the 4th day of experiment) shows wider interval of AgNPs size, 80% of AgNPs were in interval from 20 to 60 nm in diameter.
For analysing of AgNPs long-term stability, nanoparticles after 4 days experiment were used. The 4th day was considered as day 0 for subsequent experiments.
It is well-known that light acts destructively on AgNPs. IZAK-NAU et al [21] assumed that such changes in AgNPs' stability under this condition could be attributed to the elevated temperature of the dispersions exposed to daylight, which can increase the NPs collision rate and subsequently induce faster agglomeration. Additionally, daylight can cause photo-reduction of already dissolved Ag+ that consequently may lead to the production of new NPs increasing the overall sample polydispersity. Therefore, combination of dark with low (~5 °C) and room temperature was chosen for long-term stability experiments.
Figure 3 shows the UV-vis spectra and size distribution of AgNPs prepared by extract from three weeks’ old algae stored under different conditions.
The SPR bands of AgNPs prepared by 3wE extract were symmetrical, the time and temperature of storage did not influence the symmetry of SPR bands which indicated small and uniform AgNPs. However, at room temperature steady increase of absorbance, λmax, against time of experiment (from 1.206 on day 0 to 1.533 on the 300th day) and slight increase of SPR band width may indicate the onset of changes in solution (Fig. 3(a)). According to works [26,27], this behaviour could be possible if the dissolution or agglomeration of nanoparticles occurs in solution. The visual inspection of the stored solution confirmed slight sediment of AgNPs on the bottom of Erlenmeyer flask on the 300th day.
Based on the TEM micrograph the size distribution of AgNPs was determined (Fig. 3(c)). Irrespective of the storage time almost 90% of AgNPs were down to 20 nm. However, in solutions stored at room temperature slight increase of particles amount in interval from 5 to 10 nm on the 300th day of experiment was observed. Considering the slight amount of AgNPs sediment on the bottom of Erlenmeyer flask we assume that small, well dispersed particles remained in solution and some agglomerated particles settled down.

Fig. 3 UV–vis spectra of AgNPs prepared by 3wE extract stored under different conditions
The combination of dark and low temperature can guarantee better long-term stability of AgNPs (Fig. 3(b)). UV-vis for AgNPs stored at 5 °C did not change at all, no agglomeration and no significant increase in particle frequency in dependent of time were observed (Fig. 3(c)).
The UV-vis spectra and size distribution of AgNPs prepared by extract from four weeks old algae stored at different temperatures in dark are shown in Fig. 4. The absorbance of AgNPs stored at room temperature increased from 0.646 to 0.871 for day 0 and the 300th day of experiment respectively (Fig. 4(a)). The change of SPR band position or shape was not observed at low temperature (Fig. 4(b)). The AgNPs remained the same as that at the beginning of experiment.
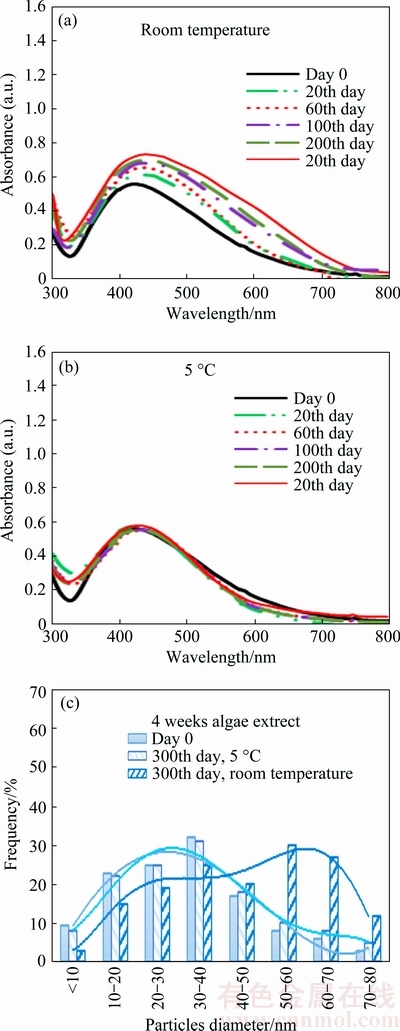
Fig. 4 UV–vis spectra of AgNPs prepared by 4wE extract stored under different conditions
The size distribution of AgNPs (Fig. 4(c)), confirmed that AgNPs are more stable at low temperature. Increase of particles size was observed only at room temperature.
Above mentioned experimental results showed that dark has a crucial influence on AgNPs long-term stability. The position of the peaks and shape of SPR bands of nanoparticles stored at low temperature can be considered stably throughout long-term stability experiment. It is clear that algae age did not influence the stability of nanoparticles. The only difference in using three or four weeks old algae extract is in decreasing of the λmax (Fig. 2). The culture age influenced only the efficiency of AgNPs production. The best efficiency of AgNP production was observed using extract prepared from three and two weeks old algae.
4 Conclusions
1) The long-term stability of Ag nanoparticles can be influenced by storage conditions.
2) Dark and low temperature positively influence the AgNPs stability.
3) The algae life cycle is important for AgNPs formation and the age of algae does not influence the long-term stability.
4) The rate of nanoparticle production and amount of AgNPs in solution can be influenced by the algae age.
Acknowledgements
This work was supported by Slovak Grant Agency (VEGA 1/0197/15), and by the Ministry of Education, Youth and Sport of the Czech Republic within the scope of project No. LO1207 of the programme NPU1.
References
[1] IDER M, ABDERRAFI K, EDDAHBI A, OUASKIT S, KASSIBA A. Rapid synthesis of silver nanoparticles by microwave-polyol method with the assistance of latex copolymer [J]. J Clust Sci, 2017, 28(3): 1025-1040.
[2] SHIM I K, LEE Y, LEE K J, JOUNG J. An organometallic route to highly monodispersed silver nanoparticles and their application to ink-jet printing [J]. Mat Chemistry and Physics, 2008, 110: 316-321.
[3] DAVENAS J, LTAIEF A, BARLIER V, BOITEUX G, BOUAZIZI A. Nanomaterials for photovoltaic conversion [J]. Mater Sci and Eng C, 2008, 28: 744-750.
[4] LAH N A C, JOHAN M R. Facile shape control synthesis and optical properties of silver nanoparticlesstabilized by Daxad 19 surfactant [J]. Appl Surf Sci, 2011, 257: 7494-7500.
[5] ESPENTI C S, RAO K S V K, RAO K M. Bio-synthesis and characterization of silver nanoparticles using Terminalia chebula leaf extract and evaluation of its antimicrobial potential [J]. Materials Letters, 2016, 174: 129-133.
[6] VENUGOBAL J, ANANDALAKSHMI K. Green synthesis of silver nanoparticles using commiphora caudata leaves extract and the study of bactericidal efficiency [J]. J Clust Sci, 2016, 27: 1683-1699.
[7] ZHANG W, ZHANG L, SUN Y. Size-controlled green synthesis of silver nanoparticles assisted by L-cysteine [J]. Front Chem Sci Eng, 2015, 9: 494-500.
[8] ROSTAMI-VARTOONI A, NASROLLAHZADEH M, ALIZADEH M. Green synthesis of perlite supported silver nanoparticles using Hamamelis virginiana leaf extract and investigation of its catalytic activity for the reduction of 4-nitrophenol and Congo red [J]. J Alloys Compd, 2016, 680: 309-314.
[9] YILMAZ M, TURKDEMIR H, AKIF KILIC M, BAYRAM E, CICEKE A, METE A, ULUG B. Biosynthesis of silver nanoparticles using leaves of Stevia rebaudiana [J]. Mat Chem Physics, 2011, 130: 1195-1202.
[10] KUMAR B, SMITA K, DEBUT A, CUMBAL L. Extracellular green synthesis of silver nanoparticles using Amazonian fruit Araza (Eugenia stipitata McVaugh) [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 2363-2371.
[11] TAHERI M M, ABDUL KADIR M R, AHMAD SHAFIAI N K, SHOKUHFAR T, ASSADIAN M, SHIRDAR M R. Green synthesis of silver nanoneedles using shallot and apricot tree gum [J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 3286-3290.
[12] ZHAO Zhong-yin, WANG Mao-hua, LIU Ting-ting. Tribulus terrestris leaf extract assisted green synthesis and gas sensing properties of Ag-coated ZnO nanoparticles [J]. Mat Letters, 2015, 158: 274-277.
[13] ROOPAN S M, MADHUMITHA R G, RAHUMAN A A, KAMARAJ C, BHARATHIA A, SURENDRA T V. Low -cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity [J]. Industrial Crops and Products, 2013, 43: 631-635.
[14] FAYAZ A M, GIRILA M, RAHMAN M, VENKATESAN R, KALAICHELVAN P T. Biosynthesis of silver and gold nanoparticles using thermophilic bacterium Geobacillus stearothermophilus [J]. Process Biochemistry, 2011, 46: 1958-1962.
[15] SOHRABNEZHAD Sh, RASSA M, SEIFI A. Green synthesis of Ag nanoparticles in montmorillonite [J]. Mat Letters, 2015, 168: 28-30.
[16] VINMATHI V, PACKIA JACOB S J. A green and facile approach for the synthesis of silver nanoparticles using aqueous extract of Ailanthus excelsa leaves, evaluation of its antibacterial and anticancer efficacy [J]. Bull Mater Sci., 2015, 38: 625-628.
[17] SIBY J, BEENA M. Microwave-assisted facile green synthesis of silver nanoparticles and spectroscopic investigation of the catalytic activity [J]. Bull Mater Sci, 2015, 38: 659-666.
[18] SHANKAR P D, SHOBANA S, KARUPPUSAMY I, PUGAZHENDHI A, RAMKUMAR V S, ARVINDNARAYAN S, KUMAR G. A review on the biosynthesis of metallic nanoparticles (gold and silver) using bio-components of microalgae: Formation mechanism and applications [J]. Enzyme Microb Technol, 2016, 95: 28-44.
[19] PEREZ G R M, ZAVALA S M A, PEREZ G S, PEREZ G C. Antidiabetic effect of compounds isolated from plants [J]. Phytomedicin, 1998, 5(1): 55-75.
[20] GORHAM J M, ROHLFING A B, LIPPA K A, MacCUSPIE R I, HEMMATI A, HOLBROOK R D. Storage wars: How citrate-capped silver nanoparticle suspensions are affected by not-so-trivial decisions [J]. J Nanopart Res, 2014, 16: 2339-2353.
[21] IZAK-NAU E, HUK A, REIDY B, UGGERUD H, VADSET M, EIDEN S, VOETZ M, HIMLY M, DUSCHL A, DUSINSKA M, LYNCH I. Impact of storage conditions and storage time on silver nanoparticles' physicochemical properties and implications for their biological effects [J]. RSC Adv, 2015, 5: 84172-84185.
[22]  R. The effect of culture age, mixing and initial silver concentration on biological formation of Ag nanoparticles [J]. Nova Biotechnol et Chimica, 2014, 13: 16-25.
R. The effect of culture age, mixing and initial silver concentration on biological formation of Ag nanoparticles [J]. Nova Biotechnol et Chimica, 2014, 13: 16-25.
[23] SHANKAR S, AHMAD A, SASTRY M. Geranium leaf assisted biosynthesis of silver nanoparticles, Synthesis, characterization and antimicrobial activity of dextran stabilized silver nanoparticles in aqueous medium [J]. Biotechnol Prog, 2003, 19: 1627-1631.
[24] BANKURA K P, MAITY D, MOLLICK M M R, MONDAL D, BHOWMICK B, BAIN M K, CHAKRABORTY A, SARKAR J, ACHARYA K, D CHATTOPADHYAY. Synthesis, characterization and antimicrobial activity of dextran stabilized silver nanoparticles in aqueous medium [J]. Carbohydrate Polymers, 2012, 89: 1159-1165.
[25] SHALIGRAM N S, BULE M, BHAMBURE R, SINGHAL R S, SINGH S K, SZAKACS G, PANDEY A. Shape-controlled synthesis and surface plasmonic properties of metallic nanostructures [J]. Process Biochem, 2009, 44: 939-943.
[26] SCHNEIDER S, HALBIG P, GRAU H, NICKEL U. Reproducible preparation of silver sols with uniform particle size for application in surface-enhanced Raman spectroscopy [J]. Photochem. Photobiol., 1994, 60: 605-610.
[27] XIA Y, HALAS N J. Shape-controlled synthesis and surface plasmonic properties of metallic nanostructures [J]. MRS Bull., 2005, 30: 338-343.
银纳米颗粒的绿色合成:藻类生命周期对银纳米颗粒合成和长期稳定性的影响
Oksana VELGOSOVA1, Anna  1, Elena
1, Elena  2, Jaroslav
2, Jaroslav  2
2
1. Institute of Materials and Quality Engineering, Faculty of Materials, Metallurgy and Recycling, Technical University of  ,
,  9/A, 040 01
9/A, 040 01  , Slovakia Republic;
, Slovakia Republic;
2. Department of Materials Engineering, Faculty of Mechanical Engineering, Czech Technical University in Prague, Karlovo nam. 13, 121 32 Prague 2, Czech Republic
摘 要:用分别培养了1、2、3 和4周的凯氏拟小球藻的四种不同的提取物,生物合成球形银纳米颗粒(AgNPs)。研究藻类生命周期对AgNPs合成的影响和不同的储存条件对AgNPs 长期稳定性的影响。结果发现,藻类的年龄会影响AgNPs 的合成率和溶液中的数量,但不影响AgNPs的长期稳定性。UV–vis 和 TEM 的结果显示,AgNPs的长期稳定性受储存温度的影响,低温更有利于其长期稳定性。储存在黑暗环境和5 °C 左右时,AgNPs 表现出最佳的长期稳定性,而与藻类年龄无关,存储6个月以后,这些AgNPs 仍然保持着球形,尺寸细小(5~20 nm),且无团聚。
关键词:银纳米颗粒;稳定性;凯氏拟小球藻
(Edited by Xiang-qun LI)
Corresponding author: Oksana VELGOSOVA; Tel: +421-55-602-2533; Fax: +421-55-602-2770; E-mail: oksana.velgosova@tuke.sk
DOI: 10.1016/S1003-6326(18)64732-6
Abstract: Spherical Ag nanoparticles (AgNPs) were biologically synthesized using four different extracts prepared from Parachlorella kessleri algae cultivated for 1, 2, 3 and 4 weeks. The influence of algae life cycle on AgNPs formation and effect of different storage conditions on AgNPs long-term stability were investigated. The age of algae influenced the rate of AgNPs synthesis and amount of AgNPs in solution. The age of algae did not influence the AgNPs long-term stability. UV–vis and TEM observation revealed that long-term stability of AgNPs can be influenced by storage temperatures, and low temperature positively influences the AgNPs stability. AgNPs stored at dark and at temperature of ~5 °C showed the best long-term stability regardless of the culture age. Such AgNPs remained spherical, fine (5-20 nm) and stable (no agglomeration) even after 6 months.


