
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 241-245 Phase equilibria of Mg-rich corner in Mg-Zn-Al ternary system at 300 ℃
REN Yu-ping, QIN Gao-wu, PEI Wen-li, LI Song, GUO Yun, ZHAO Hong-da
Key Laboratory for Anisotropy and Texture of Materials of Ministry of Education,Northeastern University, Shenyang 110819
Received 8 January 2011; accepted 26 April 2011
Abstract:
The phase equilibria and compositions in Mg-rich corner at 300 ℃ were determined in the Mg-Zn-Al ternary system through the equilibrated alloy method by using X-ray diffraction (XRD) and scanning electron microscopy (SEM) assisted with energy dispersive spectroscopy of X-ray (EDS). The results show that there exist three three-phase regions consisted of α-Mg+Mg17Al12(γ)+Al5Mg11Zn4(φ), α-Mg+Mg32(Al, Zn)49(τ)+Al5Mg11Zn4(φ) and α-Mg+MgZn+Mg32(Al, Zn)49(τ), respectively. The intermetallic compounds in equilibrium with α-Mg phase all have large composition ranges, not appear to be linear. At the same time, both zinc and aluminum are soluble in the α-Mg solid solution, with which the compounds are in equilibrium.
Key words:
Mg-Zn-Al system; isothermal section; solubility; intermetallic compound;
1 Introduction
ZA series Mg alloys have been increasingly paid much attention due to their good creep resistant property at elevated temperatures, which is better than that of AZ series Mg alloys such as AZ91D alloy [1-6]. However, the case is not well understood. ZHANG et al [2] reported that there were three types of strengthening phases, including MgZn, Mg32(Al, Zn)49(τ) and quasi-crystalline phases, but the stability of the MgZn phase was much lower than that of the Mg17Al12(γ) according to the Mg-Zn and Mg-Al binary systems [7,8]. On the other hand, the Mg-Zn based alloys are age-hardenable. However, the ZA series Mg alloys were directly formed by casting, and little work was studied on the solid solution and aging processing [9,10], because there was limited information about the phase equilibria and compositions in the Mg-rich corner of this ternary system, only two isothermal sections were experimentally determined at 335 and 320 ℃ [11-13]. ZHANG et al [9] argued that the phase constituents were α-Mg and τ phases in the as-cast ZA73 Mg alloy held at 325 ℃ for 50 h, but the as-cast ZA73 Mg alloy was located in the single phase region of α-Mg solid solution according to the isothermal sections at 335 and 320 ℃ [11-13]. This means that the τ phase is metastable and should disappear at 325 ℃ with extension of time. VOGEL et al [1] argued that the quasi-crystalline phase was metastable and transformed to the equilibrium τ phase in the as-cast ZA85 Mg alloys annealed at 170-220 ℃. If this is true, the φ phase would disappear at certain temperature between 320 and 220 ℃ [10, 13]. Moreover, it is worthy noting whether the phase structure of ZA series alloys changes during the creep test above 150 ℃ and how it affects the properties at elevated temperatures. Therefore, the isothermal sections of Mg-rich corner in this ternary system at different temperatures are both practically and theoretically important. However, the isothermal sections at lower temperatures cannot accurately be experimentally determined because of the kinetic limit. Although it is well predicted by the CALPHAD method, the more reliable phase equilibrium information and thermochemical data are required. Therefore, the purpose of the present work is to determine the phase equilibria and compositions in the Mg-rich corner at 300 ℃ in the Mg-Zn-Al ternary system in order to provide more experimental information for establishing the reliable thermodynamic database and for well understanding the relationship between microstructure and properties of the current ZA series Mg alloys.
2 Experimental
The desired alloys were prepared using high purity Mg (99.99%), Al(99.99%) and Zn (99.999%). The nominal compositions are listed in Table 1. The Mg-Zn-Al alloy ingots were melted in a high purity graphite crucible in an argon protective atmosphere by using an induction furnace. The samples were cut from the ingots, held at 300 ℃ for 720 h and finally quenched by water.
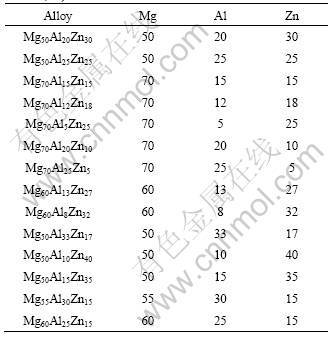
Table 1 Nominal compositions of designed alloys (molar fraction, %)
The phase constituents of the treated alloys were determined by X-ray diffraction on a Philips PW3040/60 diffractometer with Cu Kα irradiation, at a high tension of 40 kV and 40 mA as well a scanning rate of 3 (°)/min. The microstructures and compositions of the Mg-Zn-Al alloys were carried out by scanning electron microscopy with assistance of energy dispersive spectroscopy of X-ray (SEM-EDS) on a HITACHI S3400N at an accelerating potential of 20 kV.
3 Results and discussion
The equilibrium phase constituents of all alloys held at 300 ℃ for 720 h were determined by means of scanning electron microscopy (SEM) and X-ray diffractometer (XRD). It can be found that there are two kinds of alloys consisted of three or two phases. Figure 1(a) shows typical microstructure consisted of the dark, light and gray phases in the treated Mg70Al20Zn10 alloy, which is located in 3-phase? region composed of α-Mg, γ and φ phases from XRD analysis (Fig. 1(b)). According to the principle of the backscattering electron (BSE) image, the dark, light and gray phases are α-Mg, φ and γ, respectively, as shown in Fig. 1(a). Similarly, it can be obtained that the equilibrium phase constituents are α-Mg+γ+φ, α-Mg+MgZn+τ, α-Mg+φ+τ and γ+φ+τ in the treated Mg60Al25Zn15, Mg50Al10Zn40 (or Mg60Al8Zn32), Mg50Al15Zn35 (or Mg60Al13Zn27) and Mg50Al33Zn17 alloys, respectively.
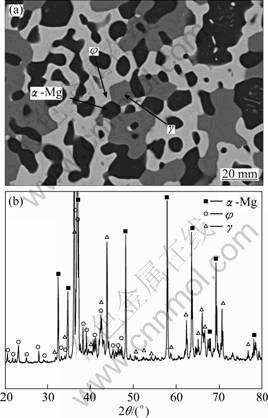
Fig. 1 BSE image (a) and XRD pattern (b) of Mg70Al20Zn10 alloy held at 300 ℃ for 720 h
The BSE image of the treated Mg70Al15Zn15 alloy is shown in Fig. 2(a). There clearly exist dark and light phases in the alloy, the equilibrium phase constituents of which are α-Mg and φ phases from the XRD analysis (Fig.2 (b)). The dark and light phases are corresponding to the α-Mg and φ phases, respectively. It can be also determined that the equilibrium phase constituents are α-Mg+φ phases in the Mg70Al12Zn18 alloy, α-Mg+γ phases in the Mg70Al25Zn5 alloy, α-Mg+MgZn phases in the Mg70Al5Zn25 alloy, φ+τ phases in the Mg50Al20Zn30 and Mg50Al25Zn25 alloys and γ+φ phases in the Mg55Al30Zn15 alloy, respectively. The compositions of the equilibrium phases in these treated alloys mentioned above were all determined by SEM-EDS, as listed in Table 2.
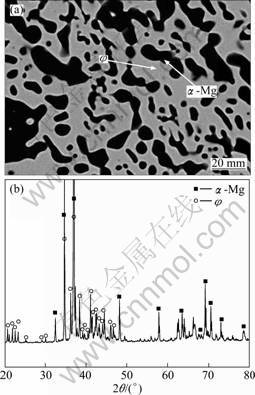
Fig. 2 BSE image (a) and XRD pattern (b) of Mg70Al15Zn15 alloy held at 300 ℃ for 720 h
The α-Mg solvus and isothermal section at 300 ℃ can be determined from the data in Table 2, as shown in Fig. 3. The superposed solubility of Zn or Al is more than that of the solely Al or Zn in the α-Mg solid solution, which is different from the systems containing two rare-earth metals of different subgroups and Mg-Sn-Y system, where the two elements are not synchronously soluble in the α-Mg solid solution[14]. This means that the solid solution strengthening can be improved in the AZ or ZA series Mg alloys. Compared with that at 320 ℃ [13], there are still four intermetallic compounds, i.e, γ, φ, τ and MgZn, in equilibrium with the α-Mg phase. The φ phase is still stable at 300 ℃ and its solubility range can be also determined, i.e. 54.2%-57.0% Mg, 14.9%- 26.9% Al and 18.3%-30.1% Zn, which is close to that at 335 and 320 ℃ [13, 15]. It implies that the composition range of the φ phase changes little with decreasing temperature from 335 to 300 ℃. Zn and Al are largely soluble in the γ and MgZn intermetallic compounds, respectively, as well as the φ and τ phases. Therefore, it is necessary to study the partition ratio of the solute atom in the α-Mg matrix and second phase for well understanding the strengthening role of the Zn or Al elements in the AZ or ZA series Mg alloys. Also, the experiment results confirm that there is still the narrow α-Mg/(α-Mg+τ) phase boundary, which is not predicted by both LIANG et al and OHNO et al [12, 16].
Table 2 Equilibrium phase constituents and compositions at 300 ℃ in Mg-Zn-Al system
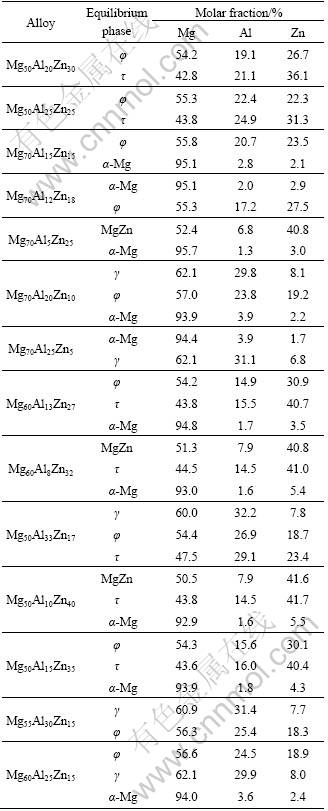
At the same time, the main AZ or ZA series Mg alloys studied currently are shown in Fig. 3(a). Only AZ31 Mg alloy is located in the α-Mg single-phase region, and ZA82 and AZ61 Mg alloys are close to the quasi-single α-Mg phase. It is suggested that the second phases are gradually dissolved in the α-Mg matrix when the three alloys are isothermally treated or thermomechanically processed at 300 ℃. Most other alloys are composed of two phases at 300 ℃, i.e, ZA122 and ZA142 alloys in the α-Mg+τ phase region, ZA102, ZA73, ZA74 and ZA104 alloys in the α-Mg+φ phase region, ZA64, AZ64 and AZ91 alloys in the α-Mg+γ phase region. There exist three alloys consisted of three phases at 300 ℃, i.e, ZA75 and ZA85 alloys in the α-Mg+φ+γ phase region and ZA144 alloy in the α-Mg+φ+τ phase region. It implies that the intermetallic compounds would dissolve in the α-Mg matrix or transform to other compounds in these alloys when treated at 300 ℃, and then the solute atoms would re-distribute, which inevitably affects the strengthening effect of the Zn or Al elements in these alloys. For example, the MgZn phase in the as-cast ZA122 and ZA142 alloys would transform to τ phase, in contrast, both τ phase in the ZA73 and ZA104 alloys and the quasi crystalline phase in the ZA74 alloy would transform to φ phase, according to the study by ZHANG et al [2]. For the ZA73 and ZA74 Mg alloys, the τ and quasi crystalline phases would dissolve in the α-Mg matrix when treated at 320 ℃ [13], and then the equilibrium phase τ and φ would precipitate when treated at 300 ℃, respectively. Therefore, it is necessary to establish a series of the isothermal sections at different temperatures, particularly at lower temperatures, in order to well understand the strengthening mechanism of the solute atoms and the relationship between composition, microstructure, process and property of the ZA series Mg alloys.
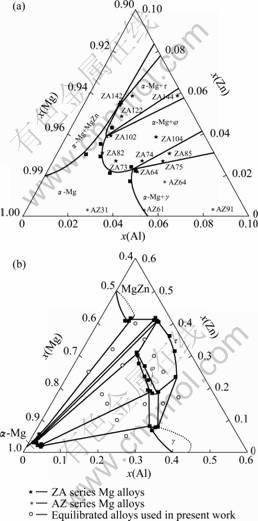
Fig. 3 Solvus of α-Mg solid solution (a) and isothermal section of Mg-rich corner (b) in Mg-Zn-Al ternary system at 300 ℃
4 Conclusions
1) The α-Mg solvus and isothermal section of Mg-rich corner at 300 ℃ are determined in the Mg-Zn-Al ternary system.
2) There exist two ternary compounds τ and φ in equilibrium with the α-Mg solid solution, except for the binary compounds γ and MgZn. The composition range of the ternary φ phase is also obtained.
3) The solubility of Zn and Al in γ, MgZn and α-Mg phases is large. In particular, the solubility of Zn in the α-Mg phases remarkably increases due to the Al addition when the α-Mg phase is in equilibrium with the MgZn intermetallic compound compared with the Mg-Zn binary system.
References
[1] VOGEL M, KRAFT O, ARZT E. Creep behavior of magnesium die-cast alloy ZA85 [J]. Scripta Mater, 2003, 48 (8): 985-990.
[2] ZHANG J, GUO Z X, PAN F S, LI Z S, LUO X D. Effect of composition on the microstructure and mechanical properties of Mg–Zn–Al alloys [J]. Mater Sci Eng A, 2007, 456(1-2): 43-51.
[3] ZHANG Z, COUTURE A, LUO A. An investigation of the properties of Mg-Zn-Al alloys [J]. Scripta Mater, 1998, 39(1): 45–53.
[4] VOGEL M, KRAFT O, DEHM G, ARZT E. Quasi-crystalline grain-boundary phase in the magnesium die-cast alloy ZA85 [J]. Scripta Mater, 2001, 45(5): 517-524.
[5] ZHANG Jing, LI Zhong-sheng, GUO Zheng-xiao, PAN Fu-sheng. Solidification microstructural constituent and its crystallographic morphology of permanent-mould-cast Mg-Zn-Al alloys [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(2): 452-458.
[6] ZHANG Z, TREMBLAY R, DUB? D, COUTURE A. Solidification microstructure of ZA102, ZA104 and ZA106 magnesium alloys and its effect on creep deformation [J]. Canadian Metallurgical Quarterly, 2000, 39(4): 503-512.
[7] OKAMOTO H. Comment on Mg-Zn (magnesium-zinc) [J]. J Phase Equilibria, 1994, 15(1): 129-130.
[8] MASSALSKI T B, OKAMOTO H. Binary alloy phase diagrams [M]. OH: Materials Park, ASM International, 1990: 169-171.
[9] ZHANG J, ZUO R L, CHEN Y X, PAN F S, LUO X D. Microstructure evolution during homogenization of a τ-type Mg–Zn–Al alloy [J]. J Alloys Comp, 2008, 448(1-2): 316–320.
[10] BALASUBRAMANI N, PILLAI U T S, PAI B C. Optimization of heat treatment parameters in ZA84 magnesium alloy [J]. J Alloys Comp, 2008, 457(1-2): 118-123.
[11] VILLARS P, PRINCE A, OKAMOTO H. Handbook of Ternary Alloy Phase Diagrams [M]. OH: Materials Park, ASM International, 1997.
[12] LIANG P, TARFA T, ROBINSON J A, WAGNER S, OCHIN P, HARMELIN M G, SEIFERT H J, LUKAS H L, ALDINGER F. Experimental investigation and thermodynamic calculation of the Al-Mg-Zn system [J]. Thermochimica Acta, 1998, 314(1-2): 87-110.
[13] REN Y P, QIN G W, PEI W L, GUO Y, ZHAO H D, LI H X, JIANG M, HAO S M. The α-Mg solvus and isothermal section of Mg-rich corner in the Mg–Zn–Al ternary system at 320 ℃ [J]. J Alloys Comp, 2009, 481(1-2): 176-181.
[14] ZHAO H D, QIN G W, REN Y P, PEI W L, GUO Y. Isothermal sections of the Mg-rich corner in the Mg-Sn-Y ternary system at 300 and 400 ℃ [J]. J Alloys Comp, 2009, 48(1-2): 140-143.
[15] DONNADIEU P, QUIVY A, TARFA T, OCHIN P, DEZELLUS A, HARMELIN M G, LIANG P, LUKAS H L, SEIFERT H J, ALDINGER F, EFFENBERG G. On the crystal structure and solubility range on the ternary phase in the Mg-Al-Zn system [J]. Z Metallkd, 1997, 88(12): 911-916.
[16] OHNO M, MIRKOVIC D, SCHMID-FETZER R. Phase equilibria and solidification of Mg-rich Mg–Al–Zn alloys [J]. Mater Sci Eng A, 2006, 421(1-2): 328-337.
Mg-Zn-Al三元系富镁角在300 ℃的相平衡
任玉平, 秦高梧, 裴文利, 李 松, 郭 运, 赵宏达
东北大学 材料各向异性与织构教育部重点实验室,沈阳 110819
摘 要:采用平衡合金法,利用X射线衍射、扫描电镜及能谱分析,确定Mg-Zn-Al三元系富镁角300 ℃ α-Mg相平衡关系和相组成。结果表明:在富镁角存在3个三相区:α-Mg+Mg17Al12(γ)+Al5Mg11Zn4(φ), α-Mg+ Mg32(Al,Zn)49(τ)+Al5Mg11Zn4(φ)和α-Mg+MgZn+Mg32(Al, Zn)49(τ)。与α-Mg相平衡的金属间化合物都具有很大的成分范围,并非呈线性。同时Zn和Al都能够溶解在α-Mg固溶体中,使金属间化合物达到相平衡。
关键词:Mg-Zn-Al系;等温截面;固溶度;金属间化合物
(Edited by FANG Jing-hua)
Foundation item: Projects (50901017, 50731002) supported by the National Natural Science Foundation of China, Project (20090042120008) supported by Doctoral Program Foundation of Institutions of Higher Education of China; Projects (N100702001, N090502002) supported by the Fundamental Research Funds of the Central Universities, China
Corresponding author: QIN Gao-wu; Tel: +86-24-83683772; E-mail: qingw@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(11)61166-7
Abstract: The phase equilibria and compositions in Mg-rich corner at 300 ℃ were determined in the Mg-Zn-Al ternary system through the equilibrated alloy method by using X-ray diffraction (XRD) and scanning electron microscopy (SEM) assisted with energy dispersive spectroscopy of X-ray (EDS). The results show that there exist three three-phase regions consisted of α-Mg+Mg17Al12(γ)+Al5Mg11Zn4(φ), α-Mg+Mg32(Al, Zn)49(τ)+Al5Mg11Zn4(φ) and α-Mg+MgZn+Mg32(Al, Zn)49(τ), respectively. The intermetallic compounds in equilibrium with α-Mg phase all have large composition ranges, not appear to be linear. At the same time, both zinc and aluminum are soluble in the α-Mg solid solution, with which the compounds are in equilibrium.


