DOI:10.19476/j.ysxb.1004.0609.2017.11.19
湘西花垣铅锌矿田方解石REE元素和Sr同位素地球化学
隗含涛1, 2,邵拥军1, 2,叶 周3,熊伊曲1, 2,周皓迪1, 2,谢友良1, 2
(1. 中南大学 有色金属成矿预测与地质环境监测教育部重点实验室,长沙 410083;
2. 中南大学 地球科学与信息物理学院,长沙 410083;
3. 湖南省地质博物馆,长沙 410004)
摘 要:
湘西花垣铅锌矿田随着杨家寨、大脑坡及清水塘等一系列大型–超大型铅锌矿床的发现,已经成为世界千万吨级铅锌矿田。通过矿脉切穿关系和不同的矿物共生组合,将花垣矿田成矿过程分为3个阶段。对矿田内主成矿期方解石的REE及Sr同位素进行了研究,并与含矿围岩及不同时代地层的REE和Sr同位素进行了对比。结果表明:方解石稀土元素特征与地层具有相似性,其中I阶段方解石REE含量较低,ΣREE为1.73×10-6~10.94×10-6,平均为4.77×10-6;LREE/HREE为6.21~10.19,平均为7.96;87Sr/86Sr比值0.70935~0.71001,平均为0.709643。II阶段方解石REE含量比I阶段高,ΣREE为24.53×10-6~67.88×10-6,平均为46.71×10-6;LREE/HREE为6.21~10.19,平均为7.96;87Sr/86Sr比值为0.70923~0.70944,平均为0.709301,比I阶段的低,说明I、II阶段成矿流体存在差异。结合围岩及不同时代地层地球化学特征认为,I阶段成矿流体主要为含矿层建造水,混有深循环热卤水;II阶段成矿流体主要为深循环热卤水,混有大气降水,从I阶段至II阶段成矿流体还原性增强。
关键词:
文章编号:1004-0609(2017)-11-2329-11 中图分类号:P597.2 文献标志码:A
湘西花垣铅锌矿田位于扬子陆块东南缘与雪峰(江南)造山带的过渡区,湘西-鄂西成矿带的中部[1],具有规模大、品位低(Pb+Zn约3%)、易开采、易选冶等特征。矿田范围内已发现矿床(点)30余处,由北往南分布有杨家寨、大脑坡、芭茅寨、李梅、土地坪、老虎冲、长登坡、清水塘等矿床,为千万吨级铅锌矿田,得到了众多地质学者的广泛关注[1-11]。虽然不同学者从矿田沉积相[2]、成矿物质来源[3-8]、矿床成因[1, 3-5]、成矿规律[5]及找矿标志[9]等方面进行了诸多研究,但对成矿流体来源的认识存在明显分歧。陈明辉等[6]对比了方解石与灰岩的δ18OPDB值认为流体来自地层水;刘文均等[10]分析了矿石及脉石矿物的δD、δ18O同位素,投影点在δD-δ18O同位素图解中与雨水线斜交,反映成矿流体主要来源与建造水有关,在后期有雨水渗入;杨绍祥等[11]将8件方解石样品的δD、δ18O值投影在δD-δ18O图解中,投影点主要落于变质水区,小部分在原生岩浆水区,认为成矿流体初始来源主要是建造水,有大气降水的加入,并可能混入少量变质水。
已有研究表明,Sr同位素对示踪流体来源及运移路径具有较好的指示意义[12-13],近年来热液方解石稀土元素也被广泛应用于成矿流体的研究[14-17],二者结合起来能够更加全面的示踪成矿流体来源,探讨成矿流体性质及演化特征等。方解石是花垣矿田与成矿关系最为密切的脉石矿物,其形成贯穿整个成矿过程。本文作者在对花垣铅锌矿田地质特征研究基础上,通过研究方解石的REE及Sr同位素特征,并与围岩及不同时代地层进行对比,探讨了矿床成矿流体来源及演化过程,有助于深入认识花垣铅锌矿田成矿机理。
1 成矿地质背景
花垣铅锌矿田位于上扬子地台东南缘的武陵山弱变形带,地跨上扬子地块和江南地块。经历了武陵、雪峰-加里东、海西、印支-喜马拉雅4个构造运动,形成了一系列规模较大的褶皱和断裂[9]。区域褶皱总体平缓,北部为燕山期桑植复向斜,南东部为加里东古丈复背斜,花垣铅锌矿田位于桑植复向斜与古丈复背斜之过渡带中的次级构造摩天岭背斜两翼。区域断裂以NEE向和NE向为主,有松桃-张家界深大断裂(花垣-张家界断裂带是其北段一部分)、保靖-铜仁-玉屏断裂带(又称为麻栗场断裂带)及松桃-水田断裂带等,控制了本区早寒武世清虚洞期以及整个下古生代的地层、岩相及矿床的产出[4]。区内广泛分布元古界板溪群、震旦系、古生界寒武系、奥陶系、志留系、泥盆系地层,尤以下古生界最为发育,沉积厚度达4000余m,岩浆活动不发育。
花垣铅锌矿田主要位于茶洞-花垣-张家界深大断裂带与保靖-铜仁-玉屏(麻栗场)断裂带之间(图1),总体走向NNE。矿田内主要出露寒武系下统石牌组含钙质、粉砂质页岩,清虚洞组白云岩(上段)和灰岩(下段);寒武系中统高台组泥质白云岩;寒武系中上统娄山关群砂屑白云岩及第四系。各地层除与第四系外,均整合接触。其中清虚洞组灰岩可细分为4个亚段,由下至上泥质含量减少,钙质含量增加。第三亚段(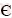 2q1-3)为浅灰-灰色巨厚层状(或块状层)泥晶-细晶藻灰岩,为铅锌矿主要赋矿层位;第四亚段(
2q1-3)为浅灰-灰色巨厚层状(或块状层)泥晶-细晶藻灰岩,为铅锌矿主要赋矿层位;第四亚段(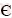 2q1-4)为浅灰-灰色厚-巨厚层状亮晶、泥晶粒屑、鲕粒灰岩,为次要赋矿层位。矿田褶皱以NE向的摩天岭背斜为代表,核部出露板溪群与震旦系,两翼为寒武系各统(群)、组,岩层产状平缓,通常只有5°~12°。矿田断裂构造发育,以NE向断裂为主,次为NNE、NW、近SN向断裂,其中与成矿关系密切的断裂为NE向花垣-张家界断裂带(F1)、两河-长乐断裂带(F3)和NNE向麻栗场断裂带(F2)(见图1)。
2q1-4)为浅灰-灰色厚-巨厚层状亮晶、泥晶粒屑、鲕粒灰岩,为次要赋矿层位。矿田褶皱以NE向的摩天岭背斜为代表,核部出露板溪群与震旦系,两翼为寒武系各统(群)、组,岩层产状平缓,通常只有5°~12°。矿田断裂构造发育,以NE向断裂为主,次为NNE、NW、近SN向断裂,其中与成矿关系密切的断裂为NE向花垣-张家界断裂带(F1)、两河-长乐断裂带(F3)和NNE向麻栗场断裂带(F2)(见图1)。
2 典型矿床地质特征
花垣铅锌矿田矿床(点)众多,矿体多为隐伏产出,主要呈层状,其次为脉状赋存于清虚洞组下段第三、第四亚段(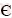 2q1-(3+4))厚层藻砂屑灰岩、鲕粒灰岩中。平面上,靠近花垣-张家界断裂的矿体中锌铅比值高,往南呈现降低趋势;垂向上,含矿层中上部铅含量高,下部铅含量较低。除铅、锌元素含量呈现一定变化趋势外,各矿床矿体、矿石及围岩蚀变等特征均具有明显相似性,现以土地坪矿床为例,介绍矿床的地质特征如下。土地坪矿床位于花垣铅锌矿田中部偏南,是目前矿田范围内开采规模最大的矿床。矿区出露地层主要为寒武系白云岩和灰岩,断裂不发育,褶皱有太阳山向斜及NNE向背斜,土地坪矿床位于二者复合部位。矿体按其形态和产状可分为两类:1) 层状矿体,与围岩产状近于一致,呈似层状、透镜状、囊状,倾角一般8°~15°,常具多层性,一般4~7层(见图2),单层矿体厚度1~5 m(见图3(a)),长数十米,沿走向常有尖灭再现现象,此类矿体规模较大,但品位较低,Zn品位(质量分数)平均为3%,Pb品位0.1%~1.5%;2) 脉状矿体,既有形态规则的脉状(见图3(b)),又有不规则的脉状和囊状,多沿规模相对较大的构造裂隙分布,走向30°~50°,近直立,宽3~20 cm,局部膨大可达50 cm,沿走向延伸长数米至数百米,常见膨大收缩或分枝复合现象,Pb+Zn品位常大于10%,最高可达50%。矿区矿物组成简单,金属矿物主要为闪锌矿,其次为方铅矿,少量黄铁矿;非金属矿物以方解石为主,白云石次之,少量重晶石、萤石、炭质沥青等。矿石结构以自形-半自形粒状结构为主,次为交代、填隙、包含等结构(见图3(g)~(i));矿石构造以花斑状(见图3(c))、斑脉状构造为主(见图3(e)),浸染状(见图3(f))、细脉状、块状、角砾状、条带状等构造次之,偶见有环带状、球粒状及晶洞状构造。矿区围岩蚀变简单,以方解石化、白云石化为主,少量重晶石化、萤石化、褪色化等低温蚀变。
2q1-(3+4))厚层藻砂屑灰岩、鲕粒灰岩中。平面上,靠近花垣-张家界断裂的矿体中锌铅比值高,往南呈现降低趋势;垂向上,含矿层中上部铅含量高,下部铅含量较低。除铅、锌元素含量呈现一定变化趋势外,各矿床矿体、矿石及围岩蚀变等特征均具有明显相似性,现以土地坪矿床为例,介绍矿床的地质特征如下。土地坪矿床位于花垣铅锌矿田中部偏南,是目前矿田范围内开采规模最大的矿床。矿区出露地层主要为寒武系白云岩和灰岩,断裂不发育,褶皱有太阳山向斜及NNE向背斜,土地坪矿床位于二者复合部位。矿体按其形态和产状可分为两类:1) 层状矿体,与围岩产状近于一致,呈似层状、透镜状、囊状,倾角一般8°~15°,常具多层性,一般4~7层(见图2),单层矿体厚度1~5 m(见图3(a)),长数十米,沿走向常有尖灭再现现象,此类矿体规模较大,但品位较低,Zn品位(质量分数)平均为3%,Pb品位0.1%~1.5%;2) 脉状矿体,既有形态规则的脉状(见图3(b)),又有不规则的脉状和囊状,多沿规模相对较大的构造裂隙分布,走向30°~50°,近直立,宽3~20 cm,局部膨大可达50 cm,沿走向延伸长数米至数百米,常见膨大收缩或分枝复合现象,Pb+Zn品位常大于10%,最高可达50%。矿区矿物组成简单,金属矿物主要为闪锌矿,其次为方铅矿,少量黄铁矿;非金属矿物以方解石为主,白云石次之,少量重晶石、萤石、炭质沥青等。矿石结构以自形-半自形粒状结构为主,次为交代、填隙、包含等结构(见图3(g)~(i));矿石构造以花斑状(见图3(c))、斑脉状构造为主(见图3(e)),浸染状(见图3(f))、细脉状、块状、角砾状、条带状等构造次之,偶见有环带状、球粒状及晶洞状构造。矿区围岩蚀变简单,以方解石化、白云石化为主,少量重晶石化、萤石化、褪色化等低温蚀变。
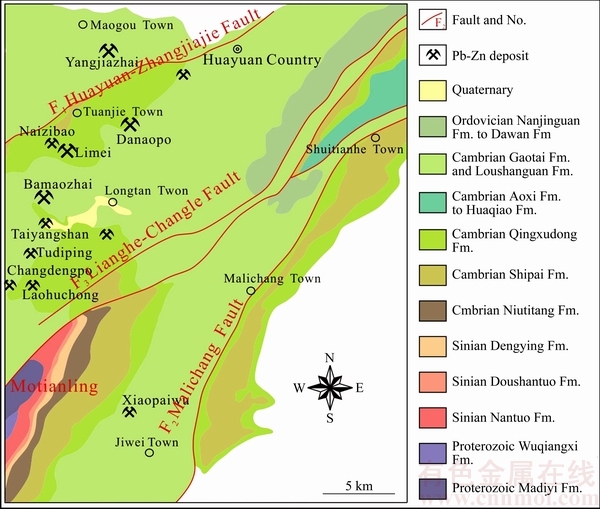
图1 花垣铅锌矿田地质简图
Fig. 1 Geological sketch map showing distribution of Pb-Zn ore deposits in Huayuan orefield, China
根据野外矿脉穿插关系以及矿物共生组合、矿石组构等特征,将花垣铅锌矿田成矿过程分为3个阶段(见图3(c)~(f)):方解石-黄铁矿-白云石-闪锌矿阶段(I),方解石-黄铁矿-重晶石-萤石-闪锌矿-方铅矿阶段(II)及方解石-方铅矿阶段(III),成矿以II阶段为主,次为I阶段,III阶段矿化最弱。I阶段闪锌矿以浅黄色-黄褐色为主,以浸染状形式分布于方解石与灰岩接触部位(即花斑状构造),或与脉石矿物沿缝合线充填呈细脉状(见图3(c));此阶段方解石多呈团斑状(多小于2 cm×2 cm×2 cm)及不规则细脉状,解理发育程度一般。II阶段闪锌矿颜色从浅黄色-黄褐色-深棕色均有,主要呈细脉状分布于灰岩与脉石矿物接触部位(即斑脉状构造),脉宽小于1 cm,其次呈浸染状分布于脉石矿物边部或其中(见图3(e));此阶段方解石以粗脉状、不规则脉状或团块状产出,菱面体节理发育。III阶段生成闪锌矿较少,方解石含量也较低,且粒径细小,解理差(见图3(f))。
3 样品采集及分析方法
本研究主要以I、II阶段方解石为研究对象。首先将矿石样品粉碎并过筛,粒度在0.18~0.25 mm之间,清洗、干燥之后在双目镜下挑选方解石单矿物样品,纯度达98%以上,供REE及Sr同位素分析。REE分析在广州澳实矿物实验室完成,采用硼酸锂熔融,等离子质谱仪进行定量分析。Sr同位素分析在核工业北京地质研究院分析测试研究中心完成。称取样品加入适量纯化的2.5 mol/L的HCl溶解,将溶好的清液移入离心管,放入离心机中离心,再用石英交换柱进行Sr的分离和纯化。Sr同位素比值测定在MAT-261固体同位素质谱仪上完成,国际标样NBS987的实测值为0.710248±0.000008。
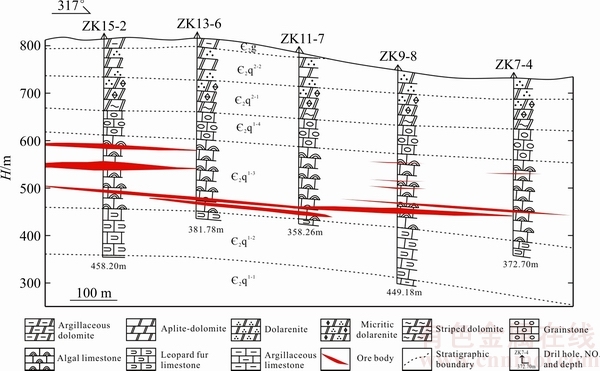
图2 土地坪铅锌矿床98号线勘探线剖面图
Fig. 2 98th prospecting line profile map of Tudiping Pb-Zn deposit

图3 花垣铅锌矿田矿体及矿石组构特征(Sp—闪锌矿,Gn—方铅矿,Py—黄铁矿)
Fig. 3 Characteristics of orebodies and ores from Huayuan Pb-Zn orefield (Sp—sphalerite, Gn—galena, Py—pyrite)
4 分析结果
4.1 稀土元素特征
方解石以及矿石和各时代地层的REE 含量、相关REE 参数如表1和2所列,球粒陨石标准化配分模式如图4所示。结果表明:I阶段方解石ΣREE含量较低,变化范围为1.73×10-6~10.94×10-6,平均为4.77×10-6; LREE/HREE介于6.21~10.19,平均为7.96;Eu异常不明显(δEu为0.54~1.29),弱的负Ce异常(δCe为0.61~1.05,平均0.87)。II阶段方解石ΣREE明显高于前者,变化范围为24.53×10-6~ 67.88×10-6,平均为46.71×10-6;LREE/HREE介于11.19~19.85,平均为16.62; Eu负异常明显(δEu为0.46~0.84,平均为0.67),弱的正Ce异常(δCe为1.05~1.09,平均为1.07)。
不同成矿阶段方解石稀土配分模式均表现为轻稀土富集右倾型[(La/Yb)N平均值分别为13.20和69.31](见图4(a)和(b)),其中I阶段方解石稀土配分模式相对平坦,II阶段方解石稀土配分模式相对陡倾,较前者更为富集轻稀土。两类方解石(La/Sm)N分别为2.61~7.17(平均4.29),2.70~5.71(平均4.22),反映二者轻稀土分馏程度相当;(Gd/Yb)N分别为1.65~2.90(平均2.16),4.39~14.97(平均10.48),说明II阶段方解石重稀土分馏程度相对较高。
方解石稀土含量和配分模式与矿石、容矿围岩及各时代地层均具有相似性(见表1和2、图4)。从稀土含量看,I阶段方解石与矿石(不同阶段矿石稀土元素变化小,未做区分)、容矿围岩(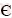 2q1-4、
2q1-4、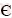 2q1-3)、含矿层顶底板地层(
2q1-3)、含矿层顶底板地层(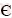 2q2-1、
2q2-1、 2q1-2)的ΣREE、LREE、HREE相当,小于II阶段方解石,明显小于清虚洞组下伏各时代地层(Pt3m~
2q1-2)的ΣREE、LREE、HREE相当,小于II阶段方解石,明显小于清虚洞组下伏各时代地层(Pt3m~ 1-2s)。从稀土配分模式看,各样品均表现为轻稀土富集型,且II阶段方解石更为富集轻稀土;矿石、容矿围岩及各时代地层整体均表现为Eu负异常,与II阶段方解石特征一致。
1-2s)。从稀土配分模式看,各样品均表现为轻稀土富集型,且II阶段方解石更为富集轻稀土;矿石、容矿围岩及各时代地层整体均表现为Eu负异常,与II阶段方解石特征一致。
4.2 Sr同位素特征
方解石及地层Sr同位素分析结果如表3所示。为获得成矿时的Sr同位素组成,将所测结果进行了校正,采用的年龄为闪锌矿Rb-Sr年龄(410±12) Ma[8],得到成矿时的Sr同位素比值记为I Sr-410。
方解石的I Sr-410为0.70923~0.71001,极差为0.00078,平均为0.70948,高于幔源锶和寒武纪海水锶的同位素比值(分别为0.7035[18]和0.709[19]);含矿层藻灰岩以及顶板白云岩和底板豹皮灰岩I Sr-410值分别为0.70886、0.70876和0.70850,均小于成矿期方解石的值;下伏石牌组、牛蹄塘组及马底驿组地层I Sr-410分别为0.72731、0.72401和0.72712,均高于成矿期方解石的值。I阶段方解石I Sr-410为0.70935~0.71001,极差为0.00066,平均为0.709643;II阶段方解石I Sr-410为0.70923~0.70944,极差为0.00021,平均为0.709301。I阶段方解石Sr同位素比值高于II阶段方解石。
表1 花垣铅锌矿田方解石稀土元素组成
Table 1 REE compositions of calcites from Huyuan Pb-Zn orefield
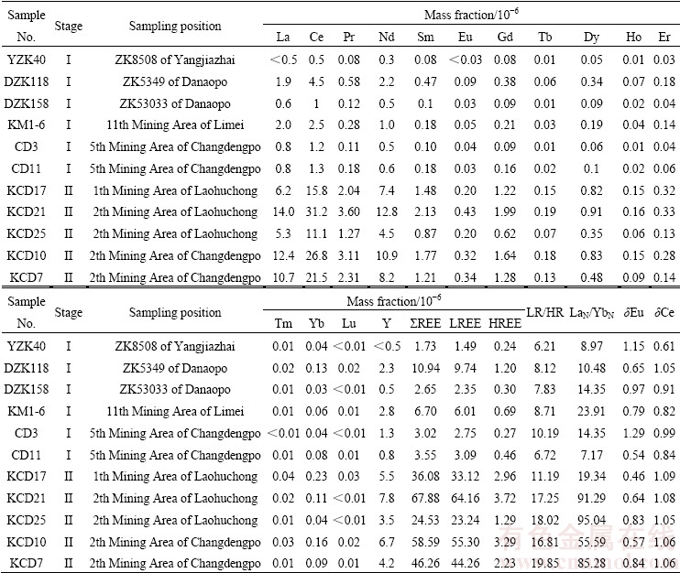
表2 花垣铅锌矿田矿石、地层稀土元素组成
Table 2 REE compositions of ores and strata from Huyuan Pb-Zn orefield
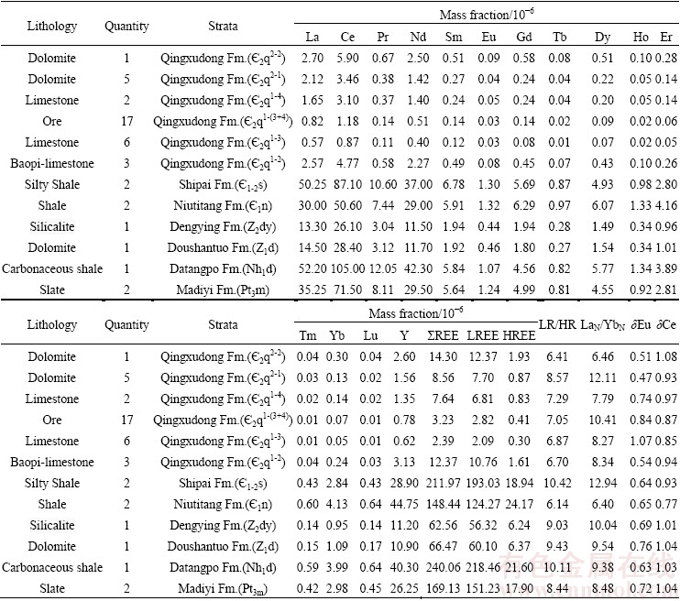
表3 花垣铅锌矿田锶同位素分析结果
Table 3 Sr isotopic analytical results from Huayuan Pb-Zn orefield
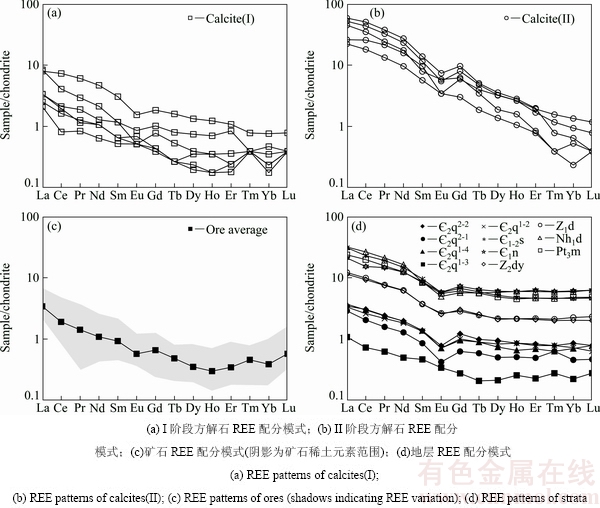
图4 花垣铅锌矿田方解石、矿石及地层稀土元素配分模式图
Fig. 4 Chondrite-normalized REE patterns of calcites, ores and strata from Huyuan Pb-Zn orefield
5 讨论
5.1 方解石的成因联系
Tb /Ca-Tb /La图是判断方解石形成与演化的有效工具[20],将两个阶段方解石的REE组成投图如图5所示,本区方解石在Tb/Ca-Tb/La图解上主要投影在热液成因区内(由于Ca与稀土含量相差5~6个数量级,在计算Yb/Ca比值时直接采用Ca的理论值),仅I阶段方解石两个样品落入靠近热液区的沉积成因区内,这可能是由于围岩提供了部分物质来源(如Ca)[16],说明花垣铅锌矿田成矿期方解石属热液成因,与地质特征相符。
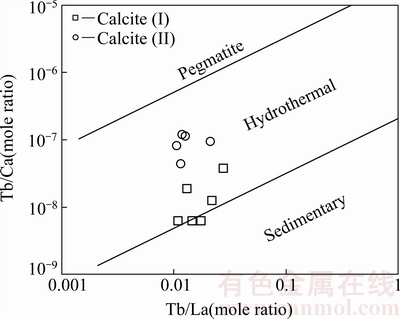
图5 方解石Tb/Ca-Tb/La图(底图据[20])
Fig. 5 Tb/Ca-Tb/La diagram of calcites from Huayuan Pb-Zn orefiled (Base maps derived from Ref. [20])
矿石及脉石矿物的REE 地球化学特征可以代表成矿流体的 REE 特征,其变化规律记录了成矿流体的来源及演化等方面的重要信息[14-17]。对比方解石、矿石及容矿围岩的ΣREE,II阶段方解石>I阶段方解石≈容矿围岩>矿石,说明成矿流体中的稀土元素主要分布在方解石中,闪锌矿、方铅矿等硫化物中稀土元素含量较低。方解石是花垣铅锌矿田最为常见的脉石矿物,其稀土元素地球化学特征可代表成矿流体的稀土元素特征。
M LLER等[20]和DAVIES等[21]系统研究了热液成矿过程中含钙矿物(方解石和萤石)的稀土元素分布特征,结果表明同一流体体系中早阶段形成的矿物ΣREE相对较高,且更为富集LREE;而晚阶段形成的矿物ΣREE相对较低,更为富集HREE。CHESLEY等[22]也发现早阶段形成的含钙矿物LREE含量高、Sm/Nd低,而晚阶段形成的矿物LREE含量低、Sm/Nd高。研究区Ⅰ阶段方解石ΣREE、LREE及LREE/HREE(平均值分别为4.77、4.24及7.96)均低于晚期形成的II阶段方解石(平均值分别为46.71、44.02及16.62),而Sm/Nd却表现为Ⅰ阶段高于Ⅱ阶段(平均值分别为0.23和0.17),说明二者流体来源可能存在差异。
LLER等[20]和DAVIES等[21]系统研究了热液成矿过程中含钙矿物(方解石和萤石)的稀土元素分布特征,结果表明同一流体体系中早阶段形成的矿物ΣREE相对较高,且更为富集LREE;而晚阶段形成的矿物ΣREE相对较低,更为富集HREE。CHESLEY等[22]也发现早阶段形成的含钙矿物LREE含量高、Sm/Nd低,而晚阶段形成的矿物LREE含量低、Sm/Nd高。研究区Ⅰ阶段方解石ΣREE、LREE及LREE/HREE(平均值分别为4.77、4.24及7.96)均低于晚期形成的II阶段方解石(平均值分别为46.71、44.02及16.62),而Sm/Nd却表现为Ⅰ阶段高于Ⅱ阶段(平均值分别为0.23和0.17),说明二者流体来源可能存在差异。
5.2 方解石REE示踪
对比方解石、矿石、围岩及各时代地层稀土元素球粒陨石标准化配分模式图可知(见图4),四者均表现为右倾的轻稀土富集型,具有明显相似性,表明成矿流体中的稀土继承了地层的稀土元素特征[14, 23]。已有的研究表明,碳酸盐地层不可能淋滤出相对富含稀土元素的流体[24]。I阶段方解石ΣREE与容矿围岩接近,而II阶段方解石ΣREE高于容矿围岩却低于含矿层下伏地层,说明与II阶段方解石有关的流体可能流经了富含REE的马底驿组(Pt3m)至石牌组( 1-2s)地层。由于Y与REE离子半径接近,通常认为二者具有相似的地球化学性质[25-27],利用Y与REE之间的变化趋势可区分不同来源的流体[23, 27]。在Y-ΣREE图中(见图6),热液方解石与沉积地层数据点线性关系明显,表明二者在成因上具有密切联系。I阶段方解石数据投点较为分散,与容矿围岩(
1-2s)地层。由于Y与REE离子半径接近,通常认为二者具有相似的地球化学性质[25-27],利用Y与REE之间的变化趋势可区分不同来源的流体[23, 27]。在Y-ΣREE图中(见图6),热液方解石与沉积地层数据点线性关系明显,表明二者在成因上具有密切联系。I阶段方解石数据投点较为分散,与容矿围岩(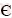 2q1-4、
2q1-4、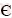 2q1-3)重叠,表明其主要来源于含矿地层的溶解,流体可能主要为含矿层中的建造水;II阶段方解石分布较为集中,位于围岩与清虚洞组下伏地层之间,说明流体可能主要为流经下伏地层向上运移的富含REE的流体,可能是地层水渗滤循环形成的热卤水。
2q1-3)重叠,表明其主要来源于含矿地层的溶解,流体可能主要为含矿层中的建造水;II阶段方解石分布较为集中,位于围岩与清虚洞组下伏地层之间,说明流体可能主要为流经下伏地层向上运移的富含REE的流体,可能是地层水渗滤循环形成的热卤水。
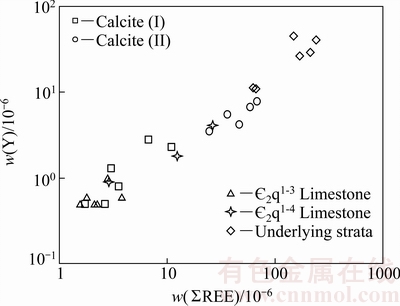
图6 花垣铅锌矿田w(Y)-w(ΣREE)变化图解
Fig. 6 Y content versus total REE content from Huayuan Pb-Zn orefiled
铕异常主要与温度关系密切,其次为fO2[28-29]。高温(>250 ℃)、强还原条件下,Eu2+较为稳定;低温和相对氧化条件下,则以Eu3+为主;在大约100~200 ℃的中等温度和中等还原条件下,Eu2+ 和Eu3+二者含量相当[28-29]。方解石中Eu主要取代Ca2+的位置,而Eu3+(离子半径为0.095 nm)相比Eu2+(离子半径为0.117 nm)更容易置换Ca2+(离子半径为0.100 nm)[30-31]。I阶段方解石Eu异常特征不明显,说明成矿流体中Eu2+ 和Eu3+含量相当,反映成矿环境为中等还原环境;II阶段方解石δEu值为0.46~0.84,表现明显的负异常特征,说明成矿时流体中Eu主要以Eu2+形式存在,反映成矿环境为温度较高的强还原环境。
铈异常取决于矿物沉淀时的氧化还原环境。还原条件下Ce以Ce3+形式随流体迁移,只有在氧逸度较高条件下,Ce3+被氧化成Ce4+,而Ce4+溶解度较小,易被氢氧化物吸附而脱离溶液体系,致使整个溶液体系呈现Ce的亏损,从该溶液中沉淀出的矿物也表现为Ce负异常[23]。研究区I阶段方解石δCe值为0.61~1.05,平均为0.87,表现为弱负异常;II阶段方解石δCe值为1.05~1.09,平均为1.07,表现为弱正异常。说明从I阶段到II阶段成矿流体还原性增强,这与II阶段生成大量硫化物的地质事实相符。
5.3 Sr同位素示踪
Sr同位素在化学与生物化学过程中,均不会产生明显的分馏[13],因此可用方解石的I Sr-410代表成矿时流体的Sr同位素组成。由于海水中Sr的残留时间远远大于海水混合时间,故海水中的Sr同位素分布在任何时代都相当均一,海相碳酸盐的Sr同位素组成具有全球对比意义。黄思静等[32]测得中寒武纪海相碳酸盐87Sr/86Sr为0.708571~0.709852,平均为0.709174,略高于本次测得的清虚洞组灰岩、白云岩的87Sr/86Sr值,但仍小于方解石87Sr/86Sr比值(平均为0.70948)。这说明围岩及顶、底板地层不足以提供成矿流体高的锶同位素比值,暗示流体应来自或流经了基底至石牌组的地层,并与其中具有高锶同位素比值的碎屑岩、页岩和泥岩等进行了水岩反应及同位素交换,从而导致沉淀出的热液矿物具有比围岩地层高的锶同位素组成。
前文已论述,稀土元素显示I阶段成矿流体可能主要为含矿层建造水,II阶段成矿流体可能主要为深循环热卤水。建造水Sr同位素比值应与寒武纪海水相当或略大,而热卤水来自或流经了富含放射性Sr的基底至石牌组地层,其Sr同位素比值应更大。TAN等[33]测得柴达木盆地卤水的87Sr/86Sr 值为0.711811~ 0.712396,平均值为0.711660,与地壳平均Sr同位素组成相近(0.711900[18]),解释为盆地热卤水与所接触的地壳岩石发生了水-岩反应而富含放射性成因Sr。
I阶段成矿流体(87Sr/86Sr平均为0.709643)相比建造水明显富含放射性成因Sr,说明其并非单一来源,可能有少量热卤水的加入。II阶段成矿流体(87Sr/86Sr平均为0.709301)相比热卤水又明显贫放射性成因Sr,说明应有低87Sr/86Sr比值的流体加入。HAN等报道了碳酸盐地区贵州贵阳雨水的87Sr/86Sr 值介于0.707934~0.709080之间,平均值为0.708219[34];贵州茂兰雨水的87Sr/86Sr 值介于 0.707463~0.712852 之间,平均值为0.709058[35]。由雨水淋滤白云岩、灰岩生成的演化水87Sr/86Sr 值虽然有所增加(分别为0.7080~0.7100,0.7075~0.7080)[36],但仍明显低于II阶段成矿流体Sr比值。湘西花垣地区出露地层主要为碳酸盐岩,与贵州类似,两地的雨水及经地层演化的大气降水应具有相似的Sr同位素组成,这就使得II阶段热卤水成矿过程中,大气降水的加入致使成矿流体Sr比值降低成为了可能。刘文均等[10]测定了闪锌矿、方解石、重晶石、萤石、白云石中的H、O同位素组成,结果也表明成矿过程中有大气降水的参与。综上所述,研究区成矿流体并非单一来源,I阶段成矿流体中混有少量热卤水,II阶段成矿流体中有大气降水参与成矿。
6 结论
1) 热液矿物方解石稀土配分曲线呈轻稀土富集的右倾型,与矿田基底及沉积地层具有相似性,在方解石Y-ΣREE图解中投点呈线性分布,表明成矿流体主要继承了流经的沉积地层的稀土元素特征。
2) I阶段方解石ΣREE低,与围岩相近,稀土配分曲线呈相对平坦的右倾型,87Sr/86Sr比值较高(高于围岩及建造水),表明成矿流体主要为含矿层建造水,有深循环形成的热卤水的混入。
3) II阶段方解石ΣREE高,高于围岩而低于含矿层下伏地层,稀土配分曲线呈相对陡倾的右倾型,87Sr/86Sr比值较低(高于围岩、建造水,低于热卤水),表明成矿流体主要为深循环形成的热卤水,有大气降水的混入。
4) I阶段方解石Eu异常不明显,Ce呈弱负异常;II阶段方解石Eu呈负异常,Ce呈弱正异常,说明成矿环境从I阶段到II阶段还原性增强。
致谢:
野外工作中得到湖南省地矿局405地质队刘伟副队长、余沛然总工程师、 曾健康副总工程师以及隋志恒、余冰、杨兵、张劲松等工程师的热情帮助;审稿人和编辑部提出了宝贵的建设性意见,谨致谢忱!
REFERENCES
[1] 段其发. 湘西-鄂西地区震旦系-寒武系层控铅锌矿成矿规律研究[D]. 中国地质大学(武汉), 2014: 106-137.
DUAN Qi-fa. The research of metallogenic regularity of stritabound zinc-lead deposits from Sinian-Cambrian in western Hunan and western Hubei[D]. Wuhan: China University of Geosciences, 2014: 106-137.
[2] 郑荣才, 曾允孚. 湘西渔塘早寒武世生物礁沉积相特征[J]. 沉积学报, 1988, 6(2): 61-67.
ZHENG Rong-cai, ZENG Yun-fu. Sedimentary characteristics of early Cambrian Yutang organic reefs in western Hunan[J]. Acta Sedimentologica Sinica, 1988, 6(2): 61-67.
[3] 舒见闻. 渔塘铅锌矿床成因初步探讨[J]. 大地构造与成矿学, 1983, 7(4): 309-320.
SHU Jian-wen. Application of metallogeny of Diwa (geodepression) theory to prospecting for rich ore in the Yutang lead- zinc sulfide deposit[J]. Geotectonica et Metallogenia, 1983, 7(4): 309-320.
[4] 彭国忠. 湖南花垣渔塘地区层控型铅锌矿床成因初探[J]. 地质科学, 1986(2): 179-186.
PENG Guo-zhong. A preliminary discussion on the origin of stratabound lead-zine ore deposits in the Yutan region of Huayuan country, Hunan Province[J]. Scientia Geologica Sinica, 1986(2): 179-186.
[5] 刘亚新, 李时谦, 徐惠长, 邓松华. 湖南省热(卤)水矿床成矿控制及成矿规律[J]. 华南地质与矿产, 2004(3): 31-37.
LIU Ya-xin, LI Shi-qian, XU Hui-chang, DENG Song-hua. Metallogenetic regularity and ore-controlling factors of hot-brine type ore deposits in Hunan Province[J]. Geology and Mineral Resources of South China, 2004(3): 31-37.
[6] 陈明辉, 胡祥昭, 鲍振襄, 包觉敏. 湖南渔塘铅锌矿集中区地质特征及成矿问题讨论[J]. 地质与勘探, 2011, 47(2): 251-260.
CHEN Ming-hui, HU Xiang-zhao, BAO Zheng-xiang, BAO Jue-ming. Geological features and metallogenesis of the Yutang Pb-Zn ore concentration belt in Hunan Province[J]. Geology and Exploration, 2011, 47(2): 251-260.
[7] 雷义均, 戴平云, 段其发, 刘阿睢, 陶 明. 鄂西—湘西北地区铅锌矿矿源层对铅锌矿床产出定位的制约[J]. 桂林理工大学学报, 2013, 33(1): 1-6.
LEI Yi-jun, DAI Ping-yun, DUAN Qi-fa, LIU A-sui, TAO Ming. Constraints of lead-zinc source bed to lead-zinc Deposits in western Hubei to northwestern Hunan[J]. Journal of Guilin University of Technology, 2013, 33(1): 1-6.
[8] 段其发, 曹 亮, 曾健康, 周 云, 汤朝阳, 李 堃. 湘西花垣矿集区狮子山铅锌矿床闪锌矿Rb-Sr 定年及地质意义[J]. 地球科学—中国地质大学学报, 2014, 39(8): 977-986.
DUAN Qi-fa, CAO Liang, ZENG Jian-kang, ZHOU Yun, TANG Chao-yang, LI Kun. Rb-Sr dating of sphalerities from Shizishan Pb-Zn deposit in Huayuan ore concentration area, Western Hunan, and its geological significance[J]. Earth Science (Journal of China University of Geosciences), 2014, 39(8): 977-986.
[9] 杨绍祥, 劳可通. 湘西北铅锌矿床的地质特征及找矿标志[J]. 地质通报, 2007, 26(7): 899-908.
YANG Shao-xiang, LAO Ke-tong. Geological characteristics and ore indicators of lead- zinc deposits in northwestern Hunan, China[J]. Geological Bulletin of China, 2007, 26(7): 899-908.
[10] 刘文均, 郑荣才. 花垣铅锌矿床成矿流体特征及动态[J]. 矿床地质, 2000, 19(2): 173-181.
LIU Wen-jun, ZHENG Rong-cai. Characyeristics and movement of ore-forming fluids in the Huayuan lead-zinc deposit[J]. Mineral Deposit, 2000, 19(2): 173-181.
[11] 杨绍祥, 劳可通. 湘西北铅锌矿床碳氢氧同位素特征及成矿环境分析[J]. 矿床地质, 2007, 26(3): 330-340.
YANG Shao-xiang, LAO Ke-tong. A tentative discussion on genesis of lead-zinc deposits in northwest Hunan[J]. Mineral Deposit, 2007, 26(3): 330-340.
[12] 沈能平, 彭建堂, 袁顺达, 张东亮, 符亚洲, 胡瑞忠. 湖北徐家山锑矿床方解石C、O、Sr同位素地球化学[J]. 地球化学, 2007, 36(5): 479-485.
SHENG Neng-ping, PENG Jian-tang, YUAN Shun-da, ZHANG Dong-liang, FU Ya-zhou, HU Rui-zhong. Carbon, oxygen and strontium isotope geochemistry of calcites from Xujiashan antimony deposit, Hubei Province[J]. Geochimica, 2007, 36(5): 479-485.
[13] 刘昊年, 黄思静, 胡作维, 吴 萌, 王庆东. 锶同位素在沉积学中的研究与进展[J]. 岩性油气藏, 2007, 19(3): 59-65.
LIU Hao-nian, HUANG Si-jing, HU Zuo-wei, WU Meng, WANG Qing-dong. Advances of strontium isotope in sedimentology[J]. Lithologic Reservoirs, 2007, 19(3): 59-65.
[14] 周家喜, 黄智龙, 周国富, 曾桥松. 黔西北天桥铅锌矿床热液方解石C、O同位素和REE地球化学[J]. 大地构造与成矿学, 2012, 36(1): 93-101.
ZHOU Jia-xi, HUANG Zhi-long, ZHOU Guo-fu, ZENG Qiao-song. C, O isotope and REE geochemistry of the hydrothermal calcites from the Tianqiao Pb-Zn ore deposit in NW Guizhou Province, China[J]. Geotectonica et Metallogenia, 2012, 36(1): 93-101.
[15] 彭建堂, 胡瑞忠, 漆亮, 赵军红, 符亚洲. 锡矿山热液方解石的REE分配模式及其制约因素[J]. 地质论评, 2004,50(1): 25–32.
PENG Jian-tang, HU Rui-zhong, QI Liang, ZHAO Jun-hong, FU Ya-zhou. REE distribution pattern for the hydrothermal calcites from the Xikuangshan antimony deposit and its constraining factors[J]. Geological Review, 2004, 50(1): 25–32.
[16] 曹华文, 裴秋明, 张寿庭, 向 辉, 张林奎, 唐 利. 河南栾川三道沟铅锌银矿床方解石地球化学特征及Sm-Nd年龄研 究[J]. 地球化学, 2016, 45(2): 144-154.
CAO Hua-wen, PEI Qiu-ming, ZHANG Shou-ting, XIANG Hui, ZHANG Lin-kui, TANG Li. Geochemistry and Sm-Nd age of calcites from the Sandaogou Pb-Zn-Ag deposit in Luanchuan, western Henan Province[J]. Geochimica, 2016, 45(2): 144-154.
[17] 邓 红, 黄智龙, 肖宪国, 丁 伟. 贵州半坡锑矿床方解石稀土元素地球化学研究[J]. 矿物学报, 2014, 34(2): 208-216.
DENG Hong, HUANG Zhi-long, Xiao Xianguo, Ding Wei. REE geochemistry of gangue calcite from Banpo Deposit in Dushan antimony ore field, Guizhou Province, China[J]. Acta mineralogical sinica. 2014, 34(2): 208-216.
[18] PALMER M R, ELDERFIELD H. Sr isotope composition of sea water over the past 75 Myr[J]. Nature, 1985, 314(6011): 526-528.
[19] BURKE W H, DENISON R E, HETHERINGTON E A, KOEPNICK R B, NELSON H F, OTTO J B. Variation of seawater 87Sr/86Sr throughout Phanerozoic time[J]. Geology, 1982, 10(10): 516-519.
[20] M LLER P, PAREKH P P, SCHNEIDER H J. The application of Tb/Ca-Tb/La abundance ratios to problems of fluorspar genesis[J]. Mineral Deposita, 1976, 11(1): 111-116.
LLER P, PAREKH P P, SCHNEIDER H J. The application of Tb/Ca-Tb/La abundance ratios to problems of fluorspar genesis[J]. Mineral Deposita, 1976, 11(1): 111-116.
[21] DAVIES J F, PREVEC S A, WHITEHEAD R E, JACKSON S E. Variations in REE and Sr-isotope chemistry of carbonate gangue, Castellanos Zn-Pb deposit, Cuba[J]. Chemical Geology, 1998, 144(1/2): 99-199.
[22] CHESLEY J T, HALLIDAY A N, SCRIVENER R C. Samarium-neodymium direct dating of fluorite mineralization [J]. Science, 1991, 252: 949-951.
[23] 刘淑文, 石 顺, 李荣西, 高云宝, 刘玲芳, 段立志, 陈宝赟, 张少妮. 扬子板块北缘马元铅锌矿床稀土元素地球化学研 究[J]. 矿床地质, 2013, 32(5): 979-988.
LIU Shu-wen, SHI Shun, LI Rong-xi, GAO Yun-bao, LIU Ling-fang, DUAN Li-zhi, CHEN Bao-yun, ZHANG Shao-ni. REE geochemistry of Mayuan Pb-Zn deposit on northern margin of Yangtze Plate[J]. Mineral Deposits, 2013, 32(5): 979-988.
[24] MICHARD A. Rare earth element systematics in hydrothermal fluids[J]. Geochimica Et Cosmochimica Acta, 1989, 53(3): 745-750.
[25] BAU M, M LLER P. Comparative study of yttrium and rare-earth element behavior in fluorine-rich hydrothermal fluids[J]. Contributions to Mineralogy and Petrology, 1995, 119(2): 213-223.
LLER P. Comparative study of yttrium and rare-earth element behavior in fluorine-rich hydrothermal fluids[J]. Contributions to Mineralogy and Petrology, 1995, 119(2): 213-223.
[26] CHERNIAK D J , ZHANG X Y, WAYNE N K, WATSON E B. Sr, Y and REE diffusion in fluorite[J]. Chemical Geology, 2001, 181(1): 99-111.
[27] SCH NENBERGER J, K
NENBERGER J, K HLER J, MARKL G. REE systematics of fluorides, calcite and siderite in peralkaline plutonic rocks from the Gardar Province, South Greenland[J]. Chemical Geology, 2008, 247(1/2): 16-35.
HLER J, MARKL G. REE systematics of fluorides, calcite and siderite in peralkaline plutonic rocks from the Gardar Province, South Greenland[J]. Chemical Geology, 2008, 247(1/2): 16-35.
[28] SVERJENSKY D A. Europium redox equilibria in aqueous solution[J]. Earth & Planetary Science Letters, 1984, 67(1): 70-78.
[29] BAU M. Rare-earth element mobility during hydrothermal and metamorphic fluid-rock in traction and the significance of the oxidation state of europium[J]. Chemical Geology, 1991, 93(3/4): 219-230.
[30] SHANNON R D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides[J]. Acta Crystallography, 1976, 32(5): 751-767.
[31] XIONG Yi-qu, SHAO Yong-jun, ZHOU Hao-di, WU Qian-hong, LIU Jian-ping, WEI Han-tao, ZHAO Rui-cheng, CAO Jing-ya. Ore-forming mechanism of quartz-vein-type W-Sn deposits of the Xitian district in SE China: Implications from the trace element analysis of wolframite and investigation of fluid inclusions[J]. Ore Geology Reviews, 2017, 83: 152-173.
[32] 黄思静, 石 和, 毛晓冬, 张 萌, 沈立成, 武文慧. 重庆秀山寒武系锶同位素演化曲线及全球对比[J]. 地质论评, 2002, 48(5): 509-516.
HUANG Si-jing, SHI He, MAO Xiao-dong, ZHANG Meng, SHEN Li-cheng, WU Wen-hui. Evolution of Sr Isotopes of the Cambrian Sections in Xiushan, Chongqing, and Related Global Correlation[J]. Geological Review, 2002, 48(5): 509-516.
[33] TAN Hong-bing, RAO Wen-bo, MA Hai-zhou, CHEN Jian-sheng, LI Ting-wei. Hydrogen, oxygen, helium and strontium isotopic constraints on the formation of oilfield waters in the western Qaidam Basin, China[J]. Asian Earth Sci, 2011, 40(2): 651-660.
[34] HAN Gui-lin, LIU Cong-Qiang. Strontium isotope and major ion chemistry of the rainwaters from Guiyang, Guizhou Province, China[J]. Science of the Toal Environment, 2006, 364(1/3): 165-174.
[35] HAN Gui-lin, TANG Yang, WU Qi-xin, TAN Qiu. Chemical and strontium isotope characterization of rainwater in karst virgin forest, Southwest China[J]. Atmos Environ, 2010, 44(2): 174-181.
[36] HAN Gui-lin, LIU Cong-Qiang. Water geochemistry controlled by carbonate dissolution: a study of the river waters draining karst-dominated terrain, Guizhou Province, China[J]. Chemical Geology, 2004, 204(1/2): 1-21.
REE and Sr isotope geochemistry of gangue calcites from Huayuan Pb-Zn orefield in western Hunan, China
WEI Han-tao1, 2, SHAO Yong-jun1, 2, YE Zhou3, XIONG Yi-qu1, 2, ZHOU Hao-di1, 2, XIE You-liang1, 2
(1. Key Laboratory of Metallogenic Prediction of Nonferrous Metals and Geological Environment Monitoring, Ministry of Education, Changsha 410083, China;
2. School of Geosciences and Info-Physics, Central South University, Changsha 410083, China;
3. The Geological Museum of Hunan, Changsha 410004, China)
Abstract: With the discovery of the large to super large Yangjiazhai, Danaopo and Qingshuitang Pb-Zn deposits, the Huayuan orefield becomes a world-class super large Pb-Zn orefield. According to the crosscutting relationship of ore veins and coexisting mineral assemblage, the mineralization process was subdivided into three stages. REE and strontium isotope of calcites were analyzed and compared with those of ore-hosting limestone and strata of different geological times. The results show that the REE patterns of calcites are similar to those of strata. Stage I calcites display low total contents of REE (ΣREE=1.73×10-6-10.94×10-6,average 4.77×10-6), 87Sr/86Sr ratios ranging from 0.70935 to 0.71001 (average 0.709643) and LREE/HREE ratios range from 6.21 to 10.19 (average 7.96). Stage II calcites display higher contents of REE (ΣREE=24.53×10-6-67.88×10-6,average 46.71×10-6) and lower 87Sr/86Sr ratios ranging from 0.70923 to 0.70944 (average 0.709301) than those of stage I calcite, and similar LREE/HREE ratios range from 6.21 to 10.19 (average 7.96). These indicate that the ore-forming fluids of stages I and II are derived from different origins. According to geochemical characteristics of wall rock and strata of different geological times, it is concluded that the ore-forming fluids of stage I are mainly connate water in ore-hosting strata, mixed by deep-source circular brine; the ore-forming fluids of stage II are mainly deep-source circular brine, mixed by meteoric water, and the reducibility increases from stage I to II.
Key words: calcite; REE; Sr isotope; ore-forming fluids; Huayuan Pb-Zn orefield
Foundation item: Project(12120114052201) supported by the Chinese Geological Survey; Project(41472302) supported by the National Natural Sciences Foundation of China; Project supported by the Development Foundation of Key Laboratory of Metallogenic Prediction of Nonferrous Metals and Geological Environment Monitor, Ministry of Education, China; Project(2015zzts069) supported by the Independent Innovation Program for Doctoral Candidates of Central South University, China
Received date: 2016-05-12; Accepted date: 2017-08-10
Corresponding author: SHAO Yong-jun; Tel: +86-13973149482; E-mail: shaoyongjun@126.com
(编辑 龙怀中)
基金项目:中国地质调查局资助项目(12120114052201);国家自然科学基金资助项目(41472302);中南大学有色金属成矿预测与地质环境监测教育部重点实验室发展基金资助项目;中南大学博士生自主探索创新资助项目(2015zzts069)
收稿日期:2016-05-12;修订日期:2017-08-10
通信作者:邵拥军,教授,博士;电话:13973149482;E-mail:shaoyongjun@126.com
摘 要:湘西花垣铅锌矿田随着杨家寨、大脑坡及清水塘等一系列大型–超大型铅锌矿床的发现,已经成为世界千万吨级铅锌矿田。通过矿脉切穿关系和不同的矿物共生组合,将花垣矿田成矿过程分为3个阶段。对矿田内主成矿期方解石的REE及Sr同位素进行了研究,并与含矿围岩及不同时代地层的REE和Sr同位素进行了对比。结果表明:方解石稀土元素特征与地层具有相似性,其中I阶段方解石REE含量较低,ΣREE为1.73×10-6~10.94×10-6,平均为4.77×10-6;LREE/HREE为6.21~10.19,平均为7.96;87Sr/86Sr比值0.70935~0.71001,平均为0.709643。II阶段方解石REE含量比I阶段高,ΣREE为24.53×10-6~67.88×10-6,平均为46.71×10-6;LREE/HREE为6.21~10.19,平均为7.96;87Sr/86Sr比值为0.70923~0.70944,平均为0.709301,比I阶段的低,说明I、II阶段成矿流体存在差异。结合围岩及不同时代地层地球化学特征认为,I阶段成矿流体主要为含矿层建造水,混有深循环热卤水;II阶段成矿流体主要为深循环热卤水,混有大气降水,从I阶段至II阶段成矿流体还原性增强。
[1] 段其发. 湘西-鄂西地区震旦系-寒武系层控铅锌矿成矿规律研究[D]. 中国地质大学(武汉), 2014: 106-137.
[2] 郑荣才, 曾允孚. 湘西渔塘早寒武世生物礁沉积相特征[J]. 沉积学报, 1988, 6(2): 61-67.
[3] 舒见闻. 渔塘铅锌矿床成因初步探讨[J]. 大地构造与成矿学, 1983, 7(4): 309-320.
[4] 彭国忠. 湖南花垣渔塘地区层控型铅锌矿床成因初探[J]. 地质科学, 1986(2): 179-186.
[5] 刘亚新, 李时谦, 徐惠长, 邓松华. 湖南省热(卤)水矿床成矿控制及成矿规律[J]. 华南地质与矿产, 2004(3): 31-37.
[6] 陈明辉, 胡祥昭, 鲍振襄, 包觉敏. 湖南渔塘铅锌矿集中区地质特征及成矿问题讨论[J]. 地质与勘探, 2011, 47(2): 251-260.
[7] 雷义均, 戴平云, 段其发, 刘阿睢, 陶 明. 鄂西—湘西北地区铅锌矿矿源层对铅锌矿床产出定位的制约[J]. 桂林理工大学学报, 2013, 33(1): 1-6.
[9] 杨绍祥, 劳可通. 湘西北铅锌矿床的地质特征及找矿标志[J]. 地质通报, 2007, 26(7): 899-908.
[10] 刘文均, 郑荣才. 花垣铅锌矿床成矿流体特征及动态[J]. 矿床地质, 2000, 19(2): 173-181.
[11] 杨绍祥, 劳可通. 湘西北铅锌矿床碳氢氧同位素特征及成矿环境分析[J]. 矿床地质, 2007, 26(3): 330-340.
[12] 沈能平, 彭建堂, 袁顺达, 张东亮, 符亚洲, 胡瑞忠. 湖北徐家山锑矿床方解石C、O、Sr同位素地球化学[J]. 地球化学, 2007, 36(5): 479-485.
[13] 刘昊年, 黄思静, 胡作维, 吴 萌, 王庆东. 锶同位素在沉积学中的研究与进展[J]. 岩性油气藏, 2007, 19(3): 59-65.
[14] 周家喜, 黄智龙, 周国富, 曾桥松. 黔西北天桥铅锌矿床热液方解石C、O同位素和REE地球化学[J]. 大地构造与成矿学, 2012, 36(1): 93-101.
[15] 彭建堂, 胡瑞忠, 漆亮, 赵军红, 符亚洲. 锡矿山热液方解石的REE分配模式及其制约因素[J]. 地质论评, 2004,50(1): 25–32.
[16] 曹华文, 裴秋明, 张寿庭, 向 辉, 张林奎, 唐 利. 河南栾川三道沟铅锌银矿床方解石地球化学特征及Sm-Nd年龄研 究[J]. 地球化学, 2016, 45(2): 144-154.
[17] 邓 红, 黄智龙, 肖宪国, 丁 伟. 贵州半坡锑矿床方解石稀土元素地球化学研究[J]. 矿物学报, 2014, 34(2): 208-216.
[23] 刘淑文, 石 顺, 李荣西, 高云宝, 刘玲芳, 段立志, 陈宝赟, 张少妮. 扬子板块北缘马元铅锌矿床稀土元素地球化学研 究[J]. 矿床地质, 2013, 32(5): 979-988.
[32] 黄思静, 石 和, 毛晓冬, 张 萌, 沈立成, 武文慧. 重庆秀山寒武系锶同位素演化曲线及全球对比[J]. 地质论评, 2002, 48(5): 509-516.


