J. Cent. South Univ. (2012) 19: 46-54
DOI: 10.1007/s11771-012-0971-z![]()
Corrosion resistance of waterborne epoxy coating pigmented by nano-sized aluminium powder on steel
LIU Jian-hua(刘建华), ZHAN Zhong-wei(詹中伟), LI Song-mei(李松梅), YU Mei(于美)
School of Materials Science and Engineering, Beihang University, Beijing 100191, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract:
A novel kind of waterborne epoxy coating pigmented by nano-sized aluminium powders on high strength steel was formulated. Several coatings with different pigment volume content (PVC) were prepared. The coating morphology was observed using scanning electron microscopy (SEM), and the electrochemical properties were investigated by electrochemical impedance spectroscopy (EIS). Immersion test and neutral salt spray test were also conducted to investigate the corrosion resistance of the coating. It is demonstrated that the critical pigment volume content (CPVC) value is between 30% and 40%. The coating with PVC of 30% exhibits good corrosion resistance in 3.5% (mass fraction) NaCl solution.
Key words:
nano-sized aluminium powder; waterborne epoxy coating; corrosion resistance; pigment volume content;
1 Introduction
Organic coating is one of the efficient methods to protect metallic substrates from corrosion [1], and the efficiency could be enhanced by adding some kinds of suitable pigments [2]. Some widely used lead-based and hexavalent chromium (VI)-contained pigments, posed environmental concerns, and were proved to be detrimental to human. Therefore, they must be removed from the coating formulation [3]. Moreover, the introduction of strict regulations in the use of volatile organic compounds (VOC) has brought about the development of waterborne coating technologies. Consequently, based on the environmental protection requirements, many novel protective coatings with waterborne binders and non-toxic anticorrosion pigments [4] have been studied extensively.
In recent years, waterborne coatings with nano- sized inorganic composites as pigments have been investigated because of their effectiveness in corrosion protection. For example, nano-TiO2 [5-7], nano-SiO2 [6-7], and nano-sized titanium powder [8] were applied to modifying organic coatings.
When coatings are formulated, one important characteristic value is pigment volume content (PVC), which strongly affects their properties [9]. Furthermore, there is a critical pigment volume content (CPVC) [10] in many coating formulations containing pigments or dyes. Below the CPVC, the pigment can uniformly distribute in the binder matrix without harming its integrality and compactness. While above the CPVC, the excessive pigment can not be fully packed by the binder, and pores and voids occur in the coating matrix.
In our previous studies, the corrosion resistance of many kinds of steel has been extensively investigated [11-14]. Besides, nano-science has also been introduced into our research to exploit valuable areas with novel sharp [15] and properties [16]. In this research, nano- sized aluminium powder was added into waterborne epoxy coatings with different pigment volume contents (PVC) from 10% to 80%. Scanning electron microscopy (SEM) and electrochemical impedance spectroscopy (EIS) were applied to determine the critical pigment volume content (CPVC) independently. The corrosion resistance of the coating with optimum PVC of 30% was investigated by immersion test and neutral salt spray test. In combination with equivalent circuit simulation method, electrochemical impedance spectroscopy was also used to reveal the corrosion behavior of the coating (PVC 30%) during the immersion in 3.5% (mass fraction) NaCl solution.
2 Experimental
2.1 Samples preparation
High strength steel panels (30CrMnSi) were used as metallic substrates with a size of 100 mm × 50 mm ×2 mm. The panels were polished with SiC waterproof abrasive papers up to grit #400, degreased with acetone and deionized water, then blown dry with clean air. The pre-treatment operations were performed prior to the application of the epoxy coating.
The coating procedure was as follows: The waterborne epoxy resin (HD-BE314, chemical purity, Beijing Jinhuili Applied Chemical Products Co. Ltd., China) was mixed with deionized water in a mass ratio of 1:1, then magnetically stirred for about 2 h until a uniform, light-yellow solution was obtained. The aluminium powder (analytical purity, median-particle- size (D50) of 60 nm, Nanjing Emperor Nano-Material Co. Ltd., China) was slowly added into a silane coupling agent solution under severe stirring for 10 min, and the molar ratio of silane-to-aluminium was 1:2. After stirring, the aluminium powder solution was ultrasonically processed for 30 min, and then mixed with the epoxy solution to get different PVC from 10% to 80% with increment of 10%. The final solution was then stirred for at least 6 h at room temperature, while several additives were added, such as defoamer, diapersant, flow agent, which were bought as commercial products and used without any further purification or dilution. The composition of the coating presented in this study is given in Table 1.
Table 1 Composition of waterborne epoxy coating

The steel panels were dip coated for two times and then dried in air for 24 h, followed by a 3 h oven-dry at 120 °C. The thickness of the coatings was (20±3) μm after drying, which was measured by a coating thickness gauge MiniTest 610 (Elektro Physik, Germany). The cross-section micrographs of the coating, as well as the related EDS measurements, were also carried out to confirm the thickness.
2.2 Testing methods
To observe the surface and cross-section morphology of the coatings, SEM analyses were performed using a JSM-5800 instrument in secondary electron mode.
The electrochemical impedance spectroscopy (EIS) was measured by electrochemical work station (Princeton 2273, USA). The electrochemical testing solution was a 3.5% NaCl aqueous solution open to air, held at room temperature (25 °C ). A three-electrode cell arrangement was used in the experiment by sticking a plastic cylinder on the sample sheet and filling it with the testing solution. The exposed sample surface area was 7 cm2. A Pt-plate and a saturated calomel electrode (SCE) were used as counter and reference electrode, respectively. All EIS measurements were performed in a frequency range from 1 MHz to 10 mHz under open circuit potential, with a 10 mV amplitude of the sinusoidal voltage signal.
The corrosion resistance was examined using immersion test and neutral salt spray test according to ASTM B117. During the immersion in 3.5% NaCl solution, several EIS measurements were carried out at different time points (0.5, 5, 24, 72, 120, 288, 400, 600, 800 h) with the frequency range from 0.1 MHz to 10 mHz. The impedance data were analyzed using ZsimpWin software to extract characteristic parameters of the coatings.
To measure the adhesion performance of the coatings, a cutting tool (295/V, Erichen, Germany) was used to make a cross-cut through the coatings till the substrate. The cutting surface was observed and evaluated according to the ASTM D 3359-02.
3 Results and discussion
3.1 Determination of CPVC
The critical pigment volume content (CPVC) is an important parameter to each coating system containing one kind or more pigments [17-22]. Many experimental methods were reported as reference method of CPVC, such as calculation of oil absorption values, internal stress measurement, hiding power, mercury porosimetry [23] and gas permeation [24]. Among these methods, the SEM observation and EIS measurement were regarded as the most reproducible methods to distinguish the undercritical from overcritical coatings.
The surface morphology of coatings containing nano-sized aluminium powder with pigment volume content (PVC) from 10% to 60% is shown in Fig. 1. The micro-pore structure is observed in the coatings with PVC of more than 40% as shown in Fig. 1(d). For coatings with PVC lower than 30%, the aluminium powders are randomly embedded in the continuously connected matrix of epoxy binder with a relatively flat surface. For coatings with PVC from 30% to 40%, the metallic powders are uniformly distributed in the coating body, and the binder adequately fills the spaces among the powder particles and the aggregates, which results in sporadic isolated holes. Therefore, it is demonstrated that the optimal CPVC value is between 30% and 40%.
Figure 2 shows the micrograph of cross-section of the coating with PVC of 30% and related EDS results. The epoxy coating appears darker than the steel substrate, which clearly distinguishes the interface. The coating tightly adheres to the substrate, without any pores or defects at the interface. The matrix of the coating is compact, indicating that the double dip-coating process does not bring in delamination inside the coating. The thickness of the coating is approximately 20 μm. The EDS linear scanning was conducted to precisely determine the interface between the substrate and the coating. The linear scanning direction is shown as the white arrow in the SEM micrograph in Fig.2. Three elements (Fe, C and Al) were chosen with their EDS results along the arrow listed in Fig. 2. The steep changes of the contents of three elements clearly show the interface labeled with the dash line.

Fig. 1 SEM micrographs of surface of coatings with different PVC: (a) 10%; (b) 30%; (c) 40%; (d) 60%

Fig. 2 SEM micrograph (a) and EDS linear scanning of cross-section of coating with PVC of 30%: (b) Fe; (c) C; (d) Al
To identify the above conclusion about the CPVC value, EIS of the coatings was measured as another independent indicator. Bode diagrams of the coatings containing nano-sized aluminium powder with PVC from 10% to 80% after an immersion time of 3 h in 3.5% NaCl solution are shown in Fig. 3, and those of 120 h are shown in Fig. 4.

Fig. 3 Bode diagrams of waterborne epoxy coatings containing nano-sized aluminium powder with different PVC after 3 h immersion in 3.5% NaCl solution: (a) Magnitude plots; (b) Phase plots
As shown in Fig. 3(a), after 3 h exposure in 3.5% NaCl solution, the Bode magnitude plots of the undercritical coatings with PVC of 10% and 20% show the same shape, and so do the curves of coatings with PVC from 50% to 80%, which are regarded to be overcritical. The spectra of the coatings with PVC of 30% and 40% have impedance between the above two groups of curves. The lower PVC coatings generally present much higher impedance than those with higher PVC, especially in the low frequency range. However, the Bode phase plots do not support such classification evidently, as shown in Fig. 3(b). All the curves have a capacitance arc in the middle frequency range (10-1- 103 Hz). The curves of the coatings with PVC lower than 40% also show a sharp salient in the high frequency range, which are much steeper than those with higher PVC.

Fig. 4 Bode diagrams of waterborne epoxy coatings containing nano-sized aluminium powder with different PVC after 120 h immersion in 3.5% NaCl solution: (a) Magnitude plots; (b) Phase plots
The Bode magnitude plots of coatings with various PVC immersed for 120 h, as shown in Fig. 4, distinguish the overcritical from the undercritical coatings much more clearly than that of 3 h immersion. The gaps between the undercritical and the overcritical coatings are significantly widened, where the curves of coatings with PVC of 30% and 40% lay in the middle. Such classification can also be derived from the Bode phase plots. The Bode phase curves with PVC of 10% and 20% appear to be similar with those after 3 h exposure. The curves with PVC higher than 40% have only one visible capacitance arc, which shift to lower frequency compared with curves in Fig. 3, and become much more flatter in the high frequency range. The curves with PVC of 30% and 40% differ from the others. They feature the salient in low frequency, which is similar with the overcritical PVC, and the soar in high frequency, which is alike with the undercritical PVC.
Summarily, based on the analysis of the SEM images and the EIS curves, the CPVC value of the waterborne epoxy coatings containing nano-sized aluminium powder can be demonstrated between 30% and 40%.
3.2 Corrosion resistance of coatings during immersion
The CPVC value can greatly affect the properties of the coatings, and is taken into consideration during the formulation. Generally, the ratio of PVC/CPVC is advised to be between 0.5 and 0.8, in order to get a better comprehensive performance [24]. Therefore, the coatings studied in the following immersion test were prepared with a PVC value of 30%.
Figure 5 shows the EIS plots of the coatings with PVC of 30% immersed in 3.5% NaCl solution for different immersion time. From these curves, it is revealed that three successive stages are distinguished during the immersion process. In the first stage of the immersion process (0.5-5 h), there is only one capacitance arc on the Nyquist plot, which implies that the coating exhibits good barrier to prohibit the permeation of the corrosive species such as water, oxygen, and other ions towards the surface of the substrate. The Bode plot also presents a simple shape indicating a time constant in this stage.
After 5 h immersion, the capacitance arc on the Nyquist plot changes as a semicircle, and another arc appears at the end of the semicircle, which implies that the reactions of the aluminium powder embedded in the coating have been detected. Moreover, a tiny tail appears at the end of the Nyquist curves, which is probably caused by the electrical conduction of the corrosion products of the aluminium powders, mainly the hydroxide and oxide of aluminium. It is generally considered that these precipitations gradually accumulate and block the micro-pores through which the corrosive media reach the interface of the coating and substrate, resulting in a kind of “healing” of the damage site of the coating. The height of the lg|Z|-plateau of Bode magnitude curves at low frequencies, which is generally used as an estimate for the pore resistance of the coating, declines with increasing the exposure time. On the Bode phase plots, the phase angle decreases greatly in the intermediate frequency range, compared with that of initial stage. A salient of the phase angle curves is observed in the low frequency range, and another relatively small one for the high frequency range. Therefore, more than two time constants are detected, which is confirmed in the following equivalent circuit simulation procedure. The EIS curves maintain the similar shape until about 400 h, which implies that in this period the corrosion behaviors of the coatings share the same mechanism.
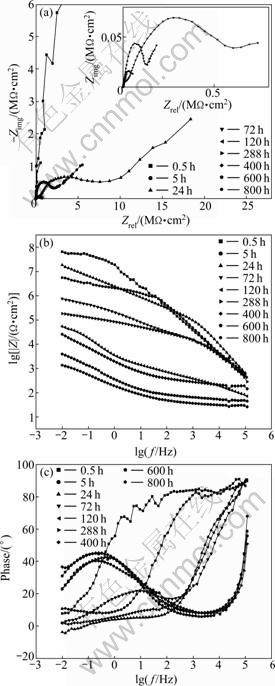
Fig. 5 EIS plots of waterborne epoxy coatings containing nano- sized aluminium powder with PVC of 30% immersed in 3.5% NaCl solution for different times: (a) Nyquist plots; (b) Bode magnitude plots; (c) Bode phase plots
After 400 h immersion, the pore resistance of the coating decreases significantly. On the Nyquist plot, the capacitive arc in the high frequency range shrinks to a small semicircle, while another one at low frequency enlarges relatively. Furthermore, the tiny tail disappears, indicating the destruction of the occluded structures produced by precipitations. A considerable change occurs for the shape of the Bode phase curves. The salient in the low frequency range becomes distinct and shifts to high degrees. The phase plateau at high frequencies, visible for the initial stage and not so visible for the middle stage, has completely disappeared, and the phase angle decreases directly and steeply to a large plateau near 0° which lasts until the middle frequencies. The above facts prove that the corrosive media such as water and ions have penetrated through the coating and reached the metallic substrate. Consequently, the semicircle in the low frequency range in the Nyquist plot is expected to change into an inclined line, which is regarded as a sign of diffusion process of substrate corrosion.
Moreover, the equivalent circuit was used to fit the experimental EIS data. As recalled by KEDDAM et al [25], an organic coating with considerable thickness cannot be simplified as an intact capacitor, and the impurities from additives ruin the perfection of the coating for most cases. Therefore, the classical equivalent circuit as shown in Fig. 6(a) cannot fully reflect the coating structure during the whole immersion, except for the initial stage. In this stage, the coating has good barrier property which can be simulated by the circuit in Fig. 6(a) including the solution resistance Rel, the coating capacitance Cc, the pore resistance of the coating Rp, the double layer capacitance Cd and the charge transfer resistance Rct. To get better simulation result, the double layer capacitance Cdl can be replaced by a constant phase element (CPE) Qdl. Using this circuit, the EIS data of the coating in initial stage are well fitted. Figure 6(b) represents a typical Nyquist plot (0.5 h) and its fitting results. From the fitting results, the characteristic parameters of the immersed coating are extracted, as listed in Table 2, together with the goodness of the fitting. The pore resistance Rp generally decreases with the immersion time in the initial stage, and so does the coating capacitance Cc. This can be explained by the water uptake and penetration of ions. These corrosive media break through the coating surface, reach the aluminium powders embedded in the coating, and react with them. The reaction products attach to the powder, which results in the increase of the charge transfer resistance Rct, and prevents the corrosion to a certain extend.
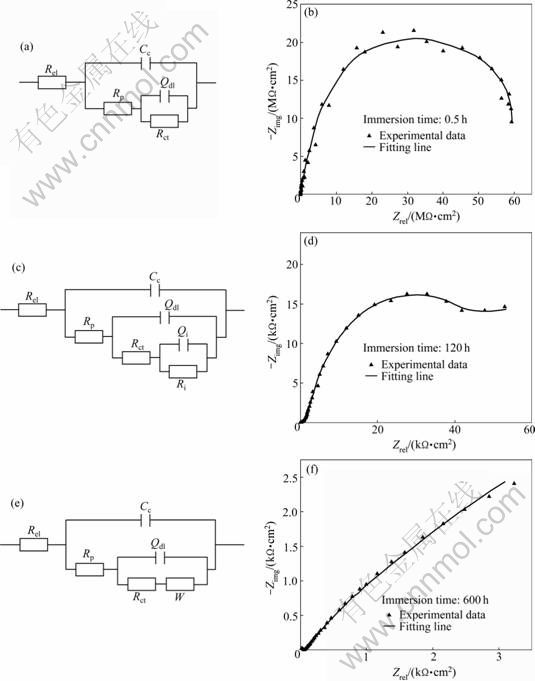
Fig. 6 Nyquist plots and fitting results using equivalent circuits of coatings immersed for different time: (a), (b) Initial stage (0.5 h); (c), (d) Middle stage (120 h); (e), (f) Final stage (600 h)
Table 2 Electrochemical parameters of coatings (PVC 30%) for different immersion time

With the increase of the immersion time, amounts of the corrosive media penetrate into the coating, and the occluded structures produced by precipitations as mentioned before start to influence the corrosion process. The dissimilar structures bring changes to the equivalent circuit. As shown in Fig. 6(c), a parallel combination of impurity structures capacitance Ci and resistance Ri is incorporated in series with Rct. Figure 6(d) shows the Nyquist plot with immersion time of 120 h and its fitting results. Three capacitive arcs appear and change the shape of the curve greatly. The arc in the high frequency range reflects the property of the unharmed coating, while the features of electrochemical reaction of aluminium powder and blocking effect of the occluded structures correspond to the ones at low frequencies. In this period, sharp decline of Rp value is clearly observed, and Rct reaches its peak as shown in Table 2 and Fig. 7, which can be explained by the continual breakage of the coating integrity and the increasingly effective cathodic protection of the aluminium powders. From the facts above, the main corrosion resistance in this period is confirmed to be from the aluminium powder reaction. Studying the evolution of coating capacitance Cc, a increscent tendency is observed, which means that water and other corrosive media penetrate much deeper in depth and larger in amount into the coating, and probably reach the metallic substrate under the coating at the end of this stage.

Fig. 7 Evolution of coating capacitance Cc, coating resistance Rp, and charge transfer resistance Rct with immersion time
Subsequently, the disbonding of the coating to the metallic substrate becomes severe due to the penetration of the corrosive media, gradually the occluded structures are overcome, and micro media-transfer pores generate at places where the coatings are broken down. The corrosion mechanism changes, and its representation in Nyquist plot is that the capacitive arc in the high frequency range shrinks in magnitude significantly, while another one at low frequencies turns to be a semicircle. In the end, the low-frequency arc has a good chance to become an inclined line, which means that the diffusion process has taken the major role of the corrosion mechanism. Therefore, a Warburg impedance W is needed to be added into the equivalent circuit, and the elements of the occluded structures should be deleted, as represented in Fig. 6(e). The Nyquist plot of coating immersed for 600 h is shown in Fig. 6(f) with its well fitted result. During the final stage, the main protective function comes from the blocking effect of the coating itself, which is incapable to well protect the substrate from corrosion. The values of Rp and Rct in Table 2 and Fig. 7 decrease sharply, and the coating capacitance Cc slowly increases.
3.3 Neutral salt spray and adhesion test
The neutral salt spray test was applied to the coatings with PVC of 30% and 40% to evaluate the corrosion resistance according to ASTM B117. The test results of the two coatings (PVC 30% and 40%) before and after 800 h neutral salt spray are shown in Fig. 8. The chromatism of the coatings appearing in Fig. 8 is caused by different shooting conditions, and not related to any property change of the coatings. It is clearly observed that the coating with PVC of 30% shows a good corrosion resistance without any visible defects in comparison with the coating of PVC 40%. On the contrary, dark red corrosion products on the surface of the coating with PVC of 40% are found, which indicates that corrosive media have penetrated the coating matrix and reached the substrate. From the results, it is clearly shown that the coating with PVC slightly lower than CPVC (i.e. PVC 30%) exhibits good corrosion resistance during long exposure in aggressive corrosion environment.
The cross-cut surfaces of the coatings with PVC of 30% (Fig. 9(a)) and 40% (Fig. 9(b)) after 400 h immersion in 3.5% NaCl solution are shown in Fig.9. The lines are clear, the edges and corners are trenchant, and no sign of flaking is detected, indicating good adhesion performance of the two coatings even after long time immersion.

Fig. 8 Appearances of coatings after neutral salt spray test with 5% NaCl solution: PVC 30% coating before (a) and after (b) 800 h salt spray; PVC 40% coating before (c) and after (d) 800 h salt spray

Fig.9 Cross-cut surfaces of coatings with PVC of 30% (a) and 40% (b) after immersion in 3.5% NaCl solution for 400 h
4 Conclusions
1) The corrosion resistance of waterborne epoxy coating can be promoted by adding nano-sized aluminium powder as pigment. Using SEM and EIS measurements, the CPVC value is confirmed between 30% and 40%.
2) The immersion corrosion process is distinguished into three successive stages. In the initial stage, the aluminium powder is wetted, and the polymer coating provides the high corrosion resistance. In the middle stage, more water and other corrosive media penetrate into the coating, and more aluminium powder takes part into the electrochemical reaction. The corrosion products of the aluminium powders precipitate and block the corrosion sites, therefore protect the substrate from further damage. in the end of the immersion, the disbonding of the coating gets severe due to the penetration of the corrosive media to the interface of coating/substrate.
References
[1] SCHOFF C K. Organic coatings: The paradoxical materials [J]. Progress in Organic Coatings, 2005, 52(1): 21-27.
[2] PERERA D Y. Effect of pigmentation on organic coating characteristics [J]. Progress in Organic Coatings, 2004, 50(4): 247-262.
[3] CHEN Xiao-ming, LI Guang-yu, LIAN Jian-she, JIANG Qing. An organic chromium-free conversion coating on AZ91D magnesium alloy [J]. Applied Surface Science, 2008, 255(1): 2322-2328.
[4] ZUBIELEWICZ M, GNOT W. Mechanisms of non-toxic anticorrosive pigments in organic waterborne coatings [J]. Progress in Organic Coatings, 2004, 49(4): 358-371.
[5] SATHIYANARAYANAN S, AZIM S S, VENKATACHARI G. Corrosion protection of magnesium ZM21 alloy with polyaniline–TiO2 composite containing coatings [J]. Progress in Organic Coatings, 2007, 59(4): 291-296.
[6] SHI Hong-wei, LIU Fu-chun, YANG Li-hong, HAN En-hou. Characterization of protective performance of epoxy reinforced with nanometer-sized TiO2 and SiO2 [J]. Progress in Organic Coatings, 2008, 62(4): 359-368.
[7] Tao Qing, Sun Zhi, Yi Chun-long. AN Yun-qi. Impact mechanism of nano-sized TiO2 and SiO2 on corrosion resistance of electric arc spraying sealing coat [J]. Procedia Earth and Planetary Science, 2009, 1(1): 851-856.
[8] ZHANG Xiu-zhi, WANG Fu-hui, DU Yuan-long. Effect of nano-sized titanium powder addition on corrosion performance of epoxy coatings [J]. Surface and Coatings Technology, 2007, 201(16/17): 7241-7245.
[9] LOBNIG R E , VILLALBA W, GOLL K, VOGELSANG J, WINKELS I, SCHMIDT R, ZANGER P, SOETEMANN J. Development of a new experimental method to determine critical pigment–volume–concentrations using impedance spectroscopy [J]. Progress in Organic Coatings, 2006, 55(4): 363-374.
[10] ASBECK W K, LOO M V. Critical pigment volume relationships [J]. Industrial and Engineering Chemistry, 1949, 41(7): 1470-1475.
[11] LIU Jian-hua, SHI Jun-xiu, LI Song-mei. Effects of electroplated coatings on corrosion behavior of Ti-1023/30CrMnSiA galvanic couple [J]. Journal of Wuhan University of Technology: Materials Science Edition, 2008, 3(5): 704-707.
[12] LI Song-mei, ZHANG Yuan-yuan, LIU Jian-hua. Corrosion behavior of steel A3 influenced by thiobacillus ferrooxidans [J]. Acta Physico-Chimica Sinica, 2008, 24(9): 1553-1557.
[13] LIU Jian-hua, LIANG Xin, LI Song-mei. Corrosion behaviors of steel A3 exposed to thiobacillus ferrooxidan [J]. Journal of Materials Science and Technology, 2008, 24(5): 766-770.
[14] LIU Jian-hua, SHANG Hai-bo, TAO Bin-wu, LI Song-mei. Corrosion behavior of high strength steels 0Cr18Ni5 and AF1410 [J]. Journal of Materials Engineering, 2004, 8: 29-31. (in Chinese)
[15] LIANG Xin, LIU Jian-hua, LI Song-mei. Preparation of new type Ni-P micro/nano metal material based on bacteria shape [J]. Journal of Materials Science and Technology, 2009, 25(1): 58-62.
[16] YU Mei, LIU Jian-hua, LI Song-mei. Fabrication and characterization of highly ordered Ni0.5Zn0.5Fe2O4 nanowire/tube arrays by sol-gel template method [J]. Journal of University of Science and Technology Beijing, 2007, 14(5): 469-472.
[17] GRUNDMEIER G, SCHMIDT W, STRATMANN M. Corrosion protection by organic coatings: Electrochemical mechanism and novel methods of investigation [J]. Electrochimica Acta, 2000, 45(15/16): 2515-2533.
[18] DEFLORIAN F, FEDRIZZI L, ROSSI S, BONORA P L. Organic coating capacitance measurement by EIS: Ideal and actual trends [J]. Electrochimica Acta, 1999, 44(24): 4243-4249.
[19] AGLAN A, ALLIE A, LUDWICK A, KOONS L. Formulation and evaluation of nano-structured polymeric coatings for corrosion protection [J]. Surface and Coatings Technology, 2007, 202(2): 370-378.
[20] VESELY D, KALENDOVA A. Anticorrosion efficiency of ZnxMgyAl2O4 core–shell spinels in organic coatings [J]. Progress in Organic Coatings, 2008, 62(1): 5-20.
[21] RISSA K, LEPIST? T, YRJ?L? K. Effect of kaolin content on structure and functional properties of water-based coatings [J]. Progress in Organic Coatings, 2006, 55(2): 137-141.
[22] VESELY D, KALENDOVA A, KALENDA P. A study of diatomite and calcined kaoline properties in anticorrosion protective coatings [J]. Progress in Organic Coatings, 2010, 68(3): 173-179.
[23] RASENBERG C J F M, HUISMAN H F. Measurement of the critical pigment volume concentration by means of mercury porosimetry [J]. Progress in Organic Coatings, 1985, 13(3/4): 223-235.
[24] KHORASSANI M, POURMAHDIAN S, AFSHAR-TAROMI F, NOURHANI A. Estimation of critical pigment volume concentration in latex paint systems using gas permeation [J]. Iranian Polymer Journal, 2005, 14 (11): 1000-1007.
[25] KITTEL J, CELATI N, KEDDAM M, TAKENOUTI H. Influence of the coating–substrate interactions on the corrosion protection: Characterisation by impedance spectroscopy of the inner and outer parts of a coating [J]. Progress in Organic Coatings, 2003, 46(2): 135-147.
(Edited by HE Yun-bin)
Foundation item: Project(51001007) supported by the National Natural Science Foundation of China; Project(2011ZE51057) supported by the Aero Science Foundation of China
Received date: 2010-10-01; Accepted date: 2011-01-23
Corresponding author: LIU Jian-hua, Professor; Tel/Fax: +86-10-82317103; E-mail: liujh@buaa.edu.cn
Abstract: A novel kind of waterborne epoxy coating pigmented by nano-sized aluminium powders on high strength steel was formulated. Several coatings with different pigment volume content (PVC) were prepared. The coating morphology was observed using scanning electron microscopy (SEM), and the electrochemical properties were investigated by electrochemical impedance spectroscopy (EIS). Immersion test and neutral salt spray test were also conducted to investigate the corrosion resistance of the coating. It is demonstrated that the critical pigment volume content (CPVC) value is between 30% and 40%. The coating with PVC of 30% exhibits good corrosion resistance in 3.5% (mass fraction) NaCl solution.


