文章编号:1004-0609(2007)10-1632-05
热浸镀铝钢扩散层的AlFe3C0.5相
张 伟1,文九巴2,王晓峰1,熊 飞1,催新安3
(1. 洛阳理工学院 材料系,洛阳 471023;
2. 河南科技大学 材料科学与工程学院,洛阳 471023;
3. 洛阳石化工程公司,洛阳 471003)
摘 要:
采用740 ℃热浸镀铝方法,在20碳钢表面制备热浸镀铝层。镀铝层经850 ℃扩散处理4 h后,采用SEM和XRD确定扩散层的相组成,采用TEM研究组成相的晶体学取向关系。分析AlFe3C0.5相的形成原因。结果表明:扩散层与基体之间存在Fe3Al、FeAl和点阵常数为0.377 nm的针叶状AlFe3C0.5相;针状AlFe3C0.5与Fe3Al相之间存在的晶体学取向关系是![]() 、
、![]() 。
。
关键词:
中图分类号:TG171; TG113 文献标识码:A
AlFe3C0.5 phase in diffusion layers of hot dip aluminized steel
ZHANG Wei1, WEN Jiu-ba2, WANG Xiao-feng1, XIONG Fei1, CUI Xin-an3
(1. Department of Materials Engineering, Luoyang Institute of Science and Technology, Luoyang 471003, China;
2. School of Materials Science and Engineering, Henan University of Science and Technology, Luoyang 471023, China;
3. Luoyang Petrochemical Engineering Corporation, Luoyang 471003, China)
Abstract: The aluminized coating on 20 carbons steel was prepared by hot dip aluminizing method at 740 ℃. After the coating diffusion treatment at 850 ℃ for 4 h, the microstructure of the interface between the diffusion layers and steel substrate was determined by scanning electronic microscopy and X-ray diffractometry, and the lattice orientation relation between constituent phases was analyzed by transmission electron microscopy. The formation mechanisms of Fe3AlC0.5 phase were also discussed. The results indicate that Fe3Al, FeAl and needle-like AlFe3C0.5 phase with lattice constant of 0.377 nm exist between the diffusion layers and steel substrate. In addition,the lattice orientation relation between AlFe3C0.5 and Fe3Al is ![]() ,
, ![]() .
.
Key words: hot dip aluminizing; diffusion layer; AlFe3C0.5 phase; crystallography
在钢材表面形成渗铝层的方法很多,如粉末包埋渗铝和热浸镀渗铝等。这些方法均可显著提高钢材的抗氧化、抗腐蚀和耐磨损等性能,已广泛应用于石油、化工、航空和冶金等领域的表面防护上[1-3]。渗铝钢的优良性能实质上是镀层组织结构的反映。因此,许多学者对渗铝层的微观组织结构进行了大量研究。研究者普遍认为:1) 碳钢粉末包埋渗铝后,其渗铝层随着铝浓度的增加,可依次得到α相、Fe3Al、FeAl、FeAl2、Fe2Al5固溶体或化合物相[3-4];2) 钢材热浸镀铝后,镀层由表面层和合金层组成。表面层由Al和FeAl3相组成,合金层由Fe2Al5和少量FeAl相组成[5-6]。经扩散处理后,扩散层物相组成与粉末包埋渗铝后相同。由基体向表面依次为α相、Fe3Al、FeAl、FeAl2和Fe2Al5等固溶体或化合物相[10-13]。针状Fe3Al相大部分沿α相的晶界呈半网络状分布,少量出现在α相晶内。Kobayashi和Yakou[14]在碳钢表面热浸镀铝,然后在 1 323 K高温下扩散1 h后,获得表层为Fe2Al5,第二层为FeAl,第三层为Fe3Al的化合物镀层。即无论是粉末包埋渗铝还是扩散处理后的热浸镀铝钢,其扩散层与基体之间的针状相均为Fe3Al相,而未发现其它严重影响渗铝层(扩散层)与基体界面结合强度的含碳铁-铝化合物相。本文作者通过SEM、TEM和XRD等研究了经扩散处理后热浸镀铝钢扩散层与基体之间的物相组成,发现扩散层与基体之间的针状相为AlFe3C0.5相,而并非Fe3Al相。由于AlFe3C0.5相的脆性比Fe3Al大,故此研究对揭示高温下热浸镀铝钢扩散层的剥落机理有重要意义。
迄今为止,虽然已有Al-Fe-C三元相图的研究成果报道[15],但对于C含量低于0.3%(质量分数)的Al-Fe-C三元合金的相变分析尚无参考依据。虽然对AlFe3C0.5在电渣熔炼获得的Al-Fe-C三元合金的研究证实其存在的真实性[16],但形成机理并不清楚。因此,本文作者还对热浸镀铝钢扩散层中AlFe3C0.5相的形成原因进行分析。
1 实验
基体材料为20碳钢,试样尺寸为30 mm×20 mm×3 mm。热浸镀铝液是含镧量为0.5%的稀土铝合金。
试样经除油、除锈、助镀处理后,在740 ℃的稀土铝合金熔液中浸镀3 min,出炉空冷,然后在850 ℃的空气炉中扩散处理4 h。试样随炉升温,随炉冷却。
用JSM-5610LV型扫描电镜对扩散层形貌进行观察。用X,Pert MPD Pro型X射线衍射仪确定扩散层与基体之间的相组成。用CM200型透射电子显微镜对试样的微结构和电子衍射花样进行分析,工作电压为160 kV。
TEM试样的制作方法如下:先沿垂直于扩散层(扩散后试样)方向切割出0.5 mm厚带有渗层的薄片,再将两块试样沿扩散层对粘在一起,用砂纸研磨至50~60 μm厚。然后用冲子冲下直径3 mm的圆片,进行离子减薄,最终得到透射电镜薄片样品。
2 结果和分析
2.1 扩散层横截面的组织形貌
热浸镀稀土铝试样经扩散处理后扩散层横截面的组织形貌如图1所示。由图可知,经扩散处理后,扩散层分3层。最外层颜色较黑,其上分布大量空洞。最外层下面是由黑逐渐变灰的次外层。次外层和基体之间是颜色较浅的过渡层。各层之间的界面毗连不平齐,呈波浪状。图2所示为扩散层的次外层与过渡层的组织形貌。可以看出,次外层与过渡层界面之间有一灰色过渡带,其上分布的针叶状相(通常认为的Fe3Al相)向基体方向伸展。过渡层由柱状组织和块状组织组成。
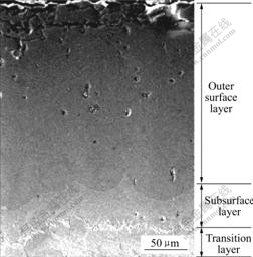
图1 20钢热浸镀稀土铝试样经850 ℃扩散处理4 h后扩散层横截面的组织形貌
Fig.1 SEM micrograph of diffusion layers of steel 20 aluminized at 850 ℃ for 4 h
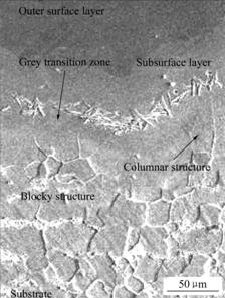
图2 扩散层的次外层与过渡层的组织形貌
Fig.2 Micrograph of subsurface layer of hot dip aluminizing steel after diffusion treatment
2.2 扩散层与基体之间的相组成
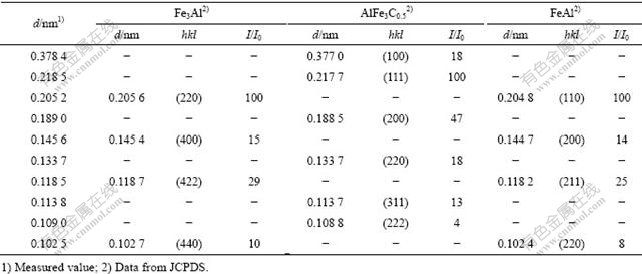
为分析扩散层与基体之间的相组成,将试样表面磨去250 μm后进行X射线衍射分析,其衍射谱见图3。将衍射结果与粉末衍射标准联合委员会(JCPDS)公布的标准粉末衍射卡进行对比(见表1)可知,扩散层的次外层与过渡层之间由Fe3Al、FeAl和AlFe3C0.5相组成。
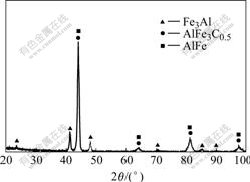
图3 扩散层表面磨掉250 μm后的X射线衍射谱
Fig.3 XRD pattern of surface of specimen after wearing down 250 μm
表1 试样扩散层表面磨掉250 μm后的X射线衍射结果
Table 1 Analysis results of X-ray diffraction on surface of specimen after wearing down 200 μm
2.3 AlFe3C0.5和Fe3Al相之间的晶体学取向关系
图4(a)所示为距扩散层外表面约230~280 μm处某一视场的透射电镜明场像。白色针状相与黑色相相互交叉。对图4(a)中区域1和2进行选区电子衍射(见图4(b))和指数标定,可确定图4(a)中区域1和2分别为Fe3Al相和AlFe3C0.5相。对照图2可知,其中的针状相实际上是AlFe3C0.5相,并非通常认为的Fe3Al相,而图2中次外层与过渡层之间的灰色过渡带应属Fe3Al相,这与文献[10-14]得出的结论完全不同。进一步分析表明, AlFe3C0.5和Fe3Al相之间存在如下的晶体学取向关系:
![]()
![]()
AlFe3C0.5相属立方晶系,a=0.377 00 nm,其硬度比Fe3Al相高,因此薛群基等[17]已采用加温燃烧合成熔化法制备出力学性能和抗磨损性能优良的AlFe3C0.5增强Fe3Al基复合材料[17]。但就热浸镀铅钢而言,脆性大的AlFe3C0.5的存在对其过渡区与基体的结合产生不利影响。
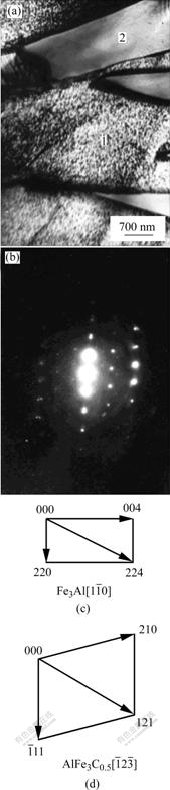
图4 距扩散层外表面约230~280 μm处某一视场的透射电镜明场像和电子衍射花样
Fig.4 TEM micrograph and SAD pattern of diffusion layers in 230-280 μm from surface: (a) TEM micrograph; (b) SAD patterns of ![]() zone of Fe3Al and
zone of Fe3Al and ![]() zone of AlFe3C0.5; (c), (d) Schematic index diagrams in Fig.4(b)
zone of AlFe3C0.5; (c), (d) Schematic index diagrams in Fig.4(b)
3 讨论
钢材热浸镀铝后,其镀铝层由表面层和合金层组成[5-6]。在高温扩散过程中,镀铝层的铝向基体方向扩散,基体中的铁向镀铝层扩散,从而在扩散层/基体界面形成铝含量不同的金属间化合物相。根据铁铝平衡状态图,紧靠基体的相应是Fe3Al相。但马臣[18]的研究表明,铝会降低碳在固态铁中的溶解度。因此,随着铝向基体方向的扩散,固溶在铁素体和Fe3C中的碳将析出,而碳原子不能穿过合金层,使碳原子浓度在合金层前沿逐渐升高。若铝原子在此方向继续扩散就必须将碳原子向更深层推移,在扩散层前沿形成碳原子的富集区。由于铁与铝具有较大的结合能,铁铝化合物的结构难以因碳原子的富集而破坏,而碳原子的半径又小,只能形成C原子择优占据Fe原子八面体间隙的间隙化合物AlFe3C0.5相。AlFe3C0.5相在Fe3Al化合物相中达到饱和时,就会析出与基体相晶体衍射结构平行的晶体相。
4 结论
1) 热浸镀铝钢经850 ℃扩散处理4 h后,其扩散层与基体之间存在FeAl、Fe3Al和点阵常数为0.377 nm的针叶状AlFe3C0.5相。
2) 针叶状AlFe3C0.5相与Fe3Al相之间存在的晶体学取向关系是:![]() ;
;![]() 。
。
REFERENCES
[1] Mahieu J, Maki J. Development of aluminized multi-phase steel with dual phase properties for high temperature corrosion resistance applications[J]. Steel Res Int, 2003, 74(4): 225-236.
[2] 郑毅然, 高文禄. 热浸镀铝钢材的应用与发展[J]. 腐蚀科学与防护技术, 1999, 11(3): 179-184.
ZHENG Yi-ran, GAO Wen-lu. Application and development of hot-dip aluminized steel[J]. Corrosion Science and Protection Technology, 1999, 11(3): 179-184.
[3] Soliman H M, Mohamed K E, Abd EI-Azim M E. Hammad F H. Oxidation resistance of the aluminide coating formed on carbon steels[J]. J Mater Sci Technol, 1997, 13: 383-388.
[4] 武汉材料保护研究所. 钢铁化学热处理金相图谱[M]. 北京: 机械工业出版社, 1980: 70-79.
Wuhan Institute of Materials Protection. metallographic patterns of chemical heat treatment[M]. Beijing: China Machine Press, 1980: 70-79.
[5] Nicholls I E. Hot-dipped aluminum coatings [J]. Corrosion Technology, 1964(10): 16-21.
[6] 夏 原, 姚 枚, 李铁藩. 热浸铝镀层形成过程的微观结构及形成机理[J]. 中国有色金属学报, 1997, 7(4): 154-158.
XIA Yuan, YAO Mei, LI Tie-fan. Coating formation process and microstructure during hot dip aluminizing (HDA) on steel[J]. The Chinese Journal of Nonferrous Metals, 1997, 7(4): 154-158.
[7] Shahverdi H R, Ghomashchi M R, Shabestan S, Hejazi J. Microstructures analysis of interfacial reaction between molten aluminum and solid iron [J]. Journal of Materials Processing Technology, 2002, 124: 345-352.
[8] Bouayad A, Gerometta Ch, Belkebir A, Ambari A. Kinetic interactions between solid iron and molten aluminum[J]. Mater Sci Eng A, 2003, 363: 53-56.
[9] Bouche K, Barbier F, Coulet A. Intermetallic compound layer growth between solid iron and molten aluminum[J]. Mater Sci Eng A, 1998, 249: 167-175.
[10] XIA Yuan, YU Sheng-xue, YAO Mei. Effect of diffusion on coating microstructure and oxidation resistance of aluminizing steel[J]. Trans Nonferrous Met Soc China, 2001, 11(6): 817-821.
[11] 魏无际, 丁 毅, 石焕荣. Q235钢热浸渗铝层的组织分析和性能[J]. 南京化工大学学报, 2000, 22(6): 10-14.
WEI Wu-ji, DING Yi, SHI Huan-rong. Investigation in microstructure and properties of hot-dip aluminizing layer of Q235steel [J]. Journal of Nanjing University of Chemical Technology, 2000, 22(6): 10-14.
[12] LI Ya-jiang, WANG Juan, ZHANG Yong-lan. Fine structures in Fe3Al alloy layer of a new hot dip aluminized steel[J]. Bulletin of Materials Science, 2002, 25(7): 635-639.
[13] LI Ya-jiang, WANG Juan, MA Hai-jun. Phase structure of Fe-Al alloy layer on the surface of a new aluminized steel[J]. Materials Research Innovations, 2005, 9(3): 74-76.
[14] Kobayashi S, Yakou T. Control of intermetallic compound layers at interface between steel and aluminum by diffusion- treatment[J]. Mater Sci Eng A, 2002, 338(1/2): 44-53.
[15] Villars P, Prince A, Okamoto H. Handbook of ternary alloy phase diagrams[M]. Materials Park, Ohio: ASM International, 1995: 89-92.
[16] Shankar R V, Baligidad R G, Raja V S. Effect of Al content on oxidation behavior of ternary Fe-Al-C alloys[J]. Intermetallics, 2002, 10(1): 73-84.
[17] 薛群基, 喇培清. 燃烧合成熔化制备块体纳米结构材料和金属间化合物基复合材料及其摩擦学性能[J]. 中国有色金属学报, 2004, 14(5): 128-138.
XUE Qun-ji, LA Pei-qin. Combustion synthesized bulk nanocrystalline materials and intermetallic matrix composites and their tribological properties[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(5): 128-138.
[18] 马 臣. 稀土对喷焊层耐腐蚀性的影响机制[J]. 中国稀土学报, 1994, (6): 23-26.
MA Chen. Influence of rare earth on corrosion resistance of spray welding coating[J]. Journal of Chinese Rare Earth Society, 1994, (6): 23-26.
基金项目:河南省重点攻关资助项目(0423023500);河南省自然科学基金资助项目(0511021600);洛阳市科技计划资助项目(040221)
收稿日期:2007-02-04;修订日期:2007-06-25
通讯作者:张 伟,高级工程师,博士;电话:0379-65928195;E-mail: weizhang57@163.com
摘 要:采用740 ℃热浸镀铝方法,在20碳钢表面制备热浸镀铝层。镀铝层经850 ℃扩散处理4 h后,采用SEM和XRD确定扩散层的相组成,采用TEM研究组成相的晶体学取向关系。分析AlFe3C0.5相的形成原因。结果表明:扩散层与基体之间存在Fe3Al、FeAl和点阵常数为0.377 nm的针叶状AlFe3C0.5相;针状AlFe3C0.5与Fe3Al相之间存在的晶体学取向关系是![]() 、
、![]() 。
。


