J. Cent. South Univ. (2017) 24: 2799-2804
DOI: https://doi.org/10.1007/s11771-017-3694-3

Copper smelter slag treatment by ammonia solution:Leaching process optimization
R. Nadirov1, L. Syzdykova2, A. Zhussupova2
1. Department of General and Inorganic Chemistry, al-Farabi Kazakh National University, Almaty, 050040, Kazakhstan;
2. Department of Physical Chemistry, Catalysis and Petrochemistry, al-Farabi Kazakh National University,Almaty, 050040, Kazakhstan
 Central South University Press and Springer-Verlag GmbH Germany,part of Springer Nature 2017
Central South University Press and Springer-Verlag GmbH Germany,part of Springer Nature 2017
Abstract:
The feasibility of copper smelter slag processing by ammonia solution treatment was investigated. The central composite rotatable design (CCRD) and approximation method were used to determine the optimum conditions of zinc and copper recovery to a solution. The experimental design was done at five levels of the four operating parameters which were the initial concentration of NH3, the initial Cl– ions concentration, leaching time and solid/liquid ratio. Two mathematical models describing dependence of metal recovery on the operating parameters were obtained. The models are successful in predicting the responses. It was found that optimal parameters for zinc and copper recovery are as follows (values for copper are given in brackets): initial CNH3 17.1% (19.9%), initial CCl– 160 g/L (160 g/L), leaching process duration 4.56 h (4.13 h), solid/liquid ratio 0.39 (0.53). The maximum Zn and Cu recoveries to solution, obtained experimentally under the conditions, are 81.16% and 56.48%, respectively.
Key words:
copper slag; ammonia solution; metal recovery; central composite design; optimization;
1 Introduction
Copper smelter slag is a by-product of the pyrometallurgical stages in the copper recovery process from sulfide materials, and mainly consists of oxides of metals and silicone, as well as considerable amounts of metallic values besides copper [1]. Pyrometallurgical, hydrometallurgical and bio-hydrometallurgical methods are the ways that can be used for non-ferrous metals recovery from copper smelter slag [2]. Today attention is focusing on hydrometallurgical methods for raw materials treatment in non-ferrous metallurgy because of relatively low environmental and operational concerns. Numerous publications have studied the leaching of base metals from metallurgical slag [3–10].
Recently, it was found that ammonia solutions containing ammonium chloride are effective for the extraction of zinc and copper from technogenic raw materials, containing zinc and copper oxides [11, 12]. Physico-chemical basis and technological principles of zinc and copper recovery by ammonia–ammonium extraction are based on the properties of the system H2O–NH3–NH4Cl containing zinc and copper amines, and the phase equilibria liquid–solid, liquid – vapor. Zinc and copper dissolution in aqueous solution of NH4OH in the absence (1) and presence (2) of Cl– ions can be described by the following schemes:
MО+4NH3(sol)+nH2O→[M(NH3)4](OH)2+(n–1)H2O (1)
МО+хNH3(sol)+2NH4Cl(sol)→[M(NH3)n]2+2Cl–+H2O(2)
where М=Cu, Zn; n=x+2.
Previously we have reported the investigation of zinc and copper recovery process from copper smelter slag of Balkhash copper plant (Kazakhstan) by ammonium chloride treatment [13]. In the present study, to avoid the using of high-temperature processes we focused on investigating the copper and zinc recovery process by ammonia solution treatment of copper smelter slag. The work is undertaken to investigate the effectiveness of four process variables, namely; an initial concentration of NH3, an initial concentration of Cl– ions, treatment process duration and solid/liquid ratio on the copper and zinc recovery to a solution from copper smelter slag.
Experimental design is widely used for regulation of the effects of parameters in many processes. The experiments in the present work are planned using central composite rotatable design to investigate the main effects of each operating factor and the interactions of these variables and to find out the factors combination that gives the maximum values of copper and zinc recovery, separately.
2 Materials and methods
2.1 Materials
Mineralogical analysis of slag sample, performed using DRON-3M model diffractometer, is presented in Table 1.
Table 1 Mineralogical analysis of slag sample (mass fraction, %)

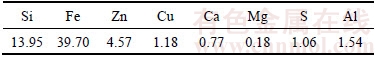
Chemical analysis of slag sample, performed using optical emission spectroscopy with inductively coupled plasma (OPTIMA 8000, Perkin Elmer), is presented in Table 2.
Table 2 Chemical analysis of slag sample (mass fraction, %)
2.2 Slag leaching
2.2.1 Experimental procedures
The slag sample produced by Balkhash copper plant (Kazakhstan) was used for the experiments. A sample of slag (10 g; –200 mesh) was placed into a glass-stopper 150-mL flask. An ammonia solution with desired concentration of NH4OH and Cl– ions was prepared by mixing distilled water and NH4Cl to the concentrated ammonia solution. The contents of the flask were well stirred using horizontal shaker at an agitation speed 200 r/min for a certain time and then were filtered. The concentration of metal ions in solution was determined by atomic adsorption spectrometer (Shimadzu AA-6200). Solid residues were analyzed by X-ray fluorescence (Spectroscan). The amount of metal recovered was estimated using Eq. (3).
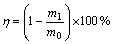 (3)
(3)
where η is the recovery; m0 and m1 correspond to metal contents of sample before and after being leaching, respectively.
2.2.2 Experimental design
Central composite rotatable design (CCRD) helps to optimize the process, affected by a number of operating parameters with the minimum numbers of experiments as well as to determine the relationship between response, namely, copper and zinc recovery to a solution, and operating factors. The three steps were used in experimental design: statistical design experiments, estimation of coefficient through a mathematical model and analysis of the model workability (Montgomery, 2001).In the present study, four operating parameters were selected as independent variables: initial concentration of NH3 (x1), initial Cl– ions concentration (x2), leaching duration (x3) and solid/liquid ratio (x4). The levels and ranges of operating parameters are shown in Table 3. The dependent output response variables were copper and zinc recovery (%).
Table 3 Independent variables and their levels used for central composite rotatable design

The matrix of CCRD is given in Table 3. According to this table, the design is composed of 24 factorial design (runs 1–16), 8 star-points (runs 17–24) and 12 replicates (runs 25–36).
The least square method was used to estimate the correlation of the independent variables and the response as a second-order polynomial equation:
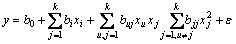 (4)
(4)
where b0 is the value for the fixed response at the central point of the experiment; bi, bj and bij are the linear, quadratic and cross-product coefficients, respectively; and ε is the residual error, estimated by the difference between the predicted and the observed value of response (y).
The dimensionless xj variables are related to the standardized forms as shown below [14]:
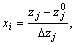 j=1, 2, 3, …, k,
j=1, 2, 3, …, k, 

where zjmax and zjmin represent the maximum and the minimum levels of factor j in natural unit. The coefficients of the fitted equation were obtained from data of Eq. (4) as follows:
B=[XTX]–1[X]TY (5)
where B is the column matrix of estimated coefficients; [XTX]–1 is the dispersion matrix; [X]T is the transpose matrix [X] and Y is the column matrix of observed values. Three known tests were used to evaluate the adequacy of the mathematical model, including Student’s t-test, R-square test and Fisher test [15]. Excel software was used for model coefficients estimation (Eq. (4)).
3 Results and discussion
3.1 Leaching process modeling
The model coefficients for copper and zinc recovery, separately, obtained from mathematical processing of data presented in Table 4, were tested for significance (t-test), at 5% of significance level and 11 degrees of freedom.
Table 4 Experimental design and results for zinc and copper recovery to solution
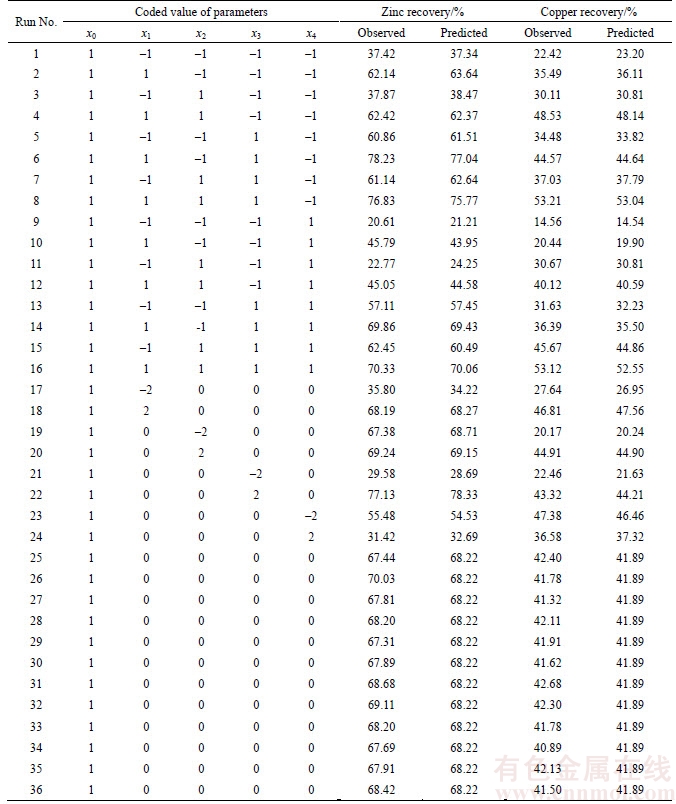
Test results showed that all individual effects for copper and zinc recovery are significant at 5% of significance level. Only the interaction (x4×x4) for copper recovery and the interactions (x2×x2), (x2×x3) for zinc recovery are not significant. Therefore, mentioned interactions were excluded from the appropriate final equations.
The second-order response function representing copper and zinc recovery to solution obtained after realizing 36 experiments and discarding the insignificant effects can be shown in coded variables as follows:
Cu recovery=41.895+5.152x1+6.165x2+5.645x3–
2.285x4+1.106x1x2–0.521x1x3–1.889x1x4–
0.910x2x3+2.165x2x4+1.768x3x4–1.159x12–
2.331x22–2.243x32 (6)
Zn recovery=68.271+8.967x1+0.44x2+12.410x3–5.461x4–
0.601x1x2–2.690x1x3–0.890x1x4+0.476x2x4+
3.020x3x4–4.030x12–3.690x32–6.166x42 (7)
The Fisher’s variance ratio test, i.e., F-test, was used for testing of equation reliability; the calculated values of F-test are presented in Table 5. The upper degree of freedom for models of copper and zinc recovery are 22 and 23, respectively. The lower degree of freedom for both models is 11. The tabulated F values for 5% of significance in the case of copper and zinc recoveries are 2.640 and 2.625, respectively. Comparison of calculated and tabulated F values for both models, separately, enables to assume the statistical significance of both models.
Table 5 Fisher test for model of copper and zinc recovery

The R2 values for Eqs. (6) and (7) were found to be 99.6%, indicating the good agreement between the experimental and the predicted values of copper and zinc recovery.
The observed values of zinc and copper recovery clearly indicate that Eqs.(6) and (7) adequately describe the leaching process.
3.2 Impact of experimental factors on zinc and copper recovery into solution
As is known, the value and signs of the regression equations coefficients provide insights the impact of the factors and their interactions on the value of the output parameter. The coefficients of regression obtained above (Eqs. (6), (7)), show that initial concentration of NH3, initial Cl– ions concentration, leaching time and solid-to- liquid ratio all have an individual effect on the zinc and copper recovery from copper slag to a solution during the ammonia–ammonium leaching. It was of interest to compare the effect of the factors significance for the zinc and copper recovery to a solution, separately. Relatively low value of the coefficient x2 in Eq. (7) indicates that Cl– ions concentration does not significantly impact zinc recovery to a solution, in comparison with copper recovery. This fact indicates the prevalence of Eq. (1) above Eq. (2) during the zinc recovery to a solution. In turn, reaction (2) requires significant recovery of copper to solution during leaching. Overall, the values of regression coefficients, excluding the factor x2, indicate that the process of zinc recovery is more sensitive to the leaching parameters than to the ones for copper recovery.
To demonstrate the effect of pairwise interactions having the most impact on metal recovery, the three-dimensional response surfaces for Zn and Cu recovery are presented in Figs. 1–4.
Each figure shows the dependence of metal recovery on two variables; while, the other two variables have been fixed at the zero level.
The value of x1x3 has a significant negative impact on the zinc recovery into solution. Opposite, the value of x3x4 has a significant impact on the process considered. The most positive impact on copper recovery has an x2x4 interaction with 2.165 of value. In its turn, the most negative impact on the mentioned metal recovery has an x1x4 interaction with –1.889 of value.

Fig. 1 Response surface for Zn-recovery at constant values of initial concentration of Cl– ions and solid-to-liquid ratio

Fig. 2 Response surface for Zn-recovery at constant values of initial concentrations of both NH3 and Cl– ions
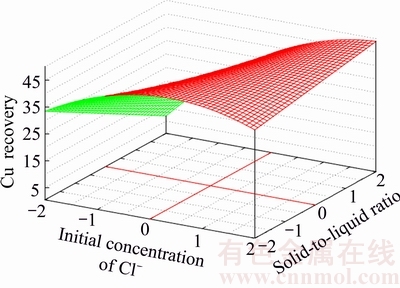
Fig. 3 Response surface for Cu-recovery at constant values of initial concentration of NH3 and leaching duration
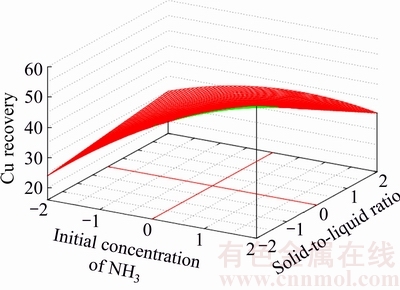
Fig. 4 Response surface for Cu-recovery at constant values of initial concentration of Cl– ions and leaching duration
3.3 Obtaining optimal parameters of leaching process
Approximation method was used to leaching process optimization [16]. The Excel software was applied at calculation process. The corresponding conditions of the best metal recovery are follows:
For zinc recovery,
x1=0.42, corresponding to initial CNH3=17.1%;
x2=2.00, corresponding to initial CCl–=160 g/L;
x3=1.59, corresponding to leaching time=4.56 h;
x4=–0.01, corresponding to solid-to-liquid ratio=0.39.
For copper recovery,
x1=1.98, corresponding to initial CNH3=19.9%;
x2=2.00, corresponding to initial CCl–=160 g/L;
x3=1.13, corresponding to leaching time=4.13 h;
x4=1.29, corresponding to solid-to-liquid =0.53.
To test the validity of the conditions found as optimal from the model, a leaching experiment was carried out.
At these optimal parameters, 80.31% of total zinc recovery and 55.62% of total copper recovery were predicted using Eqs. (6) and (7). Actually, 81.16% of zinc recovery and 56.48% of copper recovery were observed during the experiments. The results indicate that the model is successful in predicting the responses.
4 Conclusions
The present work was aimed at finding possibilities of zinc and copper concentrates obtained via copper slag treatment by ammonia solution. The statistical method called central composite rotatable design (CCRD) and approximation method were used to determine the optimum conditions of zinc and copper recovery to a solution. The experimental design was done at five levels of the operating parameters, namely the initial concentration of NH3, the initial Cl– ions concentration, leaching time and solid/liquid ratio.
The regression equations describing the effect of factors on the zinc and copper recovery to a solution were obtained. The validity of regression equation has been controlled by statistical approaches.
It was found that optimum parameters for zinc recovery are follows: initial CNH3 17.1%, initial CCl–160 g/L, leaching time 4.56 h, solid-to-liquid ratio 0.39. For copper recovery, the optimum parameters are follows: initial CNH3 19.9%, initial CCl– 160 g/L, leaching time 4.13 h, solid-to-liquid 0.53. The maximum zinc and copper recoveries to a solution obtained experimentally at the conditions were 81.16% and 56.48%, respectively.
References
[1] Davenport W G, King M, Shlesinger M, Biswas A K. Extractive metallurgy of copper [M]. Oxford: Elsevier Science Ltd., 2002.
[2] Jadhav U U, Hocheng H. A review of recovery of metals from industrial waste [J]. Journal of Achievements in Materials and Manufacturing Engineering, 2012, 54(2): 159–167.
[3] Sukla L B, Panda S C, Jena P K. Recovery of cobalt, nickel and copper from converter slag through roasting with ammonium sulphate and sulphuric acid [J]. Hydrometallurgy, 1986, 16(2): 153–165.
[4] Herreros O, Quiroz R, Manzano E, Bou C, Vinals J. Copper extraction from reverberatory and flash furnace slags by chlorine leaching [J]. Hydrometallurgy, 2012, 49(1, 2): 87–101.
[5] Banza A N, Gock E, Kongolo K. Base metals recovery from copper smelter slag by oxidizing leaching and solvent extraction [J]. Hydrometallurgy, 2002, 67(1): 63–69.
[6] Altundogan H S, Tumen F. Metal recovery from copper converter slag by roasting with ferric sulphate [J]. Hydrometallurgy, 1997, 44(1): 261–267.
[7] Arslan C, Arslan F. Recovery of copper, cobalt and zinc from copper smelter and converter slags [J]. Hydrometallurgy, 2002, 67(1): 1–7.
[8] Altundogan H S, Boyrazli M, Tumen F. A study on the sulphuric acid leaching of copper converter slag in the presence of dichromate [J]. Minerals Engineering, 2004, 17(3): 465–467.
[9] Carranza F, Iglesias N, Mazuelos A, Romero R, Forcat O. Ferric leaching of copper slag flotation tailings [J]. Minerals Engineering, 2009, 22(1): 107–110.
[10] ZHANG Y, MAN R L, NI W D, WANG H. Selective leaching of base metals from copper smelter slag [J]. Hydrometallurgy, 2010, 103(1): 25–29.
[11] Peretrutov A A, Chubenko M N, Kim P P. Physicochemical properties of eutonic aqueous solutions of zinc and copper tetraammoniates in the range 293–323 K [J]. Russian Journal of Physical Chemistry A, 2009, 83 (10): 1813–1815.
[12] Peretrutov A A, Chubenko M N, Kim P P, Yakunin Yu I. Combined solubility of copper and zinc oxides in ammonia- ammonium solutions [J]. Russian Journal of Physical Chemistry A, 2009, 83 (8): 1422–1425.
[13] Nadirov R K, Syzdykova L I, Zhussupova A K, Usserbaev M T. Recovery of value metals from copper smelter slag by ammonium chloride treatment [J]. International Journal of Mineral Processing, 2013, 124: 145–149.
[14] Box G E P, Hunter W G, Hunter J S. Statistics for experiments [M]. New York: Wiley Interscience, 1978.
[15] Montgomery D C. Design and analysis of experiments [M]. New York: John Wiley & Sons, 2001.
[16] Biegler L T, Grossmann I E, Westerberg A W. A note on approximation techniques used for process optimization [M]. Pittsburgh: Carnegie Institute of Technology, 1984.
(Edited by YANG Hua)
Cite this article as:
R. Nadirov, L. Syzdykova, A. Zhussupova. Copper smelter slag treatment by ammonia solution: Leaching process optimization [J]. Journal of Central South University, 2017, 24(12): 2799–2804.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-017-3694-3Received date: 2016-07-01; Accepted date: 2017-03-13
Corresponding author: R. Nadirov, PhD; Tel: +7–747–4520525; E-mail: nadirov.rashid@gmail.com
Abstract: The feasibility of copper smelter slag processing by ammonia solution treatment was investigated. The central composite rotatable design (CCRD) and approximation method were used to determine the optimum conditions of zinc and copper recovery to a solution. The experimental design was done at five levels of the four operating parameters which were the initial concentration of NH3, the initial Cl– ions concentration, leaching time and solid/liquid ratio. Two mathematical models describing dependence of metal recovery on the operating parameters were obtained. The models are successful in predicting the responses. It was found that optimal parameters for zinc and copper recovery are as follows (values for copper are given in brackets): initial CNH3 17.1% (19.9%), initial CCl– 160 g/L (160 g/L), leaching process duration 4.56 h (4.13 h), solid/liquid ratio 0.39 (0.53). The maximum Zn and Cu recoveries to solution, obtained experimentally under the conditions, are 81.16% and 56.48%, respectively.


