
Interposition fixing structure of TiO2 film deposited on activated carbon fibers
FU Ping-feng(傅平丰)1, LUAN Yong(栾 勇)2, DAI Xue-gang(戴学刚)2
1. Research Institute of Indoor Environment, Beijing Union University, Beijing 100083, China;
2. Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100080, China
Received 16 February 2006; accepted 29 May 2006
Abstract:
The immobilized photocatalyst, TiO2 film supported on activated carbon fibers (TiO2/ACFs) prepared with molecular adsorption-deposition (MAD), exhibits high stability in cyclic photodegradation runs. The interposition fixing structure between TiO2 film and carbon fiber was investigated by means of SEM-EDX, XRD, XPS and FTIR, and a model was proposed to explain this structure. With SEM examination of carbon fiber surface after removing the deposited TiO2 film, a residual TiO2 super-thin film was found to exist still. By determining surface groups on ACFs, titanium sulfate (Ti2(SO4)3) in burnt remainders of the TiO2/ACFs was thought to be formed with an interfacial reaction between TiO2 film and carbon fibers. These provide some evidence of firm attachment of TiO2 film to carbon fiber surface. In the consideration of characteristics of the MAD, the deposition mechanism of TiO2 film on ACFs was proposed, and the interposition fixing structure was inferred to intercrossedly form between TiO2 film and ACFs’ surface. This structure leaded to firm attachment and high stability of the TiO2 film.
Key words:
TiO2 film; activated carbon fibers; interposition fixing structure; stability;
1 Introduction
Immobilization of TiO2 film on a substrate is necessary for its applications as TiO2 suspensions have fatal limitations in difficult separation and filtration for its reuse[1]. Thus, the fixing stability of TiO2 film with its substrate is considered one of the most significant factors in its practical applications. Stable and lasting fixation of TiO2 film on its substrate will benefit photocatalytic degradation processes both technically and economically[2,3]. Therefore, it is important to study and evaluate the fixing stability of deposited TiO2 film on the substrate, which is concerned with optimizing preparation procedures of the immobilized photocatalysts and selection of a suitable substrate.
In our previous work[4], a molecular adsorption- deposition(MAD) process, using TiCl4 vapor and activated carbon fibers(ACFs) as raw material and the substrate respectively, was employed for the preparation of an ACFs supported TiO2 photocatalyst (TiO2/ACFs). The TiO2 film was deposited on the carbon fibers with a film thickness of about 100 nm. The resulted TiO2/ACFs showed high photocatalytic reactivity in the photo- catalytic degradation of methylene blue(MB) solution. The present investigation aims at examining the stability of deposited TiO2 film, and proposing a fixing mecha- nism of depositing TiO2 film on carbon fiber surfaces.
2 Experimental
The immobilized photocatalyst (TiO2/ACFs) prepared by the MAD process was described in our previous paper[4]. The stability examination of the deposited TiO2 film was carried out with cyclic photodegradaion of mechanically stirred MB solution. The reduction of MB removal rate and loss of loaded TiO2 with cyclic runs were used to characterize the stability of the deposited TiO2 film. A single photo- degradation run and measurement of TiO2 loading amount were adopted as ones in the paper[4]. The samples for the instrumental analysis included the TiO2/ACFs photocatalyst, burnt remainders of TiO2/ACFs and the naked ACFs. Here, the burnt remainders were residual powders of the TiO2/ACFs calcined in air stream. The ACFs were the viscose rayon-based ones. The morphologies of the naked ACFs and the immobilized photocatalyst were taken by using aCambridge S250 MK2 scanning electron microscope (SEM). Its attached energy dispersive X-ray spectro- scopy system with a detecting range from atomic number larger than 11(Na) (Link 860 EDS detector) was used for EDX analysis. X-ray diffraction (XRD) patterns of the photocatalyst TiO2/ACFs and the burnt remainders were obtained by using an automatic X-ray diffractometer with Cu Kα radiation (Rigaku D/Max 2200 PC, operated at 40 kV and 40 mA). XPS analysis of the naked ACFs was carried out by using an ESCALAB MarkⅡspectrophotometer. The carbon C1s electron binding energy corresponding to graphitic carbon was referenced at 284.60 eV for the calibration purpose. The FTIR spectrum of the ACFs was obtained with a Vector22 spectrophotometer using KBr wafer by adding 50 scans at a resolution of 1 cm-1.
3 Results and discussion
3.1 Stability examination of TiO2 film
As shown in Fig.1, the MB removal rate is slightly reduced with the increased cyclic photocatalytic degradation runs. However, after 15 times’ cyclic runs, the MB removal rate is still higher than 92% with the loaded TiO2 loss of less than 7%. It indicates that the deposited TiO2 film has firmly attached to the carbon fiber surface, and can not be easily exfoliated from the carbon fiber with mechanically stirred solutions for a long period.

Fig.1 Relationship of MB removal rate to cyclic photocatalytic degradation runs
3.2 Residual TiO2 super-thin film at interface of TiO2 film and carbon fiber
Fig.2 shows SEM images of a naked activated carbon fiber and the deposited TiO2 film. The single carbon fiber has a diameter of about 13 μm with the surface full of long shallow grooves. From the cross-sectional view of the TiO2 film shown in Fig.2(b), the thickness of TiO2 film can be estimated to about 100 nm, and tight attachment of the deposited TiO2 film to carbon fiber surface could be found. By comparing the surface morphology of the naked carbon fiber (Fig.2(a)) and the one after removing the deposited TiO2 film

Fig.2 SEM images of deposited TiO2 film: (a) Naked activated carbon fiber; (b) Cross-sectional view of deposited TiO2 film; (c) Carbon fiber surface after removing TiO2 film
(Fig.2(c)), a residual TiO2 super-thin film, originally existing at the interface of TiO2 film and carbon fiber surface, can be clearly observed to still cover the carbon fiber surface.
EDX analysis was performed by scanning a small area (4 μm×4 μm) on three surfaces, namely, ACFs’ surface, TiO2 film surface and the carbon fiber surface after removing the deposited TiO2 film. The analytical results are listed in Table 1. By comparing element compositions of these three surfaces, Ti is found as the dominant element on the TiO2 film, and the carbon fiber surface after removing the TiO2 film still contains a certain amount of Ti. The presence of residual titanium from EDX analysis is consistent with the direct SEM observation in Fig.2(c).
Table 1 Chemical compositions of three surfaces (mole fraction, %)
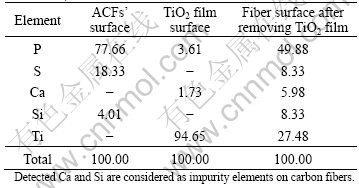
3.3 Interfacial reaction of Ti2(SO4)3 formation between TiO2 film and carbon fiber at air atmo- sphere
As shown in Fig.3, the 2θ peaks appearing at 24.34?, 32.42?, 29.36?, 21.0?, 20.24? and 14.54? in the burnt remainders of the TiO2/ACFs are attributed to the reflec-

Fig.3 XRD patterns of immobilized photocatalysts calcined in Ar gas atmosphere at 700 ℃ (a) and 900 ℃ (b), and the burnt remainders of TiO2/ACFs calcined in air stream at 700 ℃ (c) and 900 ℃ (d)
tions from (113), (116), (024), (110), (104) and (012) planes of titanium sulfate (Ti2(SO4)3), respectively [5]. For Ti2(SO4)3 is only formed in the calcination treatment in air atmosphere not in Ar gas one, it is obvious that the deposited TiO2 film has reacted with element S on the carbon fiber to form Ti2(SO4)3 in the presence of oxygen.
In order to elucidate the mechanism of Ti2(SO4)3 formation, XPS and FTIR analyses of the naked ACFs were employed to find out the chemical state and possible surface groups of sulfur, and the results are shown in Figs.4 and 5, respectively. With a binding energy of S 2p at 168.23 eV, the oxidation number of S would possibly be +4, and the chemical state of sulfur could be assigned to S═O bond of sulfino or sulfinyl group[6]. FTIR examination of surface groups on carbon fibers shows that the band at 1 090 cm-1 could be assigned to O═S═O asymmetric stretching vibration in sulfinate (![]() ) or sulfino-group (R-SO2H) by consi- dering the chemical state of sulfur[7]. On the basis of above discussion, a possible reaction mechanism is
) or sulfino-group (R-SO2H) by consi- dering the chemical state of sulfur[7]. On the basis of above discussion, a possible reaction mechanism is
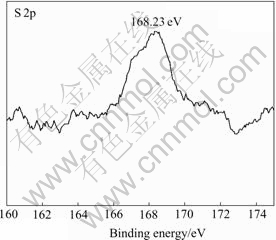
Fig.4 XPS spectrum of S 2p with high resolution

Fig.5 FTIR spectrum for ACFs
proposed to interpret the formation of Ti2(SO4)3. In the presence of oxygen, interfacial Ti cation with 3 valencies (>Ti3+) on the TiO2 surface would react with the surface sulfinate or sulfino-group on the carbon fiber surface according to the following formula:
>Ti3++R-![]() (or R-SO2H)+O2→>Ti2(SO4)3 +CO2 (1)
(or R-SO2H)+O2→>Ti2(SO4)3 +CO2 (1)
Generally, firm attachment of two solid surfaces is the prerequisite for an interfacial reaction taking place between these two surfaces. Therefore, the interfacial reaction of Ti2(SO4)3 formation between the TiO2 film and carbon fiber surface clearly exhibits that these two solid surfaces are firmly attached to each other.
3.4 Structure explanation of deposited TiO2 film on activated carbon fibers
In general, fine activated carbon fibers are highly microporous with small external surface area; the pore width of the slit-shaped micropores is in the range of 0.7-2 nm[8]. The ACFs can strongly adsorb a consi- derable amount of vapors with their micropores through a micropore filling that is regarded as the strongest physical adsorption process[9]. On the other hand, the titanium tetrachloride (TiCl4) used as the precursor is a liquid with a high vapor pressure of 1.87 kPa at 25 ℃. The TiCl4 molecule with a size of 0.132 nm is small enough to be adsorbed in the micropores of ACFs[10]. Thus, if TiCl4 vapor is adsorbed to form a film on both internal and external surfaces of ACFs, and hydrolyzed with adsorbed water, the fiber surface will be deposited with a titania layer. According to these characteristics of the MAD process and above mentioned evidences of firm attachment of TiO2 film to carbon fibers, we may propose the deposition mechanism shown in Fig.6 to explain the formation of TiO2 film on the ACFs.
According to this mechanism, lots of small needle- shaped TiO2 whiskers have formed on TiO2 film surface and implanted into the slit-shaped micropores of the ACFs. Therefore, it could be inferred that an interposition fixing structure with a thickness of micropores’ deepness between the TiO2 film and carbon fiber surface has formed with mutual insertion of the small TiO2 whiskers and micropores’ walls. This structure is thought to be very critical to keep the TiO2 film attached to carbon fibers firmly and stably.
Furthermore, the proposed deposition mechanism implies that the ACFs with larger pore volume would lead to much more completely developed interposition fixing structure, and lower vacuum pressure in a MAD apparatus before adsorbing the TiCl4 vapor would be also advantageous for developing more perfect interposition fixing structure. In a word, the more completely the interposition fixing structure is developed, the more stable the deposited TiO2 film is in the stirred solutions.
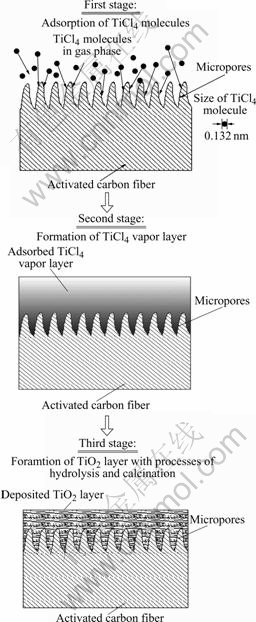
Fig.6 Deposition mechanism of TiO2 film on activated carbon fibers
4 ConclusionsThe immobilized photocatalyst prepared by the MAD process, TiO2 film anchored on activated carbon fibers (TiO2/ACFs), shows high stability in the cyclic photodegradation runs. To interpret the high stability of the deposited TiO2 film, the interposition fixing structure is studied with SEM, EDX, XRD, XPS and FTIR analyses. The results indicate that the residual TiO2 super-thin film still covers the carbon fiber surface after removing the deposited TiO2 film. Titanium sulfate (Ti2(SO4)3), in the burnt remainders of the TiO2/ACFs, is considered to be formed through the interfacial reaction of the TiO2 film with surface groups on carbon fibers. The detected and analyzed results exhibit that two solid surfaces, TiO2 film and carbon fiber surface, are tightly attached to each other. In the consideration of characteristics of the MAD process, the interposition fixing structure is inferred to be formed in the intercrossed shallow layer between TiO2 film and carbon fiber surface with the thickness of micropores’ deepness. This fixing structure leads to firm attachment and high stability of TiO2 film in the stirred solutions.
References[1] RAY A K. Design, modelling and experimentation of a new large-scale photocatalytic reactor for water treatment[J]. Chem Eng Sci, 1999, 54: 3113-3125.
[2] FABIYI M E, SKELTON R L. Photocatalytic mineralisation of methylene blue using buoyant TiO2-coated polystyrene beads [J]. J Photochem Photobio A: Chem, 2000, 132: 121-128.
[3] FAN C M, MIN Y Q, HAO X G, SUN Y P, LI X J, LI F B. Adsorption and photocatalytic degradation of phenol over TiO2/ACF [J]. Trans Nonferrous Met Soc China, 2003, 13(2): 452-456.
[4] FU P F, LUAN Y, DAI X G. Preparation of activated carbon fibers supported TiO2 photocatalyst and evaluation of its photocatalytic reactivity [J]. J Mol Catal A: Chem, 2004, 221: 81-88.
[5] HOKER J, MCCARTHY G. ICDD Card Number:22-0947 [M]. North Dakota, USA: North Dakota State University, 1991.
[6] WAGER D, RIGGS W M, DAVIS I E, MUILEUBERG G E. Handbook of X-ray Photoelectron Spectroscopy [M]. USA: Perkin Elmer Corporation Physical Electronics Division, 1979.
[7] XIE J X, CHANG J B, WANG X M. Application of IR Spectroscopy in Organic and Pharmaceutical Chemistry [M]. Beijing: Science Press, 2001, 352. (in Chinese)
[8] FREEMAN J J, GIMBLETT F G R, ROBERTS R A, SING K S W. Studies of activated charcoal cloth (I): Modification of adsorptive properties by impregnation with boron-containing compounds [J].Carbon, 1987, 25: 559-563.
[9] KANEKO K, NAKAHIGASHI Y, NAGATA K. Microporosity and adsorption characteristics against NO, SO2, and NH3 of pitch-based activated carbon fibers [J]. Carbon, 1988, 26: 327-332.
[10] MATSUMOTO A, TSUTSUMI K, KANEKO K. Titania coating of a microporous carbon surface by molecular adsorption-deposition [J]. Langmuir, 1992, 8: 2515-2520.
Corresponding author: FU Ping-feng; Tel: +86-10-62004523; E-mail: pffu@ygi.edu.cn


