J. Cent. South Univ. Technol. (2007)02-0191-05
DOI: 10.1007/s11771-007-0038-8 ![]()
Organic acids and inorganic anions in Bayer liquors by ion chromatography after solid-phase extraction
ZHONG Fu-jin(钟付金)1, CHEN Xiao-qing(陈晓青)1, ZHANG Shu-chao(张树朝)2, LI Yue-ping(李跃平)2
(1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Zhengzhou Research Institute, China Aluminum Corporation, Zhengzhou 450041, China)
Abstract:
A method for the simultaneous separation and determination of organic acids and inorganic anions in Bayer liquors was developed by gradient ion chromatography with suppressed conductivity detection. Formate, acetate, propionate, oxalate, succinate, glutarate, fluoride, chloride and sulfate were separated and determined in 33 min. The samples were pretreated with solid-phase extraction, which has high selectivity for removing a large number of metallic ions in the Bayer liquors, and filtered with a 0.45 ?m filter membrane before being injected into the ion chromatographic system. The separation of six organic acids and three inorganic anions was achieved on an IonPac AS11-HC column with KOH as the eluent, and the detection was performed by a conductivity detection mode. No interference is found in the presence of fluorate, chlorate and sulphate when organic acids are determined. The calibration graphs of peak area for all the analytes are linear over a wide range. The relative standard derivation of the peak area of analytes is less than 2.14%. Under optimum conditions the detection ranges from 0.2 to 100.0 mg/L. The average recoveries of the added standards are between 94.3% and 102.8%.
Key words:
Bayer liquors; organic acid; inorganic anion; ion chromatography; solid-phase extraction ;
1 Introduction
Bayer liquors are by-products of alumina and aluminium metal production. The Bayer process involves the extraction and precipitation of alumina from bauxite using hot sodium hydroxide. The cyclic and soluble impurities, mostly organic and inorganic ions, accumulate in the liquor stream[1-3]. Organic acids are of prime importance as their stability and removal control refinery productivity. Liquors from these processes are typically of high pH and ionic strength and contain numerous anions such as chloride, sulfate, phosphate, fluoride, oxalate, silicate, succinate, malonate and formate[1, 4]. The analysis of anions and organic acids in Bayer liquor is vital for two main reasons, namely process monitoring (including quality control and optimisation of product yield and purity) and both toxicology and environmental impact monitoring.
In recent years, several methods have been proposed for the simultaneous chromatography of organic acids and inorganic anions. However, one of the problems often encountered in organic acid determination, regardless of the technique used, is the interferences from the sample matrices. Especially in the case for industrial samples that are usually very complex in composition, sample matrices can be adsorbed onto the column. Analysis of Bayer liquor by ion chromatography(IC) is
not used routinely most probably due to co-elution of weakly retained species[5]. Furthermore, Bayer liquor is of high ionic strength and pH and is extremely difficult to separate by IC without clean-up or pre-treatment, for example by dialysis[6]. When being injected directly, untreated samples shorten column life and affect column performance[7], the latter being primarily due to severe disturbance of the acid-base equilibria in the system. Therefore, it is desirable to remove the organic matrices before analysis by ion chromatography.
Organic acids have been shown to be very harmful with regard to alumina productivity and size, especially for sodium oxalate[8-9]. A variety of methods have been utilized for the determination of oxalate in Bayer liquors, including titrimetry[10], ion chromatography[11-12], gas chromatography with dramatization[13], capillary electrophoresis [1,12,14-17], flow injection analysis[18] and high performance liquid chromatography[19-21]. Any reports on analyzing the humic acids by IC had been restricted to only analyzing oxalic acids [11-12]. But the quantitative determination was not perfect because the separation was not excellent. At present, the simultaneous and fully resolved separation of organic acids and inorganic anions in Bayer liquor using IC is not yet reported. The rapid and reliable determination of organic acids in Bayer liquor is of significant industrial importance because their presence has been implicated in the formation of small particle size hydrated alumina[22]. Therefore, setting a systematic analysis method for organics and other anions in Bayer liquor will have very important significance to conduct the study and production in alumina industry field.
In this study, a method for the simultaneous separation of fluoride, acetate, propionate, formate, chloride, glutarate, succinate, sulfate and oxalate on anion-exchange column was described. A solid-phase extraction (SPE) procedure for the removal of complex organic sample matrices was proposed.
2 Experimental
2.1 Reagents and standards
All solutions were prepared from reagent-grade chemicals in 18 MΩ water, obtained from a Milli-Q plus water purification system (Millipore, Bedford, MA, USA). Sodium fluoride, acetic acid, propionic acid, formic acid, sodium chloride, glutamic acid, succinic acid, potassium sulfate and sodium oxalate were in analytical grade. Standard solutions were prepared and diluted to 1.00 g/L and stored at 4 ℃.
2.2 Apparatus
The ion chromatography used in this work consisted of a Dionex Corporation (Sunnyvale,CA,USA) ICS-2500 system equipped with an ED50 conductivity detector. A Dionex anion self-regenerating suppressor (ASRS- ULTRA, 4 mm) was used in recycle mode to reduce eluent background conductivity. An EG50 eluent generator, a GP50 gradient pump, four eluent switches, an IonPac AS11-HC anion analytical column and IonPacAG11-HC anion guard column were used in series for the separation. Dionex chromeleon 6.60 software was used for system control and data collection. An OnGuard-H column (Dionex Corporation) was employed for the SPE process.
2.3 System equilibration and chromatographic conditions
The following procedure, not recommended by the column manufacturer, was used without any noticeable problem. When a new IonPac AS11-HC column was received, it was washed with an aqueous solution containing 20% acetonitrile (ACN) for 1 h at a flow rate of 1 mL/min, then the column was washed off with deioned(DI) water for 30 min at the same flow rate. Before each run, the impurity anions accumulated from the previous run were removed with 30 mmol/L KOH for about 30 min at a flow rate of 2 mL/min. Finally, with the suppressor and the detector turned on, the entire system was equilibrated with the initial eluent for at least 1 h.
A gradient elution was performed, using a mobile phase of aqueous potassium hydroxide (KOH) solution, at a flow rate of 1.20 mL/min. The column was thermo- treated at 30 ℃. Injection volume was 25 μL. The gradient program employed in the method is listed in Table 1.
Table 1 KOH gradient for simultaneous separation of organic acids and inorganic anions on AS11-HC column

2.4 Sample preparation
An OnGuard-H column contained mainly cation- exchange resin that was 16% cross-linked, styrene-based, sulfonic acid resin in the hydrogen form. This resin was designed to have very high selectivity for multivalent cations. It was used in the removal of high levels of metal ions from sample matrices and in the neutralization of highly alkaline samples.
The OnGuard-H column was activated with 10 mL of DI water for about 25 min. 1 mL Bayer liquors was diluted to 100 mL with DI water, shortly before solid-phase extraction. The diluted solution was then passed through the OnGuard-H column pretreated. The first 4 mL of sample was discarded before a 5 mL sample was collected for ion chromatographic analysis.
3 Results and discussion
3.1 Selection of analytical column and eluent solution
Initially, a Dionex IonPac AS11 column was considered. Using a KOH gradient, this column provides excellent resolution of most organic acids and inorganic anions, however formate and butyrate are co-elute on this column. Because it is very likely for a sample to have both acids, this column was deemed unacceptable for our purposes.
A Dionex IonPac AS11-HC column coupled with a Dionex IonPac AG11-HC guard column was found to allow almost baseline separation of six organic acids, including acetate, propionate, formate, glutarate, succinate and oxalate, commonly found in our samples. These inorganic anions were also separated within the same run. This kind of separation is impossible for ion-exclusion chromatography. The use of an AG11-HC guard column delays the elution of propionate and formate, therefore, improves the separation between acetate, propionate and formate. The IonPac AS11-HC column is also solvent compatible and offers excellent column-to-column reproducibility. All of the data presented in this work were obtained with an AS11-HC anion analytical column coupled with an AG11-HC anion guard column.
The use of low conductivity eluents to separate organic acids and inorganic anions was previously described. KHCO3, K2CO3 and KOH were considered as eluents and the best results were obtained when KOH was used. The concentration of KOH affects the retention of the analytes and also accounts for the background conductivity of the mobile phase. Lower concentrations of KOH lead to a lower background conductivity and, therefore, to an increase in the detection sensitivity and resolution. On the other hand, as KOH concentration increases, the retention time decreases. This effect is especially important in the case of binary acids. Fig.1 shows the influence of KOH concentration on the retention of the analytes, which are fluoride, acetate, propionate, formate, chloride, glutarate and succinate.
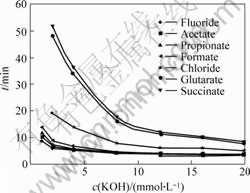
Fig.1 Effects of concentration of eluent solution (KOH) on retention time
3.2 Limits of detection and quantification, linearity and linear regression
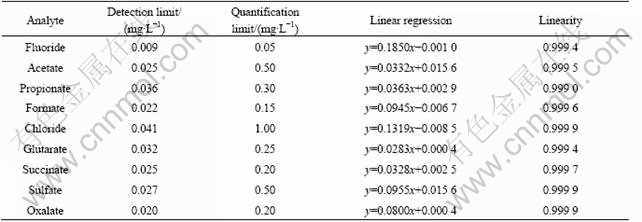
The detection limit was calculated as sb+3s, and the quantification limit was calculated as sb +10s, where sb is the average signal of ten blank injections and s is the standard deviation. Table 2 shows detection and quantification limits of organic acids analyzed. The detection limits range from 0.009 mg/L for fluoride to 0.041 mg/L for chloride and the quantification limits range from 0.05 mg/L for fluoride to 1.00 mg/L for acetate. Calibration curves were determined for seven different concentrations of a mixture of organic acids and inorganic anions standard solutions. Each calibration sample was injected in triplicate. Calibration graphs for each compound were obtained by plotting concentration against peak area and applying the least squares method. Linear regression and linearity are listed in Table 2. Each plot is linear over a wide interval from the quantification limit to at least 20 mg/L for all acids. The peak areas (y) and the theoretical concentrations of the calibration standards (x) were fit to the natural logarithm-quadratic function using the least squares regression. The results of the regression analysis were then used to back-calculate the concentration results from the peak area data, and the back-calculated concentrations and appropriate summary statistics were calculated and presented in tabular form.
Table 2 Limits of detection and quantification, linear regression and linearity for organic acids and inorganic anions analyzed
3.3 Relative standard deviation and recovery
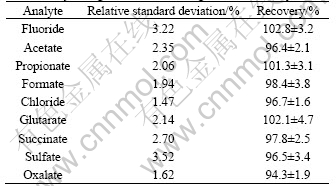
The precision study was comprised of repeatability and reproducibility studies. The repeatability was established by injecting the Bayer liquor samples seven times. The reproducibility was determined by analyzing each sample of Bayer liquors on three different days over about a week. The relative standard deviations (RSD) of the repeatability and the reproducibility are <2.14% and <3.52%, respectively. These results indicate that the present method can be used for quantitative analyses of organic acids in Bayer liquors. To establish the efficiency of the organic extraction, this procedure was also performed on a mixture of organic acids and inorganic anions added to Bayer liquors. Table 3 lists the recoveries of these organic acids and inorganic anions after applying the extraction procedure.
Table 3 Relative standard deviations of reproducibility and recovery of organic acids and inorganic anions analyzed
3.4 Organic acid and inorganic anion concentrations
of Bayer liquors analyzed
The organic acid and inorganic anion contents of two Bayer liquors are listed in Table 4. This variability could be explained by the different origins of the Bayer liquors. Chloride and sulfate concentrations are very high in Pingguo and Zhongzhou Bayer liquors. The concentrations of organic acids are lower in Zhongzhou Bayer liquor than that in Pingguo. Fig.2 shows the typical chromatogram of standards of organic acid and inorganic anion. Fig.3 shows the chromatogram of Bayer liquor sample from Pingguo Bayer liquors.
Table 4 Organic acids and inorganic anion concentrations of Bayer liquors of different origin
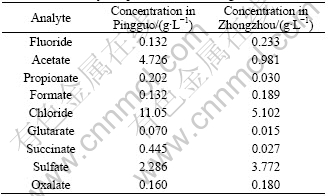
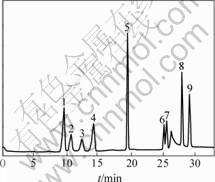
Fig.2 Ion chromatogram of standards of six organic acids and three inorganic anions
1— Fluoride; 2—Acetate; 3—Propionate; 4—Formate; 5—Chloride; 6—Glutarate ; 7—Succinate; 8—Sulfate; 9—Oxalate
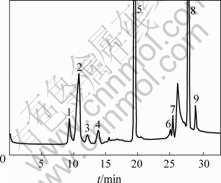
Fig.3 Ion chromatogram of Pingguo Bayer liquors
1—Fluoride; 2—Acetate; 3—Propionate; 4—Formate;
5—Chloride; 6—Glutarate ; 7—Succinate; 8—Sulfate;9—Oxalate
4 Experiment result analysis and discussion
In this study gradient profiles are sufficient to separate six organic acids and three inorganic anions in a short time. JACKSON compared the ion chromatography and capillary electrophoresis for determination of oxalate in Bayer liquors[12]. The eluent is very complicated, which consists of 1.6 mmol/L sodium tetraborate, 7.3 mmol/L boric acid, 1.6 mmol/L sodium gluconate, 5 g/L glycerin, 120 mL/L acetonitrile and 20 mL/L n-butanol at pH 8.5. Moreover, only oxalate, chloride and sulfate- are analyzed.
Our particular interest concerns the development of a simple IC procedure for the determination of acids levels in Bayer liquors. It is the first time that ion chromatography has been used to determine organic acids in Bayer liquor samples. A variety of techniques have been utilized for the determination of sodium oxalate in Bayer liquors. In contrast to these methods, the present method is simple, rapid and requires only extraction steps. A good separation can be achieved in a short separation time of 33 min.
The levels of total organic carbon (TOC) found in Bayer liquors by IC are in agreement with the volumetric (VC) analysis using potassium permanganate titration. Deviation is 2.2%-3.4%. The TOC levels determined by VC are a little higher than the result of IC. We conclude that potassium permanganate is a versatile and powerful oxidant that can be used to oxidize many substances in the process of titration, maybe because some alkyl compounds in Bayer liquors react with potassium permanganate.
5 Conclusions
1) The present method allows the simultaneous quantification of formate, acetate, propionate, oxalate, succinate, glutarate, fluoride, chloride and sulfate in Bayer liquors. The method has the advantage of measuring organic acids in a single run. The analysis is simple, rapid and does not require very complicated sample preparation steps. Only one solid-phase extraction column is used for the sample pre-treatment, thus simplifying the analytical procedure.
2) The relative standard deviations (RSD) of the repeatability and the reproducibility are <2.14% and <3.52%, respectively. The detection limits range from 0.009 mg/L for fluoride to 0.041 mg/L for chloride and the quantification limits range from 0.05 mg/L for fluoride to 1.00 mg/L for acetate. The calibration graphs of peak area for all the analytes are linear over a wide range, under optimum conditions the detection ranges from 0.2 to 100 mg/L. The average recoveries of the added standards are between 94.3% and 102.8%.
[1] GROCOTT S C, JEFFERIES L P, BOWSER T, et al. Applications of ion chromatography and capillary ion electrophoresis in the alumina and aluminium industry[J]. J Chromatogr A, 1992, 602(1/2): 257-264.
[2] ZHANG B, CHEN Q Y, ZHOU K C. Effect of modified additives on process of seeded precipitation ratio of sodium aluminate liquors[J]. J Cent South Univ: Science and Technology, 2006, 37(5): 932-936.
[3] CHEN G H, CHEN Q Y, YIN Z L, et al. Spectral dimension during the precipitation of gibbsite from the seeded caustic aluminate solutions[J]. J Cent Douth Univ: Science and Technology, 2002, 33(2): 157-159.
[4] CARDWELL T J, LAUGHTON W R. Analysis of fluoride, acetate and formate in Bayer liquors by ion chromatography[J]. J Chromatogr A, 1994, 678(2): 364-369.
[5] ROMANO J, JANDIK P , JONES W R , et al. Optimization of inorganic capillary electrophoresis for the analysis of anionic solutes in real samples[J]. J Chromatogr A, 1991, 546 (1/2): 411-421.
[6] LAKSANA S, HADDAD P R. Dialytic clean-up of alkaline samples prior to ion chromatographic analysis[J]. J Chromatogr A, 1992, 602(1/2): 57-63.
[7] HADDAD P R, JACKSON P E. Ion Chromatography—Principles and Applications[M]. Amsterdam: Elsevier, 1990.
[8] CALALO R, TRAN T. Effects of sodium oxalate on the precipitation of alumina trihydrate from synthetic sodium aluminate liquors[J]. Light Metals, 1993, 49(2): 125-133.
[9] BROWN N, COLE T J. The behaviour of sodium oxalate in a bayer alumina plant[J]. Light Metals, 1980, 15(1/2): 105-117.
[10] VOGEL A R. Textbook of Quantitative Inorganic Analysis[M]. 4th ed. Harlow: Longman, 1986: 352.
[11] BOWSER T, GROCOTT S C. Ion chromatography in the alumina and aluminum industries[C]// Proceedings of the 2nd International Alumina Quality Workshop. Perth, 1990.
[12] JACKSON P E. Analysis of oxalate in Bayer liquors: a comparison of ion chromatography and capillary electrophoresis[J]. J Chromatogr A, 1995, 693(1): 155-161.
[13] ARUDI R L, BIELSKI B H J, ALLEN A O. Search for singlet oxygen luminescence in the disproportionation of HO2/O2[J]. Photochem Photobiol, 1984, 39(4): 703-709.
[14] JACKSON P E. Capillary ion analysis: principles and applications[J]. Chem Aust, 1993, 60(1/2): 165-170.
[15] HADDAD P R, HARAKUWE A H, BUCHBERGER W. Separation of inorganic and organic anionic components of Bayer liquor by capillary zone electrophoresis I: Optimisation of resolution with electrolyte-containing surfactant mixtures[J]. J Chromatogr A, 1995, 706(1/2): 571-578.
[16] HARAKUWE A H, HADDAD P R, JACKSON P E. Quantitative determination of oxalate in Bayer liquor by capillary zone electrophoresis[J]. J Chromatogr A, 1996, 739(1/2): 399-403.
[17] CHOVANCEK M, CHOO P, MACKA M. Development of a fully buffered molybdate electrolyte for capillary electrophoresis with indirect detection and its use for analysis of anions in Bayer liquor[J]. Electrophoresis, 2004, 25(3): 437-443.
[18] BARNETT N W, LEWIS S W, PURCELL S D, et al. Determination of sodium oxalate in Bayer liquor using flow-analysis incorporating an anion exchange column and tris(2,2-bipyridyl)ruthenium(II) chemiluminescence detection[J]. Analytica Chimica Acta, 2002, 458(1/2): 291-296.
[19] WHELAN T J, WILSON M A, KAMALI KANNANGARA G S. HPLC investigation of humics found in Bayer liquors[J]. Australian Organic Geochemistry Conference, 2002, (12/15): 77-78.
[20] WHELAN T J, KAMALI KANNANGARA G S, WILSON M A. Increased resolution in high-performance liquid chromatograph spectra of high-weight organic components of Bayer liquors[J]. Ind Eng Chem Res, 2003, 42(26): 6673-6681.
[21] WHELAN T J, SHALLIKER R A, MCINTYRE C, et al. Development of a multidimensional high-performance liquid chromatography(HPLC) separation for Bayer humic substances[J]. Ind Eng Chem Res, 2005, 44(14): 3229-3237.
[22] POWER G P, TICHBON W. Sodium oxalate in the Bayer process: its origin and effects[C]// Proceedings of the 2nd International Alumina Quality Workshop. Perth: Australia, 1990.
Foundation item: Project(2005CB623702) supported by the National Key Basic Research Program of China
Received date: 2006-05-21; Accepted date: 2006-07-27
Corresponding author: CHEN Xiao-qing, Professor; Tel: +86-13974833838; E-mail:fjzhong1982@163.com
(Edited by YANG Bing)
Abstract: A method for the simultaneous separation and determination of organic acids and inorganic anions in Bayer liquors was developed by gradient ion chromatography with suppressed conductivity detection. Formate, acetate, propionate, oxalate, succinate, glutarate, fluoride, chloride and sulfate were separated and determined in 33 min. The samples were pretreated with solid-phase extraction, which has high selectivity for removing a large number of metallic ions in the Bayer liquors, and filtered with a 0.45 ?m filter membrane before being injected into the ion chromatographic system. The separation of six organic acids and three inorganic anions was achieved on an IonPac AS11-HC column with KOH as the eluent, and the detection was performed by a conductivity detection mode. No interference is found in the presence of fluorate, chlorate and sulphate when organic acids are determined. The calibration graphs of peak area for all the analytes are linear over a wide range. The relative standard derivation of the peak area of analytes is less than 2.14%. Under optimum conditions the detection ranges from 0.2 to 100.0 mg/L. The average recoveries of the added standards are between 94.3% and 102.8%.


