Trans. Nonferrous Met. Soc. China 23(2013) 2751-2758
Effects of mutation on a new strain Leptospirillum ferriphilum YXW and bioleaching of gold ore
Xue-wu YUAN, Xue-hui XIE, Feng-xia FAN, Weng-xiang ZHU, Na LIU, Jian-she LIU
College of Environmental Science and Engineering, Donghua University, Shanghai 201620, China
Received 20 September 2012; accepted 30 December 2012
Abstract:
Leptospirillum ferriphilum YXW was isolated through serial dilution from mixed microorganisms enriched in AMD from Dexing copper mine in Jiangxi Province, China. It was mutated by ultrasonic, UV and microwave to collect more efficient strain for bioleaching of gold ore. Physiological and biochemical characteristics indicate that strain YXW is a strict chemoautotrophic microorganism, and the optimal condition for its growth is temperature of 40 °C and pH 1.5. After mutation by ultrasonic, UV and microwave, the density of bacterial cells reached 9×109, 8.4×109 and 4.3×108 mL-1, increased by 291%, 265% and 87%, respectively, compared with the original culture. The bacterial total protein activity was improved by microwave and UV mutations, but was reduced by ultrasonic. Mutations had effects on bioleaching of gold ore in sequence of microwave > UV > ultrasonic. During gold ore bioleaching, the bacterial mutant after mutation by microwave had the best effect on the extraction rates of arsenic and iron, which were 19.6% and 17.7% higher than that of the original strain after bioleaching for 10 d, respectively. The results suggested that the effects of mutation on bioleaching of gold ore may not be mainly due to increase of bacterial cells density, but may be mainly attributed to the improvement of bacterial total protein activity.
Key words:
Leptospirillum ferriphilum YXW; mutation; protein activity; bioleaching; gold ore;
1 Introduction
Leptospirillum ferriphilum belonging to Leptospirillum spp. (groupⅡ) [1], is a gram-negative, moderately thermophilic, acidophilic and strictly chemolithoautotrophic bacterium. It is an important bioleaching organism in the leaching system [2,3], oxidizing ferrous iron to ferric iron for obtaining energy and fixing carbon dioxide from the atmosphere for growth [4]. For many years passed, most researches of bioleaching microorganism have been interested in Acidithiobacillus ferrooxidans, which was always considered to have the best leaching effect. However, as the rise of temperature and the concentration of ferric ion in a high level, the growth of A.ferrooxidans is inhibited obviously. Leptospirillum ferriphilum can stand extreme conditions such as low pH, high redox potential and high temperature, and it has recently received more attention [5-7]. Some studies showed that ferrous iron oxidation by Leptospirillum ferriphilum can be achieved at pH below 1.0 [8-10].
Leptospirillum spp. are strictly chemoautotrophy bacterium, and the existence of organic matter can inhibit the growth of them [11]. Therefore, Leptospirillum ferriphilum can grow very well in liquid medium, but it is difficult to grow on agarose solid medium owing to the production of inhibitors, thus, it is hard to be isolated on solid medium. So far, the double-layer plate technique [12] is considered the most effective way for isolating bioleaching microorganism under the condition of laboratory. A lot of researches in this aspect had been done and succeeded in isolating Leptospirillum ferriphilum [13,14]. Although this method is effective, the preparation of the double-layer is complicated. Serial dilution [15] is another method, which is widely applied in the isolation of organisms, a traditional method of bacteria separation and operation is very simple. Besides, serial dilution has been successfully used in isolation of Leptospirillum spp [16,17].
Leptospirillum ferriphilum is successfully used for bioleaching of many kinds of sulfide ores, and it is often dominant at the later stage of bioleaching processes [18,19]. However, long growth cycle and slow oxidation activity are the major drawbacks of bacteria, which are restrictive factors on efficient leaching effect. Mutation breeding is a frequently-used and effective method for obtaining excellent leaching microorganisms. Many studies show that mutation can enhance bioleaching activity of bacteria, but most of them focused on bacteria other than Leptospirillum ferriphilum [20-22]. The activity of protein may play a key role in bacterial oxidation activity. Leptospirillum ferriphilum obtained energy through the oxidation of ferrous ion to ferric ion, and energy transduction was catalyzed by an unknown protein, which was proposed to be the Iro protein [23]. Some studies reported that rusticyanin was a component in the oxidative respiratory chain of the bacterium [24,25], which participated in the ferrous oxidation pathway. Therefore, total protein activity was used to investigate the mechanism for improvement of bacterial oxidation activity after mutation in this work.
In this study, a new strain Leptospirillum ferriphilum YXW was isolated from bacterial mixture through serial dilution, and then it was processed by three physical mutation methods including ultrasonic, UV, and microwave, to investigate the effects of mutation on bacteria and bioleaching of gold ore.
2 Experimental
2.1 Bacteria sample and mineral sample
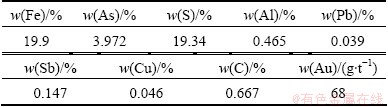
In this study, original microorganisms mixture was enriched in acid mine drainage of Dexing copper mine, Jiangxi Province, China. The gold ore samples were provided by Changchun Gold Research Institute, and they were smashed that the grain sizes were less than 74 μm in diameter. The elements analysis results are shown in Table 1. X-ray diffraction (XRD) results show that gold ore sample is mainly composed of pyrite (FeS2) and quartz (SiO2).
Table 1 Element composition of gold ore
2.2 Isolation and morphology study
9K liquid medium of 3.0 g/L (NH4)2SO4, 0.5 g/L MgSO4·7H2O, 0.5 g/L K2HPO4, 0.1 g/L KCl, 0.01 g/L Ca(NO3)2 and FeSO4·7H2O was the energy source for bacterial growth. pH value of culture was modulated to 1.5 with 5 mol/L H2SO4.
The initial microbial population was enriched with 100 mL liquid medium in 250 mL flasks, shaken at 160 r/min and incubated at temperature of 40 °C. After enrichment 4 times, serial dilution was used to isolate the target strain. The enrichment was serially diluted from 10-1 to 10-9, and then all serial dilutions were cultivated in 9K medium at 40 °C and shaken at 160 r/min. Under the conditions of the highest dilution degree, strain YXW was obtained.
Morphological characteristics and number of cells were observed with an optical microscope (Olympus CX31). Subcellular structures of the cells were examined by a scanning electron microscope (SEM).
2.3 Physiological and biochemical characteristics and substrates utilization
In order to determine the optimal pH and temperature for bacteria growth, cells were inoculated in 250 mL flasks with 100 mL 9K liquid medium. The cell density was about 1.8×107 mL-1, shaken at 160 r/min in a rotary shaker. Bacterial cultivation was performed at 40 °C with different pH of 1.0, 1.3, 1.5, 1.8, 2.0, 2.5 and 3.0; and with pH 1.5 at different temperatures of 30, 35, 40, 45 and 50 °C. After 72 h of incubation, the optical microscope was used to determine the density of cells, and potassium dichromate titration was used to calculate ferrous oxidation rate.
To study substrate utilization of the strain isolated, 9K liquid medium was supplemented with varied energy sources: FeSO4·7H2O (4.48%), sulfur (1%), gold ore (5%), pyrrhotite (5%), sodium thiosulfate(1%), peptone (0.1 %), yeast powder (0.1 %), respectively. All of these cultures were incubated in 250 mL flasks, and solutions were adjusted to pH 1.5 with 5 mol/L H2SO4. The initial cell density was about 1.8×107 mL-1. These flasks were put in a rotary shaker with a speed of 160 r/min at 40 °C. After 3 generations of the culture, the number of cells was counted by the optical microscope.
2.4 Identification of 16S rDNA
Logarithmic phase cells were collected by centrifuging at 10000 r/min, and genomic DNA was extracted using an EZ-10 Spin Column bacterial Genomic DNA Minipreps Kit (Sangon Biotech Co. Ltd., shanghai). Polymerase chain reaction (PCR) amplification of 16S rDNA of strain YXW was performed in a total volume of 50 mL using the primer 27F (5’-AGAGTTTGATCCTGGCTCAG-3’) and 1492R (5’-CGGCTACCTTGTTACGACTT-3’) [26]. PCR reaction program was carried out as followed by LIU et al [27]. PCR products were detected by 0.8% agarose gel electrophoresis and were recovered using the TIAN Gel Midi Purification Kit (Tiangen Biotech Co. Ltd., Beijing).
The 16S rDNA sequence was submitted to GenBank and analyzed by BLAST on the National Center for Biotechnology Information (NCBI). Based on the homology in 16S rDNA sequence, the relationship between strain YXW and the related species was shown by a phylogenetic development tree built by softwares Clustal X and MEGA 3.1.
2.5 Mutation
Cells in the logarithmic phase were centrifuged for 20 min (10000 r/min) and suspended in 9K basic salts medium without Fe2+, and the density of cells was adjusted to about 1.8×108 mL-1.
Mutation experiments were performed under the following conditions. For UV mutation, the power of UV lamp was 15 W. The suspended cells were taken into a plate and at a distance of 30 cm to the UV lamp, and the radiation time was 60, 120 and 180 s, respectively. After mutation, the sample was kept away from light and stored in the refrigerator at 4 °C for 12 h to prevent the bacteria from recovery with light. For microwave mutation, the bacterial suspension was treated in a microwave oven (2450 MHz, 700 W) for 10, 20 and 30 s, respectively. Bacterial mutants were transferred on the ice to remove thermal radiation, and protect enzyme from inactivation. For ultrasonic mutation, ultrasonic cell crusher was used to treat the suspended cells. The power was 500 W and frequency was 40 kHz, the treatment time was 10, 20 and 30 min, respectively. After mutation, the bacterial mutants were cultivated in 9K liquid medium under optimal growth conditions, the density of cells and rate of Fe2+ oxidation were measured every 12 h. The bacterial mutants with good oxidation activity on Fe2+ were selected in gold ore bioleaching experiments.
The bacterial mutants selected growing at the stationary growth stage in 9K medium were collected by centrifugation at 10000 r/min for 20 min, washed with distilled water and suspended in the acetate buffer. pH of suspension was adjusted to about 5.8. Cells obtained were crushed by biological cell disruption of AH-100B (ATS Engineering Inc, shanghai), centrifuged at 10000 r/min for 30 min (4 °C) to collect total protein. The total protein activity was determined by two-point inverse calibration method [28]. In this study, the amount of ferrous ions oxidation in unit time was used to weigh the activity of total protein, and a purple coordination compound was formed by combining ferrous ions non-oxidized with ferrozine. Total protein activity was monitored by measuring the absorbance of the compound in 570 nm wavelength using a UV–vis spectro- photometer (Hitachi Model U-2910). The less the absorbance of the compound, the more the activity of bacterial total protein.
2.6 Bioleaching of gold ores
Bioleaching experiments were conducted in 250 mL flasks containing 100 mL 9K liquid medium and 10% (v/v) bacteria before and after mutation. The pulp density of gold ore was 5% (w/v), and the initial pH of the culture was adjusted to 1.5 with 5 mol/L H2SO4. Flasks were incubated at 40 °C and 160 r/min in a rotary shaker. During the leaching, the total arsenic and iron in the solution were determined by ultraviolet spectro- photometry every 2 d. pH of the solution was measured by a multifunctional water quality analyzer (WTW, Germany). The amount of medium taken out must be compensated with 9K basic medium, and the evaporation of water in the flasks was supplemented with distilled water.
3 Results and discussion
3.1 Subcellular structure
The subcellular structures of cells observed under SEM are shown in Fig. 1. It is clear to find that cells are spiral-shaped, and most cells have spiral with two turns and some even up to four turns. The diameter and length of cells are 0.2-0.5 μm and 1.0-2.5 μm, respectively. The morphological characteristics and subcellular structures of cells indicated that strain YXW is similar to Leptospirillum spp.
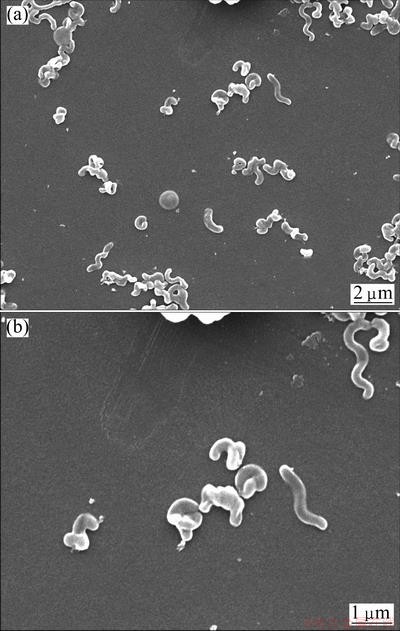
Fig. 1 SEM images of strain YXW
3.2 Physiological and biochemical characteristics
3.2.1 Optimal pH
The effects of pH on ferrous oxidation and the growth of strain YXW are shown in Fig. 2. From pH 1.0 to 2.0, the strain could grow well. The optimal pH is 1.5, at which the density of cells reaches a maximum of 2.3×108 mL-1 and Fe2+ oxidation rate is 100%.

Fig. 2 Effect of pH on ferrous oxidation and growth of strain YXW
3.2.2 Optimal temperature
The effects of temperature on ferrous oxidation and the growth of strain YXW are shown in Fig. 3. According to Fig. 3, bacteria could grow in a range temperatures 30-50 °C, and the optimal temperature is 40 °C. When cultured at 40 ℃, the number of bacteria could reach 2.3×108 cells/mL and Fe2+ oxidation rate was 100%. However, when temperature was higher than 50 °C, bacterial growth and Fe2+ oxidation were obviously inhibited. ZHANG et al [13] succeeded in isolating a new strain Leptospirillum ferriphilum, named YTW315, its capability of growth at 45 °C was strong and was inhibited at 50 °C or above, which were similar to our results.

Fig. 3 Effect of temperature on ferrous oxidation and growth of strain YXW
3.2.3 Substrate utilization characters
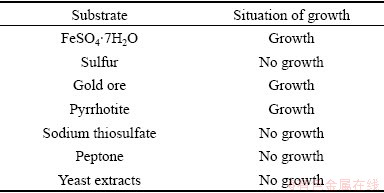
The substrate utilization characters of strain YXW are shown in Table 2. It was observed that the strain could oxidize Fe2+ and sulfide ore (gold ore and pyrrhotite), but it could not utilize thiosulfate and sulfur. Furthermore, the growth of strain YXW could be inhibited with organic matters, such as peptone and yeast extracts. These are consistent with the results reported by TUOVINEN et al [11].
Table 2 Characters of strain YXW with different substrates
3.3 Phylogenetic analysis of 16S rDNA
After electrophoresis, the agar gel was stained with ethidium bromide (EB) and an image was scanned by ChemiDoc XRS imaging system (BIO-RAD). The result is shown in Fig. 4. It shows that the length of 16S rDNA product is about 1500 base pairs (bp). The 16S rDNA was sequenced and submitted to GenBank. An accession number (JQ712516) of strain YXW was obtained.

Fig. 4 Agarose gel electrophoresis image of 16S rDNA
The phylogenetic development tree was built and the relationship chart is described in Fig. 5. It is determined that strain YXW belongs to Leptospirillum ferriphilum species, and it is most closely related to Leptospirillum ferriphilum isolating LF-104 AY485647 (similarity 100%).
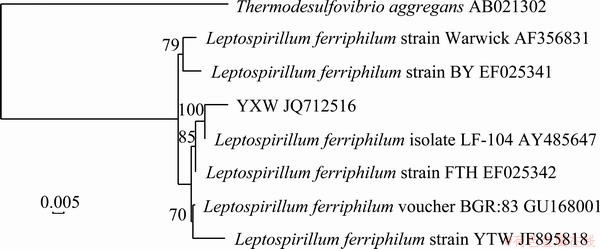
Fig. 5 Phylogenetic development tree of 16S rDNA
3.4 Influence of mutation on strain of YXW
Bacteria before and after mutation were inoculated in 250 mL flasks containing 100 mL 9K medium, shaken at 160 r/min in a rotary shaker at 40 °C. The density of cells was about 1.8×108 mL-1, and the initial pH of the culture was adjusted to 1.5 with 5 mol/L H2SO4. After 36 h, ferrous oxidation rate of bacterial mutant is shown in Table 3. It can be seen that ultrasonic 30 min, UV 120 s, microwave10 s have the best effects on Fe2+ oxidation rate among their own groups (Table 3).
Table 3 Ferrous oxidation rate of bacterial mutant after 36 h
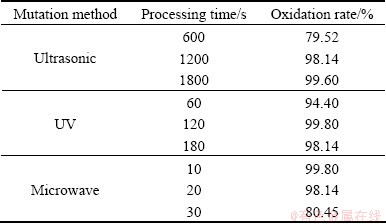
Figure 6 shows the ferrous oxidation rate of strain YXW before and after mutation during growing in 9K medium. It could be seen that ferrous ions were oxidized completely by the original strain (un-treated) which needed about 60 h, while the strain after mutation completely oxidized ferrous within 36 h. The results indicate that mutation treatment could significantly improve bacterial oxidation activity. In order to analyze the impact factors of ferrous oxidation, growth and total protein activity of strain YXW before and after mutation were studied.
The changes of growth curve and total protein activity of strain YXW before and after mutation are shown in Fig. 7. As shown in Fig. 7(a), mutation treatment made a vast enhancement in bacterial growth activity. The un-treated strain entered into the logarithmic phase at about 36 h, and the maximum concentration of cells was about 2.3×108 mL-1.

Fig. 6 Ferrous oxidation rate of strain YXW before and after mutation during growing in 9 K medium with Fe2+
Compared with the growth of the un-treated strain, the strain after mutation entered into the logarithmic phase at 24 h, which was 12 h earlier than the un-treated strain. Furthermore, mutation treatment increased cell concentration in the order of ultrasonic>UV> microwave. The density of cells reaches 9×109, 8.4×109 and 4.3×108 mL-1, which are increased by 291%, 265% and 87% after mutation by ultrasonic, UV and microwave, respectively, compared with the original culture. These results are similar to the study reported by XU et al [21], who also found that the growth of Ac. cryptum DX1-1 was enhanced by UV mutagenesis, and 20 h earlier reached the logarithmic phase.
In this study, a two-point inverse calibration method was invited to detect the total protein activity. The results are shown in Fig. 7(b). It can be seen that all the absorbance values of the purple coordination compound continuously decrease as time increases. The absorbance value of UV or microwave mutant strain is always less than that of the un-treated strain, which indicates that total protein activity of UV and microwave mutant strain are improved, compared with un-treated strain. And the change of the microwave mutant strain is more obvious. In contrast, the absorbance of ultrasonic mutant strain is higher than that of the un-treated strain, which indicates that the activity of total protein is reduced after ultrasonic mutation. The results suggest that mutation on improvement of ferrous oxidation rate can be achieved through increasing the density of cells or enhancing protein activity.
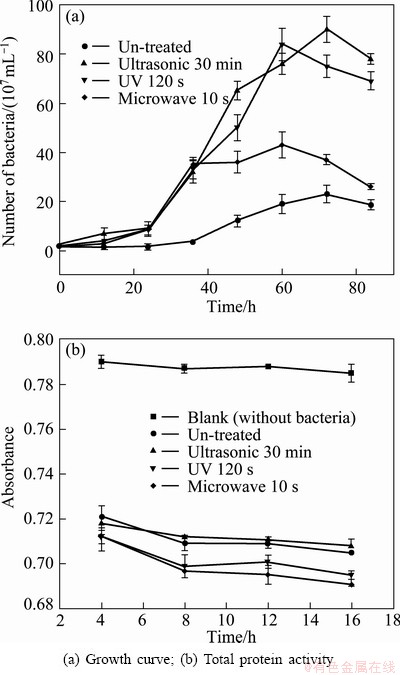
Fig. 7 Various growth patterns of strain YXW during growing in 9K medium with Fe2+
3.5 Bioleaching results of gold ore
Strains after mutation by ultrasonic 30 min, UV 120 s and microwave10 s were used for gold ore bioleaching. Figure 8 shows the concentration of arsenic and iron in the solution during bioleaching with strain YXW before and after mutation. According to Fig. 8(a), the concentration of total arsenic in the solution of mutant culture is higher than that of un-treated culture. After 10 d, the concentration of total arsenic reaches 1.98 g/L in microwave mutation, 1.89 g/L in UV mutation, 1.85 g/L in ultrasonic mutation, and 1.6 g/L in un-treated culture. As shown in Table 4, the best mutation effect of the strain for the solubilization of arsenic is microwave mutation, the extraction rate of arsenic reaches 99.7% and is 19.6% higher than that of the original strain. As shown in Fig. 8(b), the iron extraction continuously increases. In the first 10 d, the total iron concentration increases quickly, then it becomes slowly. Table 4 shows that microwave mutation is the most effective with an iron extraction rate of 76% at 10 d. In contrast, ultrasonic mutation is the least effective in leaching process with an iron extraction rate of 58.7% at 10 d, which is similar to that of the un-treated strain (58.3%).

Fig. 8 Concentration of arsenic (a) and iron (b) in solution during bioleaching with strain YXW before and after mutation
Table 4 Arsenic and iron solubilization after 10 d bioleaching with strain YXW before and after mutation
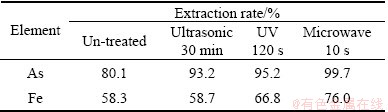
From the bioleaching results, it could be seen that all these three mutation methods could improve bioleaching effects on gold ore of strain YXW, and they were in sequence of microwave > UV > ultrasonic. According to the results studied before, we have known that microwave has the best effect on enhancement of the total protein activity, but the least increase of cell concentration. Whereas the ultrasonic has the best effect on increase of density of bacterial cells, but it can reduce the total protein activity. From these results, it can be deducted that effects of mutation on bioleaching of gold ore may mainly attribute to the improvement of total protein activity, not to the increase of cells concentration.
4 Conclusions
1) A new strain Leptospirillum ferriphilum YXW is isolated through serial dilution from mixed microorganisms enriched in acid mine drainage of Dexing copper mine, Jiangxi Province, China. The strain is the most closely related to Leptospirillum ferriphilum isolating LF-104 AY485647 (similarity 100%).
2) Physiological and biochemical studies indicate that Leptospirillum ferriphilum YXW is a strict chemoautotrophic microorganism, only oxidizes Fe2+ and sulfide ore. The optimal temperature is 40 °C and pH is 1.5.
3) Mutation treatments increase cell concentration in sequence of ultrasonic > UV > microwave. UV and microwave mutations can improve the total protein activity of strain YXW, but the ultrasonic mutation can reduce the activity of total protein.
4) Three mutation treated groups were selected for bioleaching experiments and they are ultrasonic 30 min, UV 120 s, microwave 10 s. These mutations have effects on bioleaching of gold ore in a sequence of microwave > UV > ultrasonic.
5) All the results suggest that the effects of mutation on bioleaching of gold ore may not be mainly due to increase of bacterial cells density, but to the improvement of bacterial total protein activity.
References
[1] TYSON G W, CHAPMAN J, HUGENHOLTZ P, ALLEN E E. Community structure and metabolism through reconstruction of microbial genomes from the environment [J]. Nature, 2004, 428: 37-43.
[2] TUFFIN I M, HECTOR S B, DEANE S M, RAWLINGS D E. Resistance determinants of a highly arsenic-resistant strain of Leptospirillum ferriphilum isolated from a commercial biooxidation tank [J]. Appl Environ Microbiol, 2006, 72: 2247-2253.
[3] SONG Jian, LIN Jian-qun, REN Yi-lin, LIN Jian-qiang. Competitive adsorption of binary mixture of Leptospirillum ferriphilum and Acidithiobacillus caldus onto pyrite [J]. Biotechnology and Bioprocess Engineering, 2010, 15: 923-930.
[4] GAHAN C S, SUNDKVIST J E, DOPSON M,  . Effect of chloride on ferrous iron oxidation by a Leptospirillum ferriphilum-dominated chemostat culture [J]. Biotechnology and Bioengineering, 2010, 106 (3): 422-431.
. Effect of chloride on ferrous iron oxidation by a Leptospirillum ferriphilum-dominated chemostat culture [J]. Biotechnology and Bioengineering, 2010, 106 (3): 422-431.
[5] OJUMU T V, PETERSEN J. The kinetics of ferrous ion oxidation by Leptospirillum ferriphilum in continuous culture: The effect of pH [J]. Hydrometallurgy, 2011, 106: 5-11.
[6] PATEL B C, TIPRE D R, DAVE S R. Development of Leptospirillum ferriphilum dominated consortium for ferric iron regeneration and metal bioleaching under extreme stresses [J]. Bioresource Technology, 2012, 118: 483-489.
[7] PENEV K, KARAMANEV D. Batch kinetics of ferrous iron oxidation by Leptospirillum ferriphilum at moderate to high total iron concentration [J]. Biochemical Engineering Journal, 2010, 50(1-2): 54-62.
[8] KINNUNEN P H M, PUHAKKA J A. High-rate iron oxidation at below pH 1 and at elevated iron and copper concentrations by a Leptospirillum ferriphilum dominated biofilm [J]. Process Biochemistry, 2005, 40(11): 3536-3541.
[9]  B, NURMI P, SAHINKAYA E, KAKSONEN A H, PUHAKKA J A. Temperature effects on the iron oxidation kinetics of a Leptospirillum ferriphilum dominated culture at pH below one [J]. Advanced Materials Research, 2007, 20-21: 465-468.
B, NURMI P, SAHINKAYA E, KAKSONEN A H, PUHAKKA J A. Temperature effects on the iron oxidation kinetics of a Leptospirillum ferriphilum dominated culture at pH below one [J]. Advanced Materials Research, 2007, 20-21: 465-468.
[10]  B, SAHINKAYA E, NURMI P, KAKSONEN A H, PUHAKKA J A. Kinetics of iron oxidation by Leptospirillum ferriphilum dominated culture at pH below one [J]. Biotechnology and Bioengineering, 2007, 97(5): 1121-1127.
B, SAHINKAYA E, NURMI P, KAKSONEN A H, PUHAKKA J A. Kinetics of iron oxidation by Leptospirillum ferriphilum dominated culture at pH below one [J]. Biotechnology and Bioengineering, 2007, 97(5): 1121-1127.
[11] TUOVINEN O H, NIEMELA S I, GYLLENHERG H G. Effect of mineral nutrients and organic substances on the development of Thiobacillus ferrooxidans [J]. Biotechnology and Bioengineering, 1971, 13: 517-527.
[12] JOHNSON D B, MCGINNESS S. A highly efficient and universal solid medium for growing mesophilic and moderately thermophilic, iron-oxidizing, acidophilic bacteria [J]. Journal of Microbial Methods, 1991, 13: l13-122.
[13] ZHANG Rui-yong, XIA Jin-lan, PENG Juan-hua, ZHANG Qian, ZHANG Cheng-gui, NIE Zhen-yuan, QIU Guan-zhou. A new strain Leptospirillum ferriphilum YTW315 for bioleaching of metal sulfides ores [J]. Transacitons of Nonferrous Metals Society of China, 2010, 20: 135-141.
[14] GAO Jian, ZHANG Cheng-gui, WU Xue-ling, WANG Hai-hua, QIU Guan-zhou. Isolation and identification of a strain of Leptospirillum ferriphilum from an extreme acid mine drainage site [J]. Annals of Microbiology, 2007, 57(2): 171-176.
[15] RAPPE M S, CONNON S A, VERGIN K L, GIOVANNONI S J. Cultivation of the ubiquitous SAR11 marine bacterioplankton clade [J]. Nature, 2002, 418: 630-633.
[16] GAO Jian, DING Jian-nan, KANG Jian, WU Xue-ling, QIU Guan-zhou. Identification and heavy metal toxicity assessment upon Fe2+-oxidizing ability of Leptospirillum-like bacterium isolated from acid mine drainage [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(1): 220-226. (in Chinese)
[17] TYSON G W, LO I, BAKER B J, ALLEN E E, HUGENHOLTZ P, BANFIELD J F. Genome-directed isolation of the key nitrogen fixer Leptospirillum ferrodiazotrophum sp. nov. from an acidophilic microbial community [J]. Appl Environ Microbiol, 2005, 71(10): 6319-6324.
[18] DOPSON M, LINDSTROM E B. Analysis of community composition during moderately thermophilic bioleaching of pyrite, arsenical pyrite, and chalcopyrite [J]. Microbial Ecology, 2004, 48(1): 19-28.
[19] ZENG Wei-min, QIU Guan-zhou, ZHOU Hong-bo, PENG Juan-hua, CHEN Miao, TAN S N, CHAO Wei-liang, LIU Xue-duan, ZHANG Yan-sheng. Community structure and dynamics of the free and attached microorganisms during moderately thermophilic bioleaching of chalcopyrite concentrate [J]. Bioresource Technology, 2010, 101: 7068-7075.
[20] DONG Ying-bo, LIN Hai, WANG Han, MO Xiao-lan, FU Kai-bin, WEN Hong-wei. Effects of ultraviolet irradiation on bacteria mutation and bioleaching of low-grade copper tailings [J]. Minerals Engineering, 2011, 24: 870-875.
[21] XU Ai-ling, XIA Jin-lan, ZHANG Shuai, YANG Yu, NIE Zhen-yuan, QIU Guan-zhou. Bioleaching of chalcopyrite by UV-induced mutagenized Acidiphilium cryptum and Acidithiobacillus ferrooxidans [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(2): 315-321.
[22] MENG Chun, SHI Xian-ai, LIN Hui, CHEN Jian-feng, GUO Yang-hao. UV induced mutations in Acidianus brierleyi growing in a continuous stirred tank reactor generated a strain with improved bioleaching capabilities [J]. Enzyme and Microbial Technology, 2007, 40: 1136-1140.
[23] ZENG Jia, GENG Mei-mei, LIU Yuan-dong, ZHAO Wen-jie, XIA Le-xian, LIU Jian-she, QIU Guan-zhou. Expression, purification and molecular modelling of the Iro protein from Acidithiobacillus ferrooxidans Fe-1 [J]. Protein Expression and Purification, 2007, 52: 146-152.
[24] IDA C, SASAKI K, ANDO A, BLAKE R C II, SAIKI H, OHMURAl N. Kinetic rate constant for electron transfer between ferrous Ions and novel Rusticyanin Isoform in Acidithiobacillus ferrooxidans [J]. Journal of Bioscience and Bioengineering, 2003, 95(5): 534-537.
[25]  A, DUQUESNE K, BONNEFOY V. Rusticyanin gene expression of Acidithiobacillus ferrooxidans ATCC 33020 in sulfur- and in ferrous iron media [J]. Hydrometallurgy, 2003, 71: 107-114.
A, DUQUESNE K, BONNEFOY V. Rusticyanin gene expression of Acidithiobacillus ferrooxidans ATCC 33020 in sulfur- and in ferrous iron media [J]. Hydrometallurgy, 2003, 71: 107-114.
[26] XIA Le-xian, TANG Lu, XIA Jin-lan, YIN Chu, CAI Li-yuan, ZHAO Xiao-juan, NIE Zhen-yuan, LIU Jian-she, QIU Guan-zhou. Relationships among bioleaching performance, additional elemental sulfur, microbial population dynamics and its energy metabolism in bioleaching of chalcopyrite [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(1): 192-198.
[27] LIU Jian-she, XIE Xue-hui, XIAO Sheng-mu, WANG Xiu-mei, ZHAO Wen-jie, TIAN Zhuo-li. Isolation of Leptospirillum ferriphilum by single-layered solid medium [J]. Journal of Central South University of Technology, 2007, 14(4): 467-473.
[28] XIAO Yue-sheng, ZHANG Xiao-yin. Dotection of serum ferroxidase activity by two-point inverse calibration [J]. Jiangxi Med Lab Sci, 2001, 19(6): 343-346. (in Chinese).
诱变对新菌株Leptospirillum ferriphilum YXW及浸出金矿的影响
袁学武,谢学辉,范凤霞,朱文祥,刘 娜,柳建设
东华大学 环境科学与工程学院,上海 201620
摘 要:通过稀释分离方法从江西德兴铜矿矿山废水中富集而来的混合菌中分离得到菌株Leptospirillum ferriphilum YXW,再利用超声波、紫外线和微波对其进行诱变,筛选出更高效的细菌用于金矿的浸出。生理生化特性实验显示,菌株YXW为极端化能自养型细菌,最佳的生长条件为温度40 °C,pH=1.5。诱变后,细菌浓度分别可达到9×109(超声波)、8.4×109(紫外线)和4.3×108 mL-1(微波),与原始菌相比,分别提高了291%、265%和87%。微波和紫外诱变后,细菌总蛋白活性升高,而超声诱变后,细菌总蛋白活性降低。诱变对细菌浸出金矿的影响由大到小的排列顺序是微波、紫外线、超声波。在金矿浸出过程中,微波诱变后的细菌具有最好的浸出效果。浸出10 d后,As和Fe的浸出率分别高出原始菌19.6%和17.7%。结果表明,诱变对细菌浸出金矿效果的提高,可能不在于细菌浓度的增大,而是取决于细菌总蛋白活性的提高。
关键词:Leptospirillum ferriphilum YXW;诱变;蛋白活性;生物浸出;金矿
(Edited by Xiang-qun LI)
Foundation item: Project (41073060) supported by the National Natural Science Foundation of China; Project (12ZR1440400) supported by the Shanghai Natural Science Foundation of Youth, China; Project (B604) supported by the Shanghai Leading Academic Discipline, China; Project supported by the State Environmental Protection Engineering Center for Pollution Treatment and Control in Textile Industry, China
Corresponding author: Xue-hui XIE; Tel: +86-21-67792535; Fax: +86-21-67792522; E-mail: xiexuehui@dhu.edu.cn;
Jian-she LIU; Tel: +86-21-67792523; Fax: +86-21-67792522; E-mail: liujianshe@dhu.edu.cn
DOI: 10.1016/S1003-6326(13)62793-4
Abstract: Leptospirillum ferriphilum YXW was isolated through serial dilution from mixed microorganisms enriched in AMD from Dexing copper mine in Jiangxi Province, China. It was mutated by ultrasonic, UV and microwave to collect more efficient strain for bioleaching of gold ore. Physiological and biochemical characteristics indicate that strain YXW is a strict chemoautotrophic microorganism, and the optimal condition for its growth is temperature of 40 °C and pH 1.5. After mutation by ultrasonic, UV and microwave, the density of bacterial cells reached 9×109, 8.4×109 and 4.3×108 mL-1, increased by 291%, 265% and 87%, respectively, compared with the original culture. The bacterial total protein activity was improved by microwave and UV mutations, but was reduced by ultrasonic. Mutations had effects on bioleaching of gold ore in sequence of microwave > UV > ultrasonic. During gold ore bioleaching, the bacterial mutant after mutation by microwave had the best effect on the extraction rates of arsenic and iron, which were 19.6% and 17.7% higher than that of the original strain after bioleaching for 10 d, respectively. The results suggested that the effects of mutation on bioleaching of gold ore may not be mainly due to increase of bacterial cells density, but may be mainly attributed to the improvement of bacterial total protein activity.


