Trans. Nonferrous Met. Soc. China 30(2020) 1267-1276
TLP bonding of Ti-6Al-4V to Al 2024 using thermal spray Babbitt alloy interlayer
H. NAEIMIAN, M. A. MOFID
Department of Petroleum, Mining and Material Engineering, Central Tehran Branch, Islamic Azad University, Tehran, Iran;
Received 13 September 2019; accepted 23 March 2020
Abstract:
Thermal spray assisted transient liquid phase (TLP) bonding of Ti-6Al-4V to Al2024 alloys was investigated, where the interlayer was 80 μm Babbitt thermal spray coat on Al substrate. Thermal spray creates a rough and clean surface which leads to establishing a joint with higher strength. The optimized parameters were bonding temperature of 580 °C and bonding time of 30 and 60 min. Microstructural observation together with XRD patterns confirmed the existence of Al2Cu, Al2CuMg, Cu3Ti, TiAl3, TiAl and Mg2Sn intermetallic compounds formed in Al weld side. On the other hand, Ti3Al, Sn3Ti5 and Ti3Sn intermetallic compounds formed in Ti side. With increasing bonding time from 30 to 60 min, although the interlayer was not completely consumed, the thickness of remained Babbitt interlayer decreased to approximately 15 μm. The study showed that shear strength of the joint reaches the high value of 57 MPa obtained at larger bonding time of 60 min.
Key words:
transient liquid phase; Al 2024 alloy; Ti-6Al-4V alloy; thermal spray; shear strength;
1 Introduction
Ti-6Al-4V alloy is by far the most investigated and tested titanium alloy that covers over 50% of the titanium market due to its excellent balance between high specific strength and excellent corrosion resistance [1]. However, the high cost of processing and fabrication of Ti-6Al-4V alloy has been a major factor that has limited its use. Therefore, the ability to join Al 2024 alloy to Ti-6Al-4V alloy can provide a product that is less costly, but retains the high strength and light weight properties which are necessary for the aerospace industry [2]. Diffusion bonding is a widely used technology for creating similar and dissimilar joints from challenging materials [3]. However, uncontrollable formation of TiAl and TiAl3 intermetallics caused by direct contact of Al and Ti in solid-state diffusion bonding is the main problem [4]. On the other hand, PRESCOTT and GRAHAM [5] showed that the existence of oxide film on the aluminum surface impeded good metal to metal contact. In order to overcome these deficiencies arisen during the solid state diffusion bonding process, liquid state transient liquid phase (TLP) bonding was proponed [4,6]. The presence of an interlayer between the base metals prevents from direct contact of Al and Ti, thereupon the formation of mentioned intermetallics can be controlled [7]. Furthermore, using eutectic forming interlayers at the bond interface resulted in displacing surface oxide films [6]. Another approach to solve the problem of aluminum oxide on the surfaces is coating the surfaces prior to the bonding process. ALHAZAA and KHAN [1] studied the joining of Al7075 alloy to Ti-6Al-4V alloy using Cu coatings electrodeposited directly onto the bonding surfaces. Their results indicated that Cu coatings were successful in preventing the oxidation of surfaces during the bonding process and furthermore improved the wettability on both alloy surfaces. As mentioned, when an interlayer is placed between the two alloys, it would be claimed that the formation of detrimental intermetallic compounds becomes controllable. Interlayer composition and thickness play an important role in the bonding process. Previous research on solid and liquid state diffusion bonding of Al/Ti has been reported [6-10]. Lead-free Sn-based alloys are widely used to join dissimilar alloys [11]. Sn-3.6Ag-1Cu interlayer was used by ALHAZAA et al [9] for TLP bonding of Al 7075 to Ti-6Al-4V and gave the highest bond strength of 42.3 MPa. KENEVISI et al [2,7] studied Ti/Al bonding with a 50 μm-thick Sn-based interlayer. Although their results showed that the bonding process was performed successfully, the shear strength of joint was lower than that by ALHAZAA and KHAN [1]. SAMAVATIAN et al [4] reached the maximum bond strength of 35 MPa in TLP bonding of Al 2024 to Ti-6Al-4V using pure Sn foil with a thickness of 80 μm as interlayer. Considering the works done by the researchers [2,4,7,11], it can be concluded that Sn is an appropriate interlayer to join Al alloy to Ti alloy.
As mentioned, interlayer material has an important role in the characteristics of the joint region [12]. The alloy foils are expensive and they have a complicated production process (rapid solidification). Thermal spray is economical and rapid coating process that creates a rough and clean surface. It can be concluded that applying the interlayer, as thermal spray coat, can be used for several reasons including: (1) to avoid or reduce the possibility of formation of brittle IMCs, (2) to eliminate the harmful effects of Ti and Al oxide films, (3) supplying appropriate surface roughness at the two faying surfaces, and (4) the possibility of applying interlayer to create different deposit thicknesses. Research exploration shows that there are no significant studies on the TLP process of Ti-6Al-4V to Al 2024 alloys using thermally sprayed interlayer.
The term ‘white metal’, or Babbitt, represents a set of alloys, especially based on tin (Sn) or lead (Pb), with addition of copper (Cu) and antimony (Sb). The production of Babbitt coatings through traditional techniques of thermal spray, as arc spraying process (ASP) and flame spraying (FS), requires no extensive preparation to improve the mechanical anchoring, such as the machining and preheating control as in the conventional and centrifugal casting. Thermally sprayed processes also enable high deposition rates and fabrication from thin coatings until coatings with thickness of 26 mm [13].
In this study, TLP bonding of Al/Ti alloys with Babbitt interlayer was investigated. Babbitt interlayer was applied as a thermal spray coating on aluminum substrate, between base metals. The main objective of this study is to evaluate the microstructural and mechanical properties of diffusion-bonded Al-Ti joints using the Babbitt thermal spray coat as interlayer.
2 Experimental
The base metals used in our diffusion-bonding experiments were Al 2024 and Ti-6Al-4V alloys. The accurate chemical composition of base metals is listed in Table 1. Specimens with the dimensions of 15 mm × 15 mm × 2 mm for metallography and 35 mm × 20 mm × 2 mm for shear strength test were prepared by cutting (Fig. 1). Then, flame wire- sprayed coat (Fig. 1) was applied to joining Ti-6Al-4V and Al 2024 alloys. Before spraying, aluminum substrate was prepared by blasting with 36 mesh alumina abrasive blasting grit. The Al substrate was then sprayed with Babbitt wire to a thickness of approximately 80 μm (Fig. 1). The metal alloy used in the form of solid wire was Babbitt alloy, whose chemical composition was evaluated by energy dispersive spectroscopy (EDS) described in Table 2. The stand-off distance of spraying was 150 mm. The image of Babbitt thermal spray coating with 80 μm in thickness is shown in Fig. 2. Table 3 shows the chemical composition of deposited Babbitt coating on the aluminum substrate. The Ti surfaces were prepared by conventional grinding techniques with final grinding on 1200# emery paper. The specimens were ultrasonically cleaned in an acetone bath to remove adhered contaminants and then dried in air. The optimized parameters used in our TLP bonding experiments were bonding temperature of 580 °C, bonding time of 30 and 60 min, joining pressure of 1 MPa, and heating rate of about 15 °C/min.
Table 1 Chemical composition of base metals used in this study (wt.%)
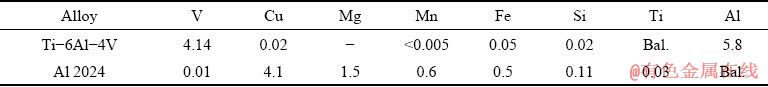
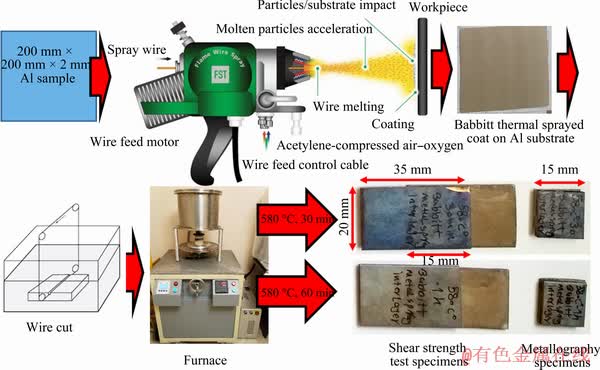
Fig. 1 Dimensions of base metals, metallography and shear strength test specimens and experimental setup for diffusion bonding with Babbitt thermal spray coat as interlayer
Table 2 Chemical composition of Babbitt solid wire with diameter of 1.2 mm (wt.%)


Fig. 2 SEM image of thermally sprayed Babbitt with 80 μm in thickness
Table 3 Chemical composition of deposited Babbitt coating on aluminum substrate (wt.%)
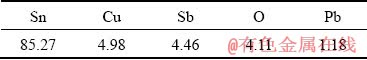
The vacuum pressure was less than 6×10-3 Pa. The assemblies were cooled in the processing chamber under vacuum. For metallurgical examination, the bonded samples were cut transversely. The etchants for Al 2024 and Ti-6Al-4V alloys were 10 mL HNO3, 5 mL HF, and 85 mL distilled water with etching time of 5 s. In order to characterize the joints and identify intermetallic compounds, scanning electron microscopy (SEM), electron dispersive spectroscopy (EDS) and X-ray diffraction (XRD) were applied. The shear strength of the specimen was measured according to the ASTM standard D1002—99 [14] at a cross-head speed of 1 mm/min. Finally, hardness measure- ments were conducted by using micro-indenter with a load of 50 g.
3 Results and discussion
3.1 Microstructure and compositional changes
Figure 3 shows the low magnification SEM images of samples bonded at a bonding temperature of 580 °C, bonding time of 30 and 60 min, with Babbitt thermal spray coat as interlayer. Bonding time is one of the most important parameters in TLP bonding process. Therefore, the microstructures of the joints welded at different time were studied. Figure 3 shows the dissolution and widening zone in the TLP bonding for the bond made at different bonding time. With the rise of bonding time from 30 min (Fig. 3(a)) to 60 min (Fig. 3(b)), the thicknesses of dissolution zones increase significantly from 200 to 570 μm because atoms are diffused evenly and fully in longer bonding time. It is observed from Fig. 3 that the width of dissolution zone is more than 200 μm, while that for Al/Ti couple bond using pure Cu [1] and Sn-3.6Ag-1Cu foils by ALHAZAA et al [9] is much lower. This can be attributed to higher diffusion potential of Ti and Al in thermally sprayed interlayer in comparison with foil interlayer, leading to faster homogenization process and wider joint region. In general, it is obvious that the structure of the thermally sprayed interlayer, which is used in this study has higher residual stresses and defects in comparison with the foil interlayer that was employed by ALHAZAA et al [1,9]. This is because of the nature of the manufacturing process. Grain boundary diffusion is a dominant factor in TLP process which occurs much faster than the bulk diffusion. The higher percentage of the grain boundary exhibits in the thermally sprayed interlayer in comparison with the foil interlayer, which increases diffusion of base metals in the liquid phase. Furthermore, imperfections (i.e. boundaries between splats, pores, voids and boundaries of un-melted particles) in the thermally sprayed interlayer (Fig. 2) act as diffusion channels. Voids could act as free surfaces. Due to the very high diffusion rate on the free surfaces (much more than the grain boundary diffusion), they can drastically increase the diffusion rate. This is responsible for the increased width of dissolution region in the case of using thermal spray coat as interlayer.

Fig. 3 Low magnification SEM images of samples bonded at bonding temperature of 580 °C, bonding time of 30 min (a) and 60 min (b) using thermally sprayed Babbitt coat as interlayer
In TLP bonding process, surface roughness is one of the most important variables that play the main role in the determination of bond strength. To create a solid-state bonding, bonding surfaces must be sufficiently close to each other to activate interatomic short-range gravity forces [15]. Early welding surfaces must be free of any surface contamination. Primarily, surface oxides on aluminum alloys are physically very sticky and are chemically very stable and insoluble in aluminum, even at high temperatures [16]. Therefore, it makes problems for the full metal-to-metal joint in the interface area. This leads to the necessity of coating the surfaces prior to the bonding process. Thermal spray is economical and rapid coating process that creates a rough and clean surface. On the other hand, the main way to overcome the problem of oxidative layers in diffusion welding is to apply relatively rough surfaces, which leads to establishing a joint with higher strength. Local plastic deformation in the early stages of joint leads to rupture of the oxide film. On uneven surfaces, bumps are more likely to deform. So, more rupture occurs in the oxide layer, and the metal-to-metal joint is improved.
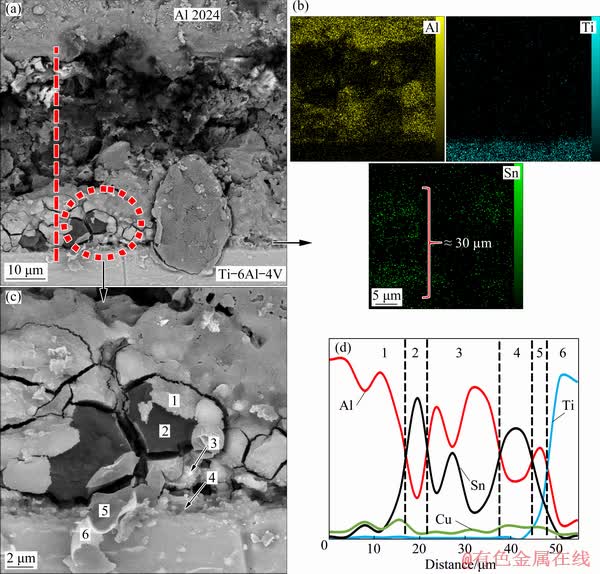
Fig. 4 BSE image of interface (a), EDS maps for Al, Ti and Sn (b), higher magnification BSE image of interface (c) and elemental distribution across interface (d), for joint made with Babbitt thermal spray coat, as interlayer with bonding time of 30 min
To investigate the microstructure more precisely, the backscattered electron image (BSE) of the interface, elemental distribution and concentration profile of the major elements (Al, Ti and Sn) across the bonding region, were taken and are shown in Figs. 4(a, c). Figure 4(a) shows that at bonding time of 30 min, different distinct reaction layers are formed inside the joint region. Figure 4(b) shows that the EDS maps correspond to Al, Ti and Sn elements of the joint formed at the bonding time of 30 min. The number of dots in each map indicates the presence of a certain element that is related to its concentration. It is apparent that inter-diffusion potential of Al and Sn is higher than that of Ti and Sn. The EDS map, corresponding to Sn element shows that although the Sn interlayer reacts with the base metals during joining, but it is not diffused completely into the base metals. The discontinuous remaining Sn layer (Fig. 4(b)) with the thickness of approximately 30 μm shows that the bonding time of 30 min was not sufficient to consume the initial Babbitt interlayer (with the thickness of approximately 80 μm). The EDS line scan of elements perpendicular to the interface (Fig. 4(d)) for this bond clearly shows the width of the interface region and non-uniform distribution of elements in this region. The inhomogeneity of the compositions suggests that the interface region contains various intermetallic phases. The distributions of Al and Ti gradually change, in opposite directions, across the interface between the Al base and Babbitt interlayer. Such gradual distributions indicate that Al and Ti diffuse fast into the interlayer during 30 min of bonding time. However, the steeper distribution curve of Ti at the interface between the Babbitt interlayer and Ti base, confirms that inter-diffusion of Sn and Ti is slower at this bonding temperature. There are six phase regions in 30 min bonded specimen. Region 1 is Al base alloy material. Region 2 that is close to the Al base, is identified as Sn-based interlayer, although the diffusion of Sn, Cu and Al makes the interlayer discontinuous. Region 3 is Al-based solid-solution. The chemical composition obtained for region 3 suggests the formation of Al-based solid-solution that contains few Sn due to solid-state diffusion during the heating stage. Region 4 is the remaining part of the interlayer and thus, mainly consists of tin. There is a considerable amount of tin in the joint region which could be due to the presence of the remaining tin that did not diffuse away from the joint center. In addition, diffusion of tin through aluminum is clearly observed. Region 5 is rich in aluminum, titanium and tin. The inhomogeneity of the compositions suggests that the joint region contains various intermetallic phases such as Al3Ti, AlTi and Sn3Ti5 intermetallics. Region 6 is Ti base alloy material. Selected regions in a bond made for 30 min (see Fig. 4(c)) were analyzed using EDS at regions marked as 1, 2, 3, 4, 5, and 6 and the elemental compositions are shown in Table 4. Region 1 mainly consists of 55.51 wt.% of aluminum and 31.97 wt.% of tin and 5.17 wt.% of magnesium. Region 2 is also rich in aluminum and tin but the aluminum content is reduced to 42.11 wt.% and tin content is increased to 47.46 wt.% and has 5.65 wt.% of magnesium and 2.17 wt.% of copper. Region 3 consists mainly of tin and aluminum with some concentrations of magnesium (7.04 wt.%) and titanium (4.59 wt.%). The inhomogeneity of the compositions suggests that the joint region contains various intermetallic phases. Regions 4-6 (Fig. 4(c)) are located at the Ti-6Al-4V interface and therefore, these regions could be responsible for joint formation at the titanium side. Region 4 is close to the Ti-6Al-4V interface and consists mainly of titanium (45.47 wt.%) and aluminum (38.71 wt.%) with tin (5.6 wt.%) and copper (2.99 wt.%) which can be Ti3Sn or AlTi. Region 5 consists mainly of tin (46.83 wt.%), aluminum (33.14 wt.%) and titanium (12.89 wt.%) with magnesium (3.53 wt.%) and copper (1.53 wt.%). So, it is most likely Al3Ti intermetallic. Region 6 consists mainly of aluminum (32.64 wt.%), tin (28.71 wt.%) and titanium (25.85 wt.%) with magnesium (5.25 wt.%) and copper (3.21 wt.%). The inhomogeneity of the compositions suggests that the joint region contains various intermetallic phases such as Al3Ti or Ti3Sn.
Table 4 EDS analysis of selected regions for bond made with bonding time of 30 min shown in Fig. 4(c) (wt.%)
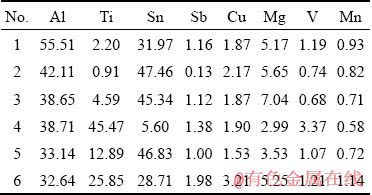
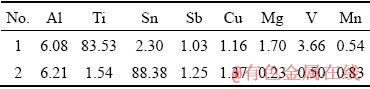
Figure 5 shows the microstructure and elemental distributions of the joint formed at 580 °C for 60 min. The number of dots in each map indicates the presence of a certain element that is related to its concentration. The EDS maps for Ti and V (Figs. 5(b, c)) show that there is a considerable amount of titanium and vanadium in far distance from the interface. When the bonding time is increased from 30 to 60 min, migration of alloying elements between both alloys is more due to the higher diffusivity at longer time. More uniform and complete inter-diffusion of Al and Ti into each other is apparent in comparison with that of the bond made at the bonding time of 30 min. The EDS map, correspond to Sn element (Fig. 5(d)) shows that with increasing bonding time to 60 min, although the interlayer is not completely consumed, but the thickness of remained Babbitt interlayer decreases to approximately 15 μm. The EDS analysis of selected regions in Fig. 5(a) is represented in Table 5. As marked in Fig. 5(a), Region 1 consists mainly of titanium (83.53 wt.%), aluminum (6.08 wt.%) and vanadium (3.66 wt.%). This indicates that the molten tin could diffuse along the grain boundaries of Al alloy. The trace of Ti indicates that with increasing bonding time, the diffusion of Ti toward the interface occurred. The substantial presence of tin in Region 2 (88.38 wt.%) indicates that the low amount of interlayer is likewise remained at the interface after 60 min of bonding time. During bonding process, mating surfaces are deformed plastically which caused waviness at the interface (Fig. 5(a)). The mating interface consists of a thin and less waviness at lower bonding temperature (Fig. 4(a)).
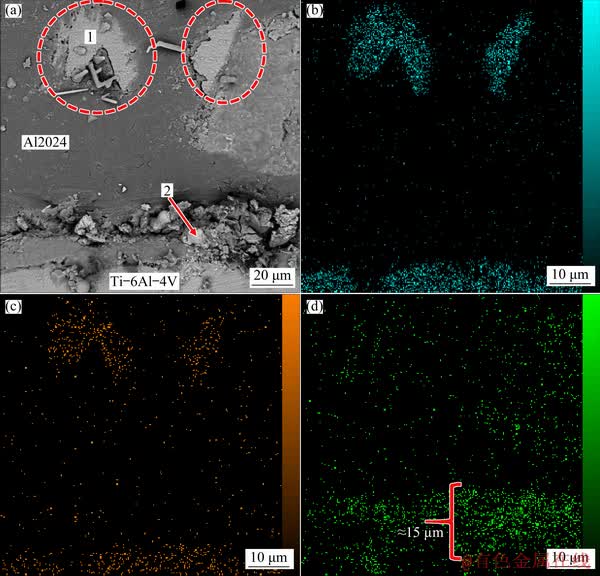
Fig. 5 Microstructure of joint formed at 580 °C for 60 min (a) and elemental distribution of Ti (b), V (c) and Sn (d)
Table 5 EDS analysis of selected regions for bond made for bonding time of 60 min shown in Fig. 5 (wt.%)
3.2 Identification of intermetallic compounds
The EDS results showed that the Al, Ti, Sn, Cu, V and Mg are present in the joint region of the bonds, indicating the probable formation of different intermetallics. In order to prove the formation of various intermetallic compounds in the joint region, XRD analysis was employed. Fracture surfaces of a bond made at 580 °C for 30 and 60 min bonding time were analyzed by XRD and the results are shown in Fig. 6. Microstructure observation together with XRD patterns confirms the existence of Al2Cu, Al2CuMg, Cu3Ti, TiAl3, TiAl and Mg2Sn intermetallic compounds formed in Al weld side. On the other hand, the peak intensities are weak for Al-containing phases (namely Al2Cu, Al2CuMg and TiAl3) at the Ti side because concentration of Al is more in Al 2024 weld side than Ti-6Al-4V side. But Ti3Al, Sn3Ti5 and Ti3Sn intermetallic compounds only formed in Ti side.
3.3 Mechanical characterization of joints
Microhardness profiles of the bonds made for 30 and 60 min are illustrated in Fig. 7. The differences between the hardness values across the joint region for the bonds made at different bonding time can be attributed to the gradual homogenization of the joint region with increasing bonding time. Figure 7 shows that the hardness of the joint at the distance of 100 μm from the center for a bond made for 30 min is HV 124 while it is HV 141 for bond made for 60 min. As bonding time increases, the hardness value of the joint interface increases too. This can be an explanation to the formation of intermetallic compounds at the joint center. The low hardness (HV 49.6) for bond made for 30 min is due to the presence of tin in the joint center.

Fig. 6 XRD patterns of fracture surfaces of Al/Babbitt/Ti bonded at bonding temperature of 580 °C for bonding time of 30 min (a, b) and 60 min (c, d)
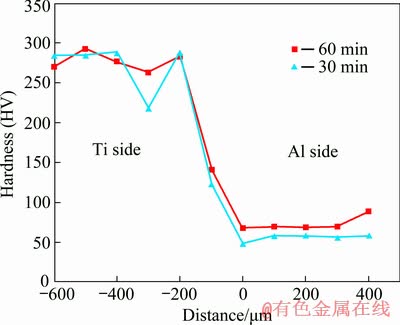
Fig. 7 Microhardness profiles across joint region for bonds made at bonding time of 30 and 60 min
Shear strength of the TLP bonds was determined by the single lap shear test. Illustration of the joint geometry prepared for performing the shear strength tests is shown in Fig. 1. The room temperature shear strength of the TLP bonded joints with the change in bonding time is shown in Fig. 8. In both samples, failure occurred across the bonded interface. Nevertheless, the maximum shear strength of 57 MPa is obtained at 60 min. The shear strength increases with increasing bonding time. The increase in the bond strength was caused by the intermetallic formation at the Ti side of the joint interface. XRD patterns confirm the formation of intermetallic compounds like TiAl, Ti3Al, TiCu3, Sn3Ti5 and Ti3Sn during the bonding process in the joint made for bonding time of 60 min (Fig. 6(d)). On the other hand, there are weak peak intensities of TiAl, Ti3Al, TiCu3, Sn3Ti5 and Ti3Sn for the joint made for bonding time of 30 min (Fig. 6(b)) due to lower diffusion time of Ti and Al in the joint region. The maximum shear strength value recorded in this work is higher than the works that have used Sn-3.6Ag-1Cu, Sn-Ag-Bi, Cu and Sn-Zn-Bi foil interlayers to join Al alloy to Ti alloy [1,2,9].
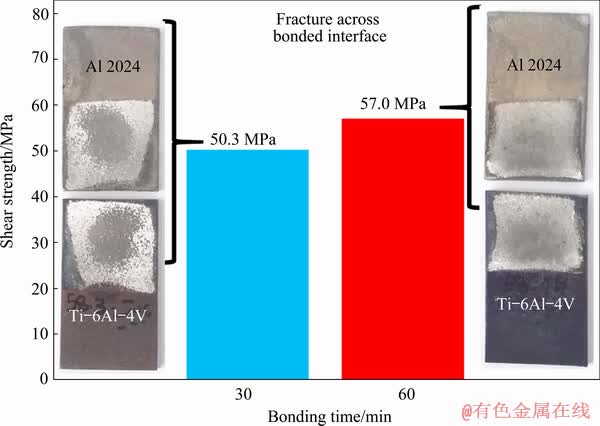
Fig. 8 Results of shear strength as function of bonding time
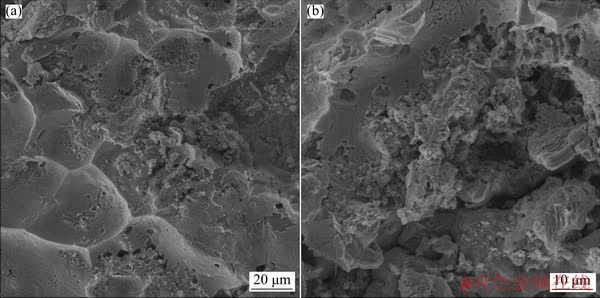
Fig. 9 SEM images of Al 2024 alloy surface (a) and Ti-6Al-4V alloy surface (b) of 60 min bonded-specimen
The fracture surface for Al 2024 and Ti–6Al–4V sides for the bond made for the bonding time of 60 min is illustrated in Fig. 9. Figure 9(a) shows that brittle faceted features indicate brittle fracture mode on the Al 2024 surface. Also, the formation of the voids and the crack propagation occurred along the intermetallic phases which were formed at the bond interface. In both cases, fracture occurs in the diffusion area (area of surface transport), because this area includes brittle intermetallic compounds. Figure 9(b) shows the mixed failure showing brittle and ductile fracture modes simultaneously at the SEM fractograph in Ti side of 60 min-bonded specimen.
4 Conclusions
(1) TLP bonding of Al 2024 alloy to Ti-6Al-4V alloy using Babbitt thermal spray coat as an interlayer was carried out successfully at 580 °C for bonding time of 30 and 60 min.
(2) Thermal spray, creates a rough and clean surface which leads to establishing a joint with higher strength. The maximum shear strength of 57 MPa is obtained at bonding time of 60 min.
(3) More uniform and complete inter-diffusion of Al and Ti into each other took place for the bond made for bonding time of 60 min in comparison with that of the bond made for bonding time of 30 min.
(4) Microstructure observation together with XRD patterns confirms the existence of Al2Cu, Al2CuMg, Cu3Ti, TiAl3, TiAl and Mg2Sn intermetallic compounds formed in Al weld side. On the other hand, Ti3Al, Sn3Ti5 and Ti3Sn intermetallic compounds formed in Ti alloy weld side.
References
[1] ALHAZAA A N, KHAN T I. Diffusion bonding of Al7075 to Ti-6Al-4V using Cu coatings and Sn-3.6Ag-1Cu interlayers [J]. Journal of Alloys and Compounds, 2010, 494: 351-358.
[2] KENEVISI M S, MOUSAVI KHOIE S M. An investigation on microstructure and mechanical properties of Al7075 to Ti-6Al-4V transient liquid phase (TLP) bonded joint [J]. Materials and Design, 2012, 38: 19-25.
[3] HABISCH S, BOHME M, PETER S, GRUND T, MAYR P. The effect of interlayer materials on the joint properties of diffusion-bonded aluminium and magnesium, metals [J]. Metals, 2018, 8: 138. DOI: 10.3390/met8020138.
[4] SAMAVATIAN M, HALVAEE A, AMADEH A, KHODABANDEH A. An investigation on microstructure evolution and mechanical properties during liquid state diffusion bonding of Al2024 to Ti-6Al-4V [J]. Materials Characterization, 2014, 98: 113-118.
[5] PRESCOTT R, GRAHAM M J. Formation of aluminum oxide scales on high-temperature alloys [J]. Oxidation of Metals, 1992, 38: 233-254.
[6] ANBARZADEH A, SABET H, ABBASI M. Effects of successive-stage transient liquid phase (S-TLP) on microstructure and mechanical properties of Al2024 to Ti-6Al-4V joint [J]. Materials Letters, 2016, 178: 280-283.
[7] KENEVISI M S, MOUSAVI KHOIE S M, ALAEI M. Microstructural evaluation and mechanical properties of the diffusion bonded Al/Ti alloys joint [J]. Mechanics of Materials, 2013, 64: 69-75.
[8] SAMAVATIAN M, HALVAEE A, AMADEH A, KHODABANDEH A. Transient liquid phase bonding of Al 2024 to Ti-6Al-4V alloy using Cu-Zn interlayer [J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 770-775.
[9] ALHAZAA A N, KHAN T I, HAQ I. Transient liquid phase (TLP) bonding of Al7075 to Ti-6Al-4V alloy [J]. Materials Characterization, 2010, 61: 312-317.
[10] CHEN X, YAN J, REN S, WEI J, WANG Q. Microstructure and mechanical properties of Ti–6Al–4V/Al1060 joints by ultrasonic-assisted brazing in air [J]. Materials Letters, 2013, 95: 197-200.
[11] VIANCO P, REJENT J, GRANT R. Development of Sn-based, low melting temperature Pb-free solder alloys [J]. Materials Transactions, 2004, 45: 765–75.
[12] DAVOODI A, SALIMIJAZI H, EDRIS H, MOSTAGHIMI J. Study of TLP bonding of Ti-6Al-4V alloy produced by vacuum plasma spray forming and forging [J]. Materials & Design, 2017, 121: 355-366.
[13] JUNIOR P R, PUKASIEWICZ A G, Evaluation of microstructure, mechanical and tribological properties of a Babbitt alloy deposited by arc and flame spray processes [J]. Tribology International, 2018, 131: 148-157.
[14] ASTM standard D1002. Standard test method for apparent shear strength of single-lap-joint adhesively bonded metal specimens by tension loading (metal-to-metal) [S]. 1999.
[15] JAFARIAN M, SABOKTAKIN RIZI M, HONARMAND M, JAVADINEJAD H R, GHAHERI A, BAHRAMIPOUR M T, EBRAHIMHAN M, Effect of thermal tempering on microstructure and mechanical properties of Mg-AZ31/ Al-6061 diffusion bonding [J]. Materials Science and Engineering A, 2016, 666: 372–379.
[16] FERNANDUS M J, SENTHILKUMAR T, BALASUBRAMANIA V, Developing temperature-time and pressure-time diagrams for diffusion bonding AZ80 magnesium and AA6061 aluminium alloys [J]. Materials & Design, 2011, 32: 1651–1656.
热喷涂巴氏合金中间层瞬间液相连接Ti-6Al-4V和Al 2024合金
H. NAEIMIAN, M. A. MOFID
Department of Petroleum, Mining and Material Engineering, Central Tehran Branch, Islamic Azad University, Tehran, Iran
摘 要:研究热喷涂辅助瞬间液相(TLP)扩散连接Ti-6Al-4V和 Al 2024合金,在铝基体上热喷涂80 μm厚的巴氏合金作为中间层。热喷涂会产生粗糙清洁的表面,使得接头强度更高。采用的优化参数为:连接温度580 °C,保温时间30和60 min。显微组织观察和XRD衍射谱证明在Al焊缝处形成Al2Cu、 Al2CuMg、Cu3Ti、TiAl3、TiAl和Mg2Sn 金属间化合物。另一方面,在Ti合金一侧形成Ti3Al、Sn3Ti5 和Ti3Sn金属间化合物。随着连接时间从30 min增加到60 min,尽管巴氏合金中间层没有被完全消耗,但是其剩余厚度下降到大约15 μm。研究表明,在60 min较长连接时间下,接头的剪切强度达到57 MPa的较高值。
关键词:瞬间液相;Al 2024合金;Ti-6Al-4V合金;热喷涂;剪切强度
(Edited by Xiang-qun LI)
Corresponding author: M. A. MOFID; Tel/Fax: +98-2166400079; E-mail: moh.ammar_mofid@iauctb.ac.ir
DOI: 10.1016/S1003-6326(20)65294-3
Abstract: Thermal spray assisted transient liquid phase (TLP) bonding of Ti-6Al-4V to Al2024 alloys was investigated, where the interlayer was 80 μm Babbitt thermal spray coat on Al substrate. Thermal spray creates a rough and clean surface which leads to establishing a joint with higher strength. The optimized parameters were bonding temperature of 580 °C and bonding time of 30 and 60 min. Microstructural observation together with XRD patterns confirmed the existence of Al2Cu, Al2CuMg, Cu3Ti, TiAl3, TiAl and Mg2Sn intermetallic compounds formed in Al weld side. On the other hand, Ti3Al, Sn3Ti5 and Ti3Sn intermetallic compounds formed in Ti side. With increasing bonding time from 30 to 60 min, although the interlayer was not completely consumed, the thickness of remained Babbitt interlayer decreased to approximately 15 μm. The study showed that shear strength of the joint reaches the high value of 57 MPa obtained at larger bonding time of 60 min.


