DOI: 10.11817/j.ysxb.1004.0609.2021-36552
不同纳米碳材料增强镁基复合材料的显微组织与力学性能
袁秋红,周国华,廖 琳,晏旭辉,彭路南,马广祥,杨春材,王启儒
(宜春学院 物理科学与工程技术学院,宜春336000)
摘 要:
以AZ91合金为基体,采用液态分散技术+粉末冶金工艺+热处理工艺制备了四种纳米碳材料(碳纳米管、包覆氧化镁碳纳米管、石墨烯纳米片和氧化石墨烯)增强的镁基复合材料;测试了复合材料的力学性能,并利用光学显微镜、X射线衍射仪、扫描电子显微镜、透射电子显微镜等对复合材料微观组织、界面结构和断口形貌进行了表征及分析。结果表明:制备的四种复合材料料中,氧化石墨烯增强的镁基复合材料屈服强度和伸长率最好,分别为(312±4.5) MPa和11.3%±0.2%,比AZ91基体分别提高了85.7%和61.4%,表明四种纳米碳材料增强体中,氧化石墨烯更有益于提高镁合金的力学性能,有利于制备高性能镁基复合材料。
关键词:
文章编号:1004-0609(2021)-01-0030-12 中图分类号:TG146.2;TB333 文献标志码:A
引文格式:袁秋红, 周国华, 廖 琳, 等. 不同纳米碳材料增强镁基复合材料的显微组织与力学性能[J]. 中国有色金属学报, 2021, 31(1): 30-41. DOI: 10.11817/j.ysxb.1004.0609.2021-36552
YUAN Qiu-hong, ZHOU Guo-hua, LIAO Lin, et al. Microstructures and mechanical properties of Mg-based composites reinforced with different nano-carbon materials[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(1): 30-41. DOI: 10.11817/j.ysxb.1004.0609.2021-36552
碳纳米管(Carbon nanotubes, CNTs)和石墨烯(Graphene, GN)是纳米碳材料的明星成员,它们具有超强的力学性能、优异的导热导电等性能[1-3],其拉伸强度超过50 GPa,弹性模量在1 TPa以上[4-5],是复合材料优良的增强体[6-7]。镁合金,密度小,比强度、比刚度高,是一种潜力巨大的金属结构材料[8-9]。然而,镁合金强度低、塑性和耐腐蚀性差等缺点,严重制约了其在工程领域中的广泛应用。在镁基体中添加力学性能优异的增强体,如碳纳米管、石墨烯,能有效提高镁基体的强度、塑性等力学性能[10-12],是目前镁基复合材料研究热点之一。碳纳米管和石墨烯都是纳米级碳材料,当添加到镁基体中,制备镁基复合材料过程中,都面临着两大难题[13-15]:一是两种纳米碳材料比表面积大,导致它们极难均匀分散到镁基体中;二是碳材料与众多金属材料不浸润,致使碳增强体与镁基体的界面结合较弱。
据文献报道[16-18],纳米氧化镁可有效细化镁基体组织、改善纳米碳材料与镁基体的界面结合质量,进而提高镁合金的综合力学性能。在碳纳米管表面包覆MgO纳米颗粒,可有效提高碳纳米管与镁基体的界面结合强度,改善镁合金的力学性能[19]。该复合材料中,纳米MgO是通过直接在镁基体中添加包覆MgO碳管,进而实现碳管与镁基体良好界面的形成。以氧化石墨烯(Graphene oxide, GO)为起始增强相,借助其丰富的含氧官能团与镁基体发生界面反应可实现MgO的原位自生成,能有效提高石墨烯与镁基体的界面结合质量,获得力学性能更优异的镁基复合材料[20]。以上工艺都是借助MgO的桥接作用实现纳米碳材料(碳纳米管/石墨烯)与镁基体的界面结合质量的提高,进而提高镁合金的力学性能。然而,增强体的尺度、形状不同,对镁基复合材料的增强效果差别很大。碳纳米管是一维结构纳米碳材料,而石墨烯则是二维结构纳米碳材料,两者的尺寸、形态等都差异较大,添加到镁基体中,其增强效果差异较大。目前,不同结构纳米碳材料及其改性材料对镁基复合材料的组织和力学性能的影响差异等相关报道还非常少。鉴于此,结合纳米碳材料增强镁基复合材料目前存在的工艺难题,本文采用碳纳米管、包覆氧化镁碳纳米管(MgO-coated CNTs, MgO@CNTs)、石墨烯纳米片(Graphene nanosheets, GNS)和氧化石墨烯为增强体,制备了四种纳米碳材料增强的镁基复合材料。对比分析了四种纳米碳材料对复合材料组织和力学性能的影响差异,为制备高性能纳米碳增强镁基复合材料提供新思路。
1 实验
1.1 实验材料
基体材料选用粒度小于48 mm的商用AZ91镁合金粉。增强体为碳纳米管、包覆氧化镁碳纳米管、石墨烯纳米片和氧化石墨烯,如图1所示。图1(a)所示为碳纳米管的微观形貌,其直径为5~30 nm。图1(b)显示许多纳米颗粒包覆在碳纳米管表面,结合能谱图(见图1(c))可确定,这些纳米颗粒为氧化镁纳米颗粒,其包覆工艺可参考前期研究工作。图1(d)、(e)、(e′)和(f)、(g)、(g′)分别为GNS和GO的微观形貌,图中均可观察到片状、褶皱形貌的GNS和GO,线条A和线条B分别是它们的厚度测量值,表明GNS厚度为5 nm,约为15层(单层石墨烯厚度为0.335 nm[21]),GO厚度为1.2 nm,约为1~2层(单层GO厚度为0.6~1.2 nm)。
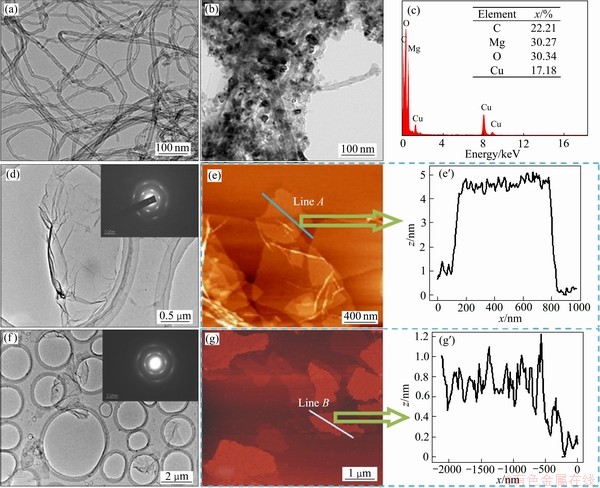
图1 碳纳米管和包覆氧化镁碳管TEM像、包覆氧化镁碳管能谱图、石墨烯纳米片和氧化石墨烯TEM和AFM形貌
Fig. 1 TEM images of CNTs (a) and MgO@CNTs (b), EDX results of MgO@CNTs (c), TEM and AFM images of GNS ((d), (e), (e′)) and GO ((f), (g), (g′))
1.2 复合材料制备工艺
纳米碳-镁基复合材料的制备工艺分为以下三步进行。
1.2.1 液态分散
将一定量纳米碳材料添加到适量的无水乙醇溶液中,经超声分散处理,获得分散状态较好的纳米碳材料乙醇悬浮液。同时,在真空手套箱中称取一定量AZ91镁合金粉添加到乙醇中形成混合浆液。然后,将上述两种溶液混合在一起,并经超声处理+机械搅拌得到AZ91镁合金粉-纳米碳材料混合浆料。最后,经过滤和真空干燥获得AZ91镁合金粉-纳米碳材料混合粉末。
1.2.2 冷压和烧结
将上述AZ91镁合金粉-纳米碳材料复合粉体与适量的硬脂酸(0.5%)混合均匀后,装入到d 40 mm、高200 mm的模具中,预压后连同模具一起从真空手套箱中取出,再在液压机上进行冷压成型,获得复合材料生坯。将复合材料生坯放入电阻炉中,在氩气气氛保护下600 ℃烧结2 h,得到烧结态复合材料,如图2(a)所示。
1.2.3 热挤压+热处理
将烧结态复合材料用铝箔纸包好,放入电阻炉中,413 ℃条件下,保温5 h进行均匀化处理后,在型号为HM035-200的四柱液压机上进行热挤压成型获得挤压态复合材料,如图2(b)所示。热挤压温度、挤压速率和挤压比分别为:380 ℃、0.3 mm/s和10:1。最后,将该复合材料在413 ℃条件下,保温18 h进行T4固溶处理。
采用上述工艺,本文制备了CNTs、MgO@ CNTs、GNS和GO含量(质量分数)分别为3.0%、3.0%、0.5%和0.5%的四种AZ91镁合金复合材料。将复合材料加工成直径为d 7 mm,标距为35 mm的拉伸试样(见图2(c)),并采用万能材料试验机(TXYA105C)测试复合材料的室温力学性能。利用光学显微镜(MA200)、X射线衍射仪(D8SOCur)、扫描电子显微镜(ZEISS ΣIGMA)、透射电子显微镜(JEM-2100)和能谱仪对复合材料显微组织、界面结构和断口形貌进行了表征与分析。
2 结果与分析
2.1 分散性分析
图3所示为AZ91合金粉与四种纳米碳材料混合后的SEM像。从图3(a)和(b)中可观察到“根须状”CNTs(MgO@CNTs)和团聚的CNTs(MgO@ CNTs)分布在合金粉颗粒表面。对比图3(a)和(b)发现,CNTs的团聚更为严重,表明包覆MgO碳纳米管具有更好的分散性。由图3(c)可见,GNS一部分分散在镁合金粉颗粒表面,一部分则散落在合金粉颗粒之间,分散效果较好。由图3(d)可见,GO则几乎都包覆在镁合金粉颗粒表面,其分散效果非常好。图3(c)和(d)中合金粉颗粒表面或颗粒间都未观察到团聚的GNS或GO,表明GNS/GO比CNTs和MgO@CNTs具有更好的分散性。此外,对比图3(c)和(d)发现,GO基本包覆在合金粉颗粒表面,而GNS则一部分吸附在合金粉颗粒表面,另一部分则分布在合金粉颗粒间,表明GO在镁合金粉中的分散性更好。

图2 复合材料实物图
Fig. 2 Photographs of as-sintered(a), as-extruded(b) and tensile specimen composites(c)
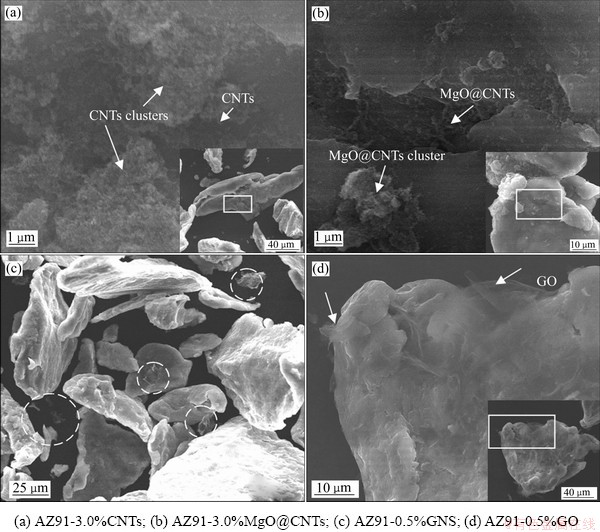
图3 复合材料混合粉末SEM像
Fig. 3 SEM images of mixed composites powders
CNTs具有较高的表面能,使其极容易团聚[22],当经过表明包覆MgO处理后,其表面能有所降低,其分散性得到一定程度的改善。与GNS相比,GO因含有丰富的含氧官能团,使其极容易在水或乙醇中剥离成单层或少层氧化石墨烯,进而与合金粉形成非常均匀的分散[23]。因此,与GNS相比,GO具有更好的分散性。
2.2 复合材料显微组织
图4所示为复合材料的金相组织和晶粒尺寸分布图。由图4可见,AZ91合金、AZ91-3.0% CNTs、AZ91-3.0%MgO@CNTs、AZ91-0.5%GNS和AZ91-0.5%GO复合材料的平均晶粒尺寸分别为(41.6±3.5) μm、(32.8±2.6) μm、(24.4±1.5) μm、(27.8±5) μm和(22.4±2) μm。在镁熔体凝固过程中,碳纳米管、石墨烯等纳米材料,一方面可形作为异质形核颗粒,提高了形核率[24];另一方面,分布较均匀的纳米碳材料能有效地阻碍镁晶粒的长大[25],两者的共同作用有效细化了镁基体的晶粒组织。对比四种纳米碳材料发现,GO的晶粒细化效果最好,其次是MgO@CNTs,再次是GNS,晶粒细化效果最差的是CNTs。据报道,MgO纳米颗粒可与镁基体形成半共格界面,进而降低了形核能,提高晶粒细化效果[17]。GO具有优异的分散性,当添加到镁基体中,其含氧官能团与镁基体发反应,生成了界面产物MgO,进而有效细化了镁基体晶粒组织[20]。CNTs经表面包覆MgO改性后,由于MgO的存在,其晶粒细化效果得到进一步的改善。与CNTs相比,GO为二维结构纳米碳材料且其平面尺寸为微米级别,使其在阻碍镁晶粒长大的作用效果更显著。因此,相比其他三种纳米碳材料,GO对镁基体的晶粒细化效果最好。
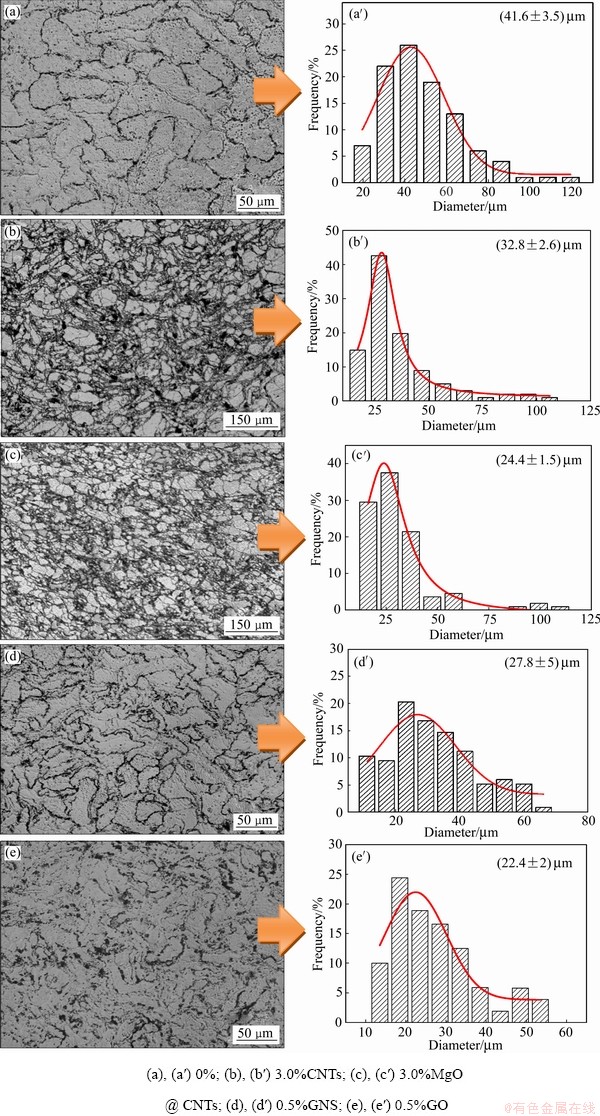
图4 AZ91复合材料显微组织与晶粒分布图
Fig. 4 Microstructures and grain size distributions of AZ91 composites
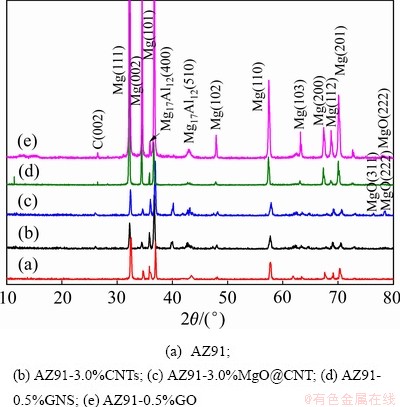
图5 AZ91复合材料XRD谱
Fig. 5 XRD patterns of AZ91 composites
图5所示为AZ91复合材料的XRD谱。由图5可见,基体材料主要由α-Mg和β-Mg17Al12相组成。当加入纳米碳材料后,四种复合材料均观察到了微弱的C(002)特征峰,表明四种复合材料中都含有纳米碳材料(碳纳米管/石墨烯)。此外,在AZ91-3.0%MgO@CNTs和AZ91-0.5%GO复合材料中还可以观察到微弱的MgO特征峰,表明两种复合材料中都含有MgO。
2.3 四种复合材料界面结构对比分析
图6所示为AZ91-3.0%MgO@CNTs[19]、AZ91- 0.5%GO[20]和AZ91-0.5%GNS复合材料的TEM像。由图6(a)~(c)可见,AZ91-0.3%MgO@CNTs和AZ91- 0.5%GO两种复合材料的界面上存在MgO纳米颗粒(见虚线圆所示),这与XRD分析的结果相吻合。由图6(d)可见,AZ91-0.5%GNS复合材料的GNS/α- Mg界面、干净、整洁且清晰。
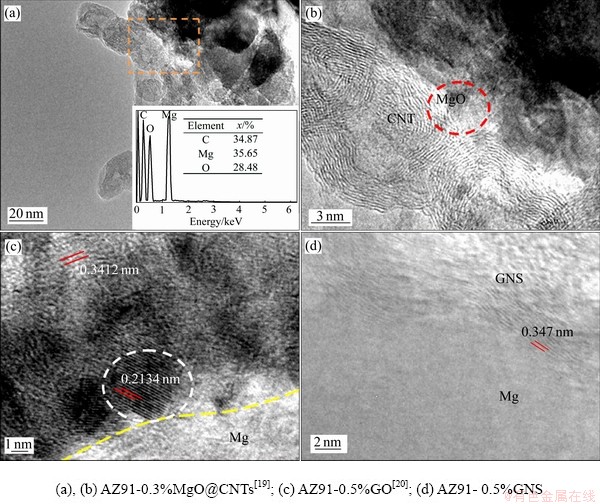
图6 AZ91复合材料TEM像
Fig. 6 TEM images of AZ91 composites
前期研究发现:CNTs/α-Mg界面为非共格界面结合,界面结合较弱。在MgO@CNTs增强的复合材料中,由于CNTs与MgO则形成了纳米级接触界面和扩散结合界面两种较强的结合界面,MgO与镁基体形成了半共格界面,使得CNTs与镁基体界面结合较强[19]。在GO增强的复合材料中,由于GO含氧官能团(—COOH,—OH和C—O—C)与Mg发生界面反应,形成了C—O—Mg化学键和MgO/α-Mg半共格界面,使石墨烯与镁基体形成强界面结合[20]。与CNTs相比,GNS为二维平面状结构且平面尺寸达到了微米级别,与镁基体的接触面积较大。因此,与CNTs/α-Mg界面相比,GNS/α-Mg界面结合更好。同样,与MgO@CNTs相比,GO与镁基体的界面更强,这与该复合材料具有更高力学性能相吻合。
综上可知,四种纳米碳材料(CNTs/MgO@ CNTs/GNS/GO)中,GO与镁的界面结合质量最好,与AZ91-0.5%GO复合材料具有最高的强度和塑性相吻合。
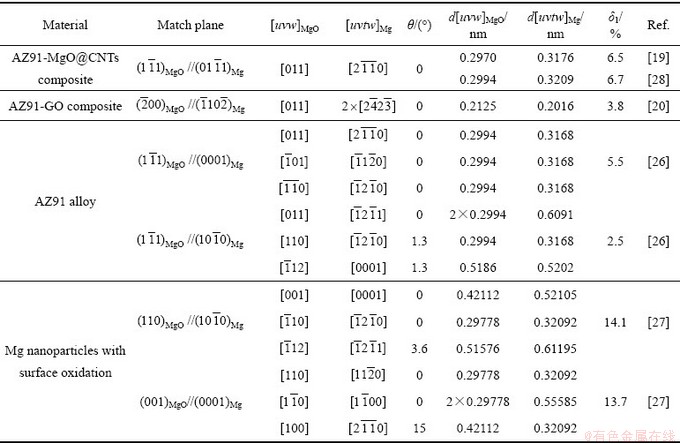
为探明复合材料中MgO/α-Mg界面特征,表1列出了目前MgO/α-Mg界面的取向关系的相关实验结果。由表1可见,AZ91-3.0%MgO@CNTs复合材料中,MgO/α-Mg界面的晶体取向关系为 //
//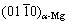 和[011]MgO//
和[011]MgO// ,其原子错配度为6.5%;沿着密排方向[011]MgO(或
,其原子错配度为6.5%;沿着密排方向[011]MgO(或 ),Mg
),Mg 与MgO
与MgO 晶面形成了半共格结合界面。AZ91-0.5%GO复合材料中,MgO/α-Mg界面的晶体取向关系为
晶面形成了半共格结合界面。AZ91-0.5%GO复合材料中,MgO/α-Mg界面的晶体取向关系为 //
// 和[011]MgO//
和[011]MgO// ,其晶格错配度为3.8%;沿着密排方向 ([011]MgO或
,其晶格错配度为3.8%;沿着密排方向 ([011]MgO或 ),Mg
),Mg 与MgO
与MgO 晶面形成了半共格结合界面。
晶面形成了半共格结合界面。
WANG等[26]采用强烈剪切变形处理技术制备的AZ91合金中,发现界面MgO/α-Mg存在两种晶体取向关系(见表1):1)  //
//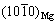 和[011] MgO//
和[011] MgO//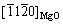 ,原子错配度为2.5%。2)
,原子错配度为2.5%。2)  //(0001)Mg和
//(0001)Mg和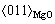 //
//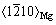 ,原子错配度为5.5%,两者均为半共格结合界面。
,原子错配度为5.5%,两者均为半共格结合界面。
KOOI等[27]对Mg纳米颗粒进行表面氧化后,发现界面MgO/α-Mg存在两种晶体取向关系(见表1):1) (110)MgO//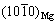 和
和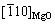 //
// ,原子错配度为14.1%。2) (001)MgO//(0001)Mg和[110]MgO//
,原子错配度为14.1%。2) (001)MgO//(0001)Mg和[110]MgO// ,原子错配度为13.7%。两者均为非共格结合界面。
,原子错配度为13.7%。两者均为非共格结合界面。
综上分析可知,MgO/α-Mg界面存在多种晶体取向关系,其形成与材料的成形工艺密切相关。AZ91-0.5%GO复合材料中,GO上的含氧官能团与镁发生了界面反应,生成的界面产物MgO与镁基体形成半共格结合界面,而AZ91-MgO@CNTs复合材料中,则不存在这一特征,两种复合材料MgO与α-Mg的界面形成条件完全不同,其晶体取向关系存在较大差异。
表1 MgO/α-Mg界面晶体取向关系及其晶格错配度
Table 1 Orientation relationship of MgO/α-Mg interface and its lattice disregistries
2.4 复合材料力学性能分析
图7所示为四种复合材料的强度(屈服强度YS/抗拉强度UTS)和伸长率对比图,相关数据如表2所示。同时,表2中给出了其他镁基复合材料的力学性能。图7和表2显示,AZ91基体、AZ91-3.0% CNTs、AZ91-3.0%MgO@CNTs、AZ91-0.5%GNS和AZ91-0.5%GO复合材料的屈服强度分别为(168±5.0) MPa、(312±4.5) MPa,(296±3.7) MPa,(284±4.6) MPa 和(250±3.8) MPa,对应伸长率则分别为(7.0±0.2)%、(9.4±0.1)%、(8.6±0.1)%、(8.7±0.1)%和(11.3±0.2)%。AZ91-0.5%GO复合材料的强度和塑性最好,表明与其他三种纳米碳材料相比,GO的强化效果最好。
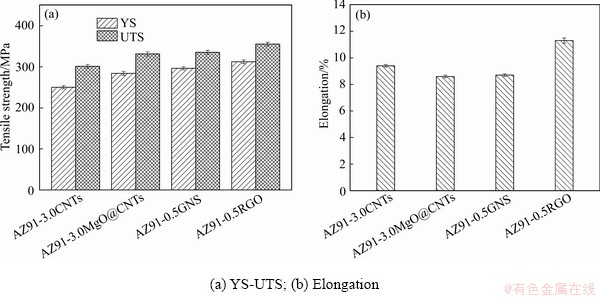
图7 复合材料力学性能
Fig. 7 Mechanical properties of composites
表2 镁基复合材料力学性能对比
Table 2 Comparison in mechanical properties of Mg-based composites
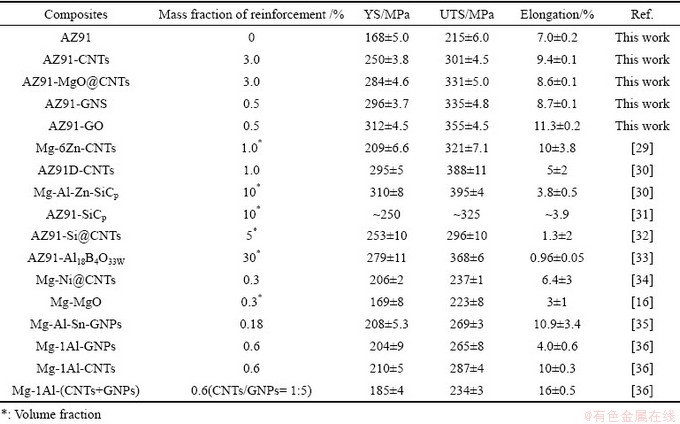
综上分析可知,采用粉末冶金+热挤压+T4固溶处理,以GO为起始增强相制备的AZ91-RGO镁基复合材料展现出优异的强韧性。GO因具有优异的分散性且与镁基体形成强界面结合,将有可能替代CNTs和GNS制备出性能更优异的纳米碳/镁基复合材料。
如图8所示,颗粒或纤维增强的镁基复合材料具有较高的强度,但塑性性能往往较差。纳米碳材料增强的镁基复合材料则具有较好的强度和塑性性能,特别是GO增强的镁基复合材料,要高于其他镁基复合材料的屈服强度和塑性,表明GO具有更好的强化效果。
2.5 复合材料拉伸断口形貌
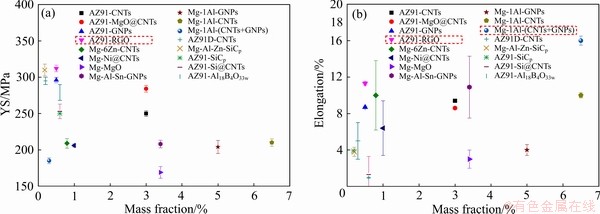
图8 复合材料屈服强度和伸长率度对比
Fig. 8 Comparison of yield strength (a) and elongation (b) of composites

图9 复合材料断口SEM像[19-20]
Fig. 9 SEM images of fracture surfaces of composites[19-20]
图9所示为复合材料断口SEM像。由图9(a)可见,裂纹处存在被拔出的碳纳米管(见粗虚箭头),表明碳纳米管与镁基体的界面结合不牢固,碳管的强化效果有限。由图9(b)可见,复合材料裂纹处则可观察到被拉断的碳纳米管,表明包覆MgO碳管与镁基体的界面结合较紧密,应力转移强化效果较好,复合材料力学性能更高。由图9(c)可见,断口形貌上分布着较多的微观裂纹,其原因是实验用石墨烯纳米片层数较多,片层之间结合力弱(范德华力),导致拉伸过程中,裂纹极容易在石墨烯纳米片层之间萌生,并迅速扩展而形成微裂纹。图9(d)中,断口形貌几乎未观察到微裂纹的存在,其原因是实验用氧化石墨烯较薄,层数较少,且与镁基体反应生成的MgO有效的提高了界面结合质量,其强化效果较好。
3 结论
1) 四种复合材料中,AZ91-0.5%GO复合材料的屈服强度和伸长率最高,分别为(312±4.5) MPa和11.3%±0.2%,表明GO的强化效果最好。
2) 纳米MgO可与镁基体形成半共格界面,能有效提高纳米碳材料与镁基体之间的界面结合质量,有利于提高复合材料的综合力学性能。
3) 与GNS、MgO@CNTs和CNTs相比,采用GO为起始增强更有利于提高镁合金的强度和塑性,有利于制备高性能镁基复合材料。
REFERENCES
[1] ZHU H, SUHR J, MA R. An overview of two dimensional materials[J]. Journal of Materiomics, 2018, 4(2): 81-82.
[2] KUILA T, BOSE S, MISHRA A K, et al. Chemical functionalization of graphene and its applications[J]. Progress in Materials Science, 2012, 57(7): 1061-1105.
[3] BALANDIN A A. Thermal properties of graphene and nanostructured carbon materials[J]. Nature Materials, 2011, 10(8): 569-581.
[4] FALKOVSKY L. Physical properties of graphene[J]. Uspekhi Fizicheskikh Nauk, 2012, 182(11): 1223-1234.
[5] SALVETAT J P, BRIGGS G A D, BONARD J M, et al. Elastic and shear moduli of single-walled carbon nanotube ropes[J]. Physical Review Letters, 1999, 82(5): 944.
[6] HUANG X, QI X, BOEY F, et al. Graphene-based composites[J]. Chemical Society Reviews, 2012, 41(2): 666-686.
[7] 何 阳, 袁秋红, 罗 岚, 等. 镁基复合材料研究进展及新思路[J]. 航空材料学报, 2018, 38(4): 26-36.
HE Yang, YUAN Qiu-hong, LUO Lan, et al. Current study and novel ideas on magnesium matrix composites[J]. Journal of Aeronautical Materials, 2018, 38(4): 26-36.
[8] FRIEDRICH H E, MORDIKE B L. Magnesium technology[M]. Berlin: Springer-Verlag, 2006.
[9] FU D M, YUAN Q H, ZENG X S, et al. Fabrication of carbon nanotube reinforced AZ91D composite with superior mechanical properties[J]. Transactions of Nonferrous Metals Society of China, 2017, 27(8): 1716-1724.
[10] LI Z, ZHANG C, YE Y, et al. Plastic deformation mechanism of MWCNTs/AZ91D nanocomposites with high ductility at room temperature[J]. Materials Characterization, 2020, 159: 110020.
[11] VAHEDI F, ZAREI-HANZAKI A, SALANDARI-RABORI A, et al. Microstructural evolution and mechanical properties of thermomechanically processed AZ31 magnesium alloy reinforced by micro-graphite and nano-graphene particles[J]. Journal of Alloys and Compounds, 2020, 815: 152231.
[12] XIANG Y, WANG X, HU X, et al. Achieving ultra-high strengthening and toughening efficiency in carbon nanotubes/magnesium composites via constructing micro-nano layered structure[J]. Composites Part A: Applied Science and Manufacturing, 2019, 119: 225-234.
[13] YUAN Q, ZENG X, WANG Y, et al. Microstructure and mechanical properties of Mg-4.0Zn alloy reinforced by NiO-coated CNTs[J]. Journal of Materials Science & Technology, 2017, 33(5): 452-460.
[14] YUAN Q H, HUANG H Q, WANG W C, et al. Achieving high stability of MgO/carbon nanotube interface via the co-deposition technique[J]. Journal of Alloys and Compounds, 2020, 824: 153889.
[15] 袁秋红, 曾效舒, 刘 勇, 等. 碳纳米管增强镁基复合材料弹性模量的研究进展[J], 中国有色金属学报, 2015, 25(1): 86-97.
YUAN Qiu-hong, ZENG Xiao-shu, LIU Yong, et al. Research progress of elastic modulus of magnesium matrix composite reinforced by carbon nanotubes[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(1): 86-97.
[16] GOH C, GUPTA M, WEI J, et al. Characterization of high performance Mg/MgO nanocomposites[J]. Journal of Composite Materials, 2007, 41(19): 2325-2335.
[17] FAN Z, WANG Y, XIA M, et al. Enhanced heterogeneous nucleation in AZ91D alloy by intensive melt shearing[J]. Acta Materialia, 2009, 57(16): 4891-4901.
[18] RAMEZANZADE S, EBRAHIMI G R, TORABI PARIZI M, et al. Synergetic effect of GNPs and MgOs on the mechanical properties of Mg-Sr-Ca alloy[J]. Materials Science and Engineering A, 2019, 761: 138025.
[19] YUAN Q H, ZENG X S, LIU Y, et al. Microstructure and mechanical properties of AZ91 alloy reinforced by carbon nanotubes coated with MgO[J]. Carbon, 2016, 96: 843-855.
[20] YUAN Q H, QIU Z Q, ZHOU G H, et al. Interfacial design and strengthening mechanisms of AZ91 alloy reinforced with in-situ reduced graphene oxide[J]. Materials Characterization, 2018, 138: 215-228.
[21] 杨永岗, 陈成猛, 温月芳, 等. 氧化石墨烯及其与聚合物的复合[J]. 新型炭材料, 2008, 23(3): 193-200.
YANG Yong-gang, CHEN Cheng-meng, WENYue-fang, et al. Oxidized graphene and graphene based polymer composite[J]. New Carbon Materials, 2008, 23(3): 193-200.
[22] LI H, KANG J, HE C, et al. Mechanical properties and interfacial analysis of aluminum matrix composites reinforced by carbon nanotubes with diverse structures[J]. Materials Science and Engineering A, 2013, 577: 120-124.
[23] GAO X, YUE H, GUO E, et al. Preparation and tensile properties of homogeneously dispersed graphene reinforced aluminum matrix composites[J]. Materials & Design, 2016, 94: 54-60.
[24] 袁秋红, 周国华, 廖 琳. 石墨烯纳米片/AZ91镁基复合材料的显微组织与力学性能[J]. 材料导报, 2018, 32(10): 1663-1667.
YUAN Qiu-hong, ZHOU Guo-hua, LIAO Lin. Microstructure and mechanical properties of graphene nanosheets reinforced AZ91 alloy matrix composite[J]. Materials Reports, 2018, 32(10): 1663-1667.
[25] XIANG S, WANG X, GUPTA M, et al. Graphene nanoplatelets induced heterogeneous bimodal structural magnesium matrix composites with enhanced mechanical properties[J]. Scientific Reports, 2016, 6: 38824.
[26] WANG Y, FAN Z, ZHOU X, THOMPSON G. Characterisation of magnesium oxide and its interface with α-Mg in Mg-Al-based alloys[J]. Philosophical Magazine Letters, 2011, 91: 516-29
[27] KOOI B J, PALASANTZAS G, DE HOSSON J T M. Gas-phase synthesis of magnesium nanoparticles: A high-resolution transmission electron microscopy study[J]. Applied Physics Letters, 2006, 89(16): 161914.
[28] JCPDS. International Centre for Diffraction Data[DB]. PCDFWIN V. 2.3, 2002.
[29] LI C D, WANG X J, LIU W Q, et al. Microstructure and strengthening mechanism of carbon nanotubes reinforced magnesium matrix composite[J]. Materials Science and Engineering A, 2014, 597: 264-269.
[30] SHIMIZU Y, MIKI S, SOGA T, et al. Multi-walled carbon nanotube-reinforced magnesium alloy composites[J]. Scripta Materialia, 2008, 58(4): 267-270.
[31] WANG X J, XU L, HU X S, et al. Influences of extrusion parameters on microstructure and mechanical properties of particulate reinforced magnesium matrix composites[J]. Materials Science and Engineering A, 2011, 528(21): 6387-6392.
[32] PARK Y, CHO K, PARK I, et al. Fabrication and mechanical properties of magnesium matrix composite reinforced with Si coated carbon nanotubes[J]. Procedia Engineering, 2011, 10: 1446-1450.
[33] ZHENG M Y, WU K, LIANG M, et al. The effect of thermal exposure on the interface and mechanical properties of Al18B4O33w/AZ91 magnesium matrix composite[J]. Materials Science and Engineering A, 2004, 372(1/2): 66-74.
[34] NAI M H, WEI J, GUPTA M. Interface tailoring to enhance mechanical properties of carbon nanotube reinforced magnesium composites[J]. Materials & Design, 2014, 60: 490-495.
[35] RASHAD M, PAN F, ASIF M, et al. Powder metallurgy of Mg-1%Al-1%Sn alloy reinforced with low content of graphene nanoplatelets (GNPs)[J]. Journal of Industrial and Engineering Chemistry, 2014, 20(6): 4250-4255.
[36] RASHAD M, PAN F, TANG A, et al. Synergetic effect of graphene nanoplatelets (GNPs) and multi-walled carbon nanotube (MW-CNTs) on mechanical properties of pure magnesium[J]. Journal of Alloys and Compounds, 2014, 603: 111-118.
Microstructures and mechanical properties of Mg-based composites reinforced with different nano-carbon materials
YUAN Qiu-hong, ZHOU Guo-hua, LIAO Lin, YAN Xu-hui, PENG Lu-lan, MA Guang-xiang, YANG Chun-cai, WANG Qi-ru
(Physical Science and Technology College, Yichun University, Yichun 336000, China)
Abstract: Mg-based composites, using AZ91 alloy as matrix and reinforced with four kinds of nano-carbon materials (CNTs, MgO-coated CNT, GNS and GO), were fabricated by liquid dispersion technique, powder metallurgy process and T4 heat treatment, respectively. The mechanical properties of the composites were tested. The microstructure, interface structure and fractographs of the composites were characterized and via optical microscopy, X-ray diffraction (XRD), transmission electron microscopy (TEM) and scanning electron microscopy (SEM) equipped with energy-dispersive X-ray spectroscopy (EDX). The results show that the composite with GO exhibits the best mechanical properties among the four as-synthesized composites. The yield strength and elongation of the composite with GO are (312±4.5) MPa and 11.3%±0.2%, which are enhanced by 85.7% and 61.4%, respectively, comparing with AZ91 alloy. It means that GO is the best reinforcement among the four nano-carbon materials to strengthen the mechanical properties of AZ91 alloy, which is beneficial to fabricate the Mg-based composites with high performance.
Key words: Mg-based composite; nano-carbon materials; interfacial bonding; mechanical properties
Foundation item: Project(51761037) supported by the National Natural Science Foundation of China; Projects (GJJ180851, GJJ180836) supported by the Science and Technology Research Project of Jiangxi Provincial Department of Education, China; Project(JXJG-19-15-8) supported by the Key Educational Reform Project of Jiangxi Provincial Depart of Education, China
Received date: 2020-03-02; Accepted date: 2020-06-22
Corresponding author: ZHOU Guo-hua; Tel: +86-7795-3201571; E-mail: spark382001@aliyun.com
(编辑 何学锋)
基金项目:国家自然科学基金资助项目(51761037);江西省教育厅科技项目(GJJ180851,GJJ180836);江西省教育厅教学改革重点项目(JXJG-19-15-8)
收稿日期:2020-03-02;修订日期:2020-06-22
通信作者:周国华,教授,博士;电话:07795-3201571;E-mail:spark382001@aliyun.com
摘 要:以AZ91合金为基体,采用液态分散技术+粉末冶金工艺+热处理工艺制备了四种纳米碳材料(碳纳米管、包覆氧化镁碳纳米管、石墨烯纳米片和氧化石墨烯)增强的镁基复合材料;测试了复合材料的力学性能,并利用光学显微镜、X射线衍射仪、扫描电子显微镜、透射电子显微镜等对复合材料微观组织、界面结构和断口形貌进行了表征及分析。结果表明:制备的四种复合材料料中,氧化石墨烯增强的镁基复合材料屈服强度和伸长率最好,分别为(312±4.5) MPa和11.3%±0.2%,比AZ91基体分别提高了85.7%和61.4%,表明四种纳米碳材料增强体中,氧化石墨烯更有益于提高镁合金的力学性能,有利于制备高性能镁基复合材料。
[7] 何 阳, 袁秋红, 罗 岚, 等. 镁基复合材料研究进展及新思路[J]. 航空材料学报, 2018, 38(4): 26-36.
[8] FRIEDRICH H E, MORDIKE B L. Magnesium technology[M]. Berlin: Springer-Verlag, 2006.
[15] 袁秋红, 曾效舒, 刘 勇, 等. 碳纳米管增强镁基复合材料弹性模量的研究进展[J], 中国有色金属学报, 2015, 25(1): 86-97.
[21] 杨永岗, 陈成猛, 温月芳, 等. 氧化石墨烯及其与聚合物的复合[J]. 新型炭材料, 2008, 23(3): 193-200.
[24] 袁秋红, 周国华, 廖 琳. 石墨烯纳米片/AZ91镁基复合材料的显微组织与力学性能[J]. 材料导报, 2018, 32(10): 1663-1667.
[28] JCPDS. International Centre for Diffraction Data[DB]. PCDFWIN V. 2.3, 2002.


