文章编号:1004-0609(2016)-10-2205-09
二进料口分馏萃取体系理论与动态特性
钟学明
(南昌航空大学 环境与化学工程学院,南昌 330036)
摘 要:
介绍二进料口分馏萃取理论的核心内容,包括各段级数的计算方法等。与传统分馏萃取相比较,二进料口的化工试剂消耗下降且其降幅随进料比增加而增大,当进料比为1时,洗酸的消耗下降了50%。采用动态模拟法研究二进料口分馏萃取体系的产品纯度变化、各段积累量和级样分布的特性。研究表明:二进料口分馏萃取体系的2个产品均能够较快地达到纯度设计指标,即更容易同时获得2个高纯产品,是制备高纯产品的良好技术方法。在二进料口分馏萃取体系中,组分在萃取段和洗涤段的积累只需较少的萃取排数即可直接进入稳态;但是,当萃取排数较低时组分在萃洗段的积累则先进入假稳态,当萃取排数较高时才真正进入稳态。二进料口分馏萃取稳态的级样分布遵循递增规则和递减规则,而且每条分布曲线分别在每个进料级均各有一个转折点。
关键词:
中图分类号:TF8 文献标志码:A
分馏萃取是一种十分重要的现代串级萃取分离技术,具有产品纯度高、组分收率高、生产规模大、分离成本低等突出优点,常用于稀土元素[1-5]、锕系元 素[6-8]、镉钴镍[9-10]等难分离元素的湿法冶炼。1978~ 1986年,徐光宪等[11-16]创建了串级萃取理论Ⅰ~Ⅷ,不仅揭示了分馏萃取体系的基本性质与规律,而且为研究分馏萃取体系和设计工艺提供了基本方法。1987年之后我国在该领域陆续取得一系列研究成果,比如多组分稀土分馏萃取工艺的高精度设计理论[17-20]提高了多组分分馏萃取工艺设计的精度,四出口萃取方法[21]、分馏萃取流程优化选择理论[22]和模糊分组-超链接萃取理论[23-25]降低了稀土分离工艺和流程的酸碱消耗,分馏萃取“无效区”理论[26]、相邻级杂质比变化理论[27]和水相返混理论[28]为提高分馏萃取工艺的调控水平奠定了理论基础,异价金属离子的工艺设计理论[29]为精确设计异价金属离子的分离工艺提供了方法。但是,现有分离萃取理论研究集中于一个进料口的传统分馏萃取体系,疏于研究原料对分馏萃取的影响,2个及多个进料口的分馏萃取理论的研究则尚未见报道。
自然界的矿物不仅种类繁多,而且元素含量变化甚大。例如,以稀土元素的摩尔分数计,氟碳铈矿的轻稀土元素的摩尔分数为97%、中重稀土的摩尔分数为3%;高钇稀土矿的轻稀土元素的摩尔分数为6%、中重稀土的摩尔分数为94%。显然,这些稀土原料之间在元素成分上具有一致性,但是在元素含量上具有差异性。然而,传统分馏萃取体系只设有一个进料口,必定只能处理一种稀土料液。面对稀土元素相同但是其含量有较大差异的两种原料,现行方案是建立两条不同的分馏萃取工艺生产线,分别进行萃取分离。然而,现有方案存在重复建设、酸碱消耗高、稀土元素收率低等不足[30-31]。那么,是否可以在一个分馏萃取工艺体系中同时处理元素含量不同的两种原料呢?为此,2013年提出了二进料口分馏萃取新方法,不但可以在一个分离萃取体系中处理元素相同但含量相异的两种料液,而且其化工试剂的消耗量有显著下降[30]。实际上,原料之间元素相同而各元素的含量存在一定差异、甚至差异显著是常见现象。因此,开展二进料口分馏萃取理论与体系特性的研究是必要的。
1 动态模拟实验方法
与人工分液漏斗实验法相比较,计算机动态模拟法具有结果更准确、信息更丰富等优点[32]。实际上,李标国等[33]建立的动态模拟法是替代人工分液漏斗实验、系统研究分馏萃取体系特性的唯一可靠方法[32]。
采用VC++ 6.0编写了二进料口分馏萃取体系计算机动态模拟计算程序。二进料口分馏萃取计算机动态模拟实验步骤依次为:1) 输入自变量:输入第一种料液和第二种料液中易萃组分A和难萃组分B的摩尔分数、分离系数、分离指标、进料比r(计算程序设定第二种料液的进料量为1 mol)、极值公式指数k[34]。2) 计算因变量:由计算机按照程序计算出水相出口分数,有机相出口分数,萃取段、萃洗段和洗涤段的纯化倍数,洗涤量,萃取量,萃取段、萃洗段和洗涤段的混合萃取比与级数。3) 输入控制参数:输入萃取排数或排级比。4) 模拟动态过程:由计算机按照程序完成填料、萃取平衡和物料转移及进料操作的运算[33]。萃取段和邻近萃取段的萃洗段一半级数用第一种料液填充、萃洗段的另一半级数(即邻近洗涤段的那部分)和洗涤段用第二种料液填充。在第一进料口(即图1中第n级)进r mol第一种料液,在第二进料口(即图1中第n+t级)进1 mol第二种料液。5) 数据输出:输出各级有机相和水相中稀土元素的摩尔分数或质量。
2 二进料口分馏萃取理论
2.1 二进料口分馏萃取体系的物料分布
二种水性料液中均含有易萃组分A和难萃组分B,第一种料液F1中的A组分的摩尔分数为fA,1,B组分的摩尔分数为fB,1;第二种料液F2中的A组分的摩尔分数为fA,2,B组分的摩尔分数为fB,2。摩尔分数的大小关系为fA,1<fA,2,fB,1>fB,2。二进料口分馏萃取体系由萃取段、萃洗段(中间段)和洗涤段三部分构成,其中萃取段级数为n,萃洗段级数为t,洗涤段级数为m。第一种料液的进料量为r mol/min,第二种料液的进料量为1 mol/min。萃取体系的物料萃取量为s mol/min,物料洗涤量为w mol/min,水相出口的物料
流量为Ma mol/min,有机相出口的物料流量为Mo mol/min,则皂化有机相二进料口分馏萃取体系各级物料分布如图1所示。图1中,水相的流动方向是从右向左流动,有机相的流动方向是从左向右流动。萃余液水相出口在第1级,负载有机相出口在第n+t+m级。
2.2 二进料口分馏萃取体系的萃取分离机理
以酸性膦(磷)类萃取剂HR分离稀土为例,难萃组分皂化有机相BR3从第1级进入分馏萃取体系(皂化目的是提高分馏萃取的萃取分离效率),在萃取段和萃洗段的离子交换反应为


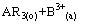 (1)
(1)
式中:下标“o”和“a”分别表示有机相和水相。在洗涤段,目的是洗涤有机相中过量皂化的组分B,洗涤反应为
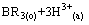

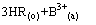 (2)
(2)
二进料口分馏萃取通过反应(1)和(2)实现组分A与B的分离。
2.3 二进料口分馏萃取体系各段的级数计算
1) 纯化倍数
用PA表示有机相出口产品A的纯度,PB表示水相出口产品B的纯度。那么,洗涤段的纯化倍数a为
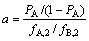 (3)
(3)
萃取段的纯化倍数b为
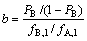 (4)
(4)
萃洗段的纯化倍数c为
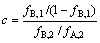 (5)
(5)
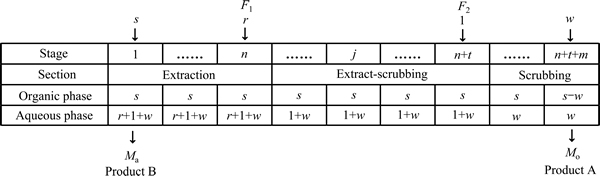
图1 皂化有机相二进料口分馏萃取体系的物料分布
Fig. 1 Material distribution in fractional extraction system with two inlets using saponified organic phase
2) 出口流量
当水相出口和有机相产品均为纯产品时,二进料口分馏萃取体系第一级的水相中的物料出口流量Ma为
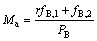 (6)
(6)
最后一级负载有机相中的物料出口流量Mo则为:
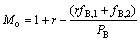 (7)
(7)
3) 产品产率
A产品的产率RA为
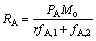 (8)
(8)
B产品的产率RB为
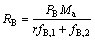 (9)
(9)
4) 洗涤量和萃取量
二进料口分馏萃取体系的洗涤量可以采用极值公式[34]来确定,洗涤量为
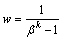 (0<k<1) (10)
(0<k<1) (10)
式中:β为组分A和B之间的分离系数,k为指数[34]。
萃取量s为
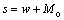 (11)
(11)
5) 各段混合萃取比
根据混合萃取比的定义[34]且结合图1可知,萃取段混合萃取比E1,萃洗段混合萃取比E2,洗涤段混合萃取比E3分别为
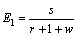 (E1<1) (12)
(E1<1) (12)
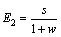 (13)
(13)
 (E3>1) (14)
(E3>1) (14)
6) 萃取级数
萃取段级数近似公式为
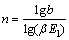 (15)
(15)
洗涤段级数近似公式为
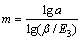 (16)
(16)
当E2≤1时,萃洗段级数近似公式为
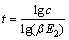 (17)
(17)
当E2>1时,则萃洗段级数近似公式为
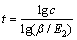 (18)
(18)
篇幅所限,在此本文作者仅介绍计算萃取级数的近似公式。根据串级萃取理论可知,精确公式是在近似公式后面加上修正项。修正项的计算方法,请参见串级萃取理论[32, 34]。
2.4 酸碱消耗的分析比较
根据极值公式[34]可知,2个只分离一种稀土料液的传统分馏萃取工艺、二进料口分馏萃取工艺的洗涤量均为w。因此,与2个传统分馏萃取工艺相比较,二进料口分馏萃取工艺一元洗酸的消耗量下降值Δw为
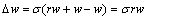 (19)
(19)
式中:σ为金属离子携带的正电荷数。而洗酸消耗下降百分率λ则为
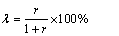 (20)
(20)
由式(20)可知,洗酸消耗下降百分率λ随着进料比r 的增大而增大。当进料比r=1时,洗酸消耗量理论上可以下降50%。不难看出,二进料口分馏萃取分离工艺的优势十分明显。
当制备两种纯产品时,分离第一种料液的分馏萃取工艺的萃取量s1、分离第二种料液的分馏萃取工艺的萃取量s2、二进料口分馏萃取工艺的洗涤量s分别为
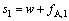 (21)
(21)
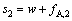 (22)
(22)
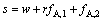 (23)
(23)
根据式(21)、(22)和(23),二进料口分馏萃取工艺一元皂化碱的消耗量下降值Δs为
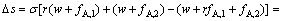
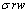 (24)
(24)
皂化碱消耗下降百分率ξ为
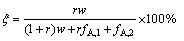 (25)
(25)
2.5 二进料口分馏萃取酸碱消耗降低的理论分析
分离是以消耗能源为代价的熵减过程。采用酸性萃取剂分离稀土时,不可避免需要消耗酸和碱即消耗化学能。以图1中萃洗段中间的第j(n<j<n+t,例如,j=n+t/2)级为界,左边的第1级至第j级在结构上等同于分离第一种料液的传统分馏萃取体系,但是节约了洗酸(原因是来自第j+1级的水相起洗酸的作用);右边的第j+1级至第n+t+m级等同于分离第二种料液的传统分馏萃取体系,但是节约了皂化碱(原因是来自第j级的有机相起皂化有机相的作用)。由此可知,二进料口分馏萃取使用一份皂化碱和一份洗涤酸分离两种稀土料液,这是二进料口分馏萃取酸碱消耗下降的根本原因。
此外,由第1级至第j级构成的“分馏萃取体系”,在第n+1级至第j级发生离子交换反应(1),是一个标准的满载分馏萃取体系,消除了洗酸副作用使酸碱消耗进一步下降[30, 35]。
2.6 进料比的取值范围
从图1可知,当进料比r=0时,二进料口分馏萃取体系退化为传统分馏萃取体系。因此,二进料口分馏萃取体系的下限值为r>0。由式(20)和(25)可知,随着进料比r的增大,酸碱的消耗下降。研究大量二进料分馏萃取体系发现,进料比r的下限值为:
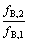 <
<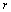 (26)
(26)
式(26)为经验公式。
由式(12)和(15)可知,随着进料比r的增大,萃取段级数增加。因此,进料比r存在一个上限值。由式(16)得:
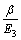 >1 (27)
>1 (27)
将式(14)代入式(27),整理得:
 >
>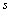 (28)
(28)
将式(23)代入(28),整理得:
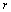 <
<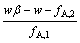 (29)
(29)
由式(29)可知,进料比r的取值将受到分离系数、洗涤量和料液组成等因素的限制。当进料比r满足式(26)和(29)时二进料口分馏萃取工艺的酸碱消耗较低,处于优化状态。
3 二进料口分馏萃取体系的动态特性
以动态模拟二进料口P507分馏萃取~Nd/Sm~分组分离氟碳铈矿和高钇稀土矿为例。易萃组分A代表中重稀土,难萃组分B表示轻稀土。氟碳铈矿料液为第一种料液,A的摩尔分数fA,1=0.03,B的摩尔分数fB,1=0.97;高钇稀土矿料液为第二种料液,A组分的摩尔分数为fA,2=0.94,B组分的摩尔分数fB,2=0.06。组分A与B之间的分离系数为10.6,进料量之比r=1,指数k=0.8。分离指标为A产品纯度PA=0.99999,B产品纯度PB=0.999999。
3.1 产品纯度的变化特性
分馏萃取体系的产品纯度变化特性见图2。图2的横坐标为萃取排数的对数,纵坐标为-lg(1-P)。纵坐标的含义为产品达到几个9,例如-lg(1-P)=5,表示产品纯度P=0.99999。因此,图2反映了给定萃取排数时分馏萃取体系产品能够达到的理论纯度。从图 2(a)中可以看出,二进料口分馏萃取体系的A产品在萃取排数为252排、B产品纯度为40排时分别达到纯度设计指标。由此可知,二进料口分馏萃取体系的2个产品均能够很快地达到纯度设计指标。从图2(b)中可以看出,单独分离第一种料液的传统分馏萃取体系的A产品在萃取排数约为399000排、B产品纯度为399排时分别达到纯度设计指标。从图2(c)中可以看出,单独分离第二种料液的传统分馏萃取体系的A产品在萃取排数为159排、B产品纯度约为317000排时分别达到纯度设计指标。由于进料量、皂化量和洗涤量的波动,原料组成的细小变化,以及工艺参数调节等因素的影响,实际运行的分馏萃取工艺体系很难以达到萃取排数大于1×105排的理论纯度。或者说,萃取排数小于1×105排的理论纯度才更有实际意义。因此,与传统的分馏萃取工艺体系相比,二进料口分馏萃取体系更容易同时获得2个高纯产品。此外,二进料口分馏萃取体系中A产品纯度随萃取排数的变化,与分离第二种料液的传统分馏萃取体系中A产品纯度变化相似,均在萃取排数为1000时,A产品纯度出现峰值;二进料口分馏萃取体系中B产品纯度的变化,则与分离第一种料液的传统分馏萃取体系中B产品纯度变化有些相似。
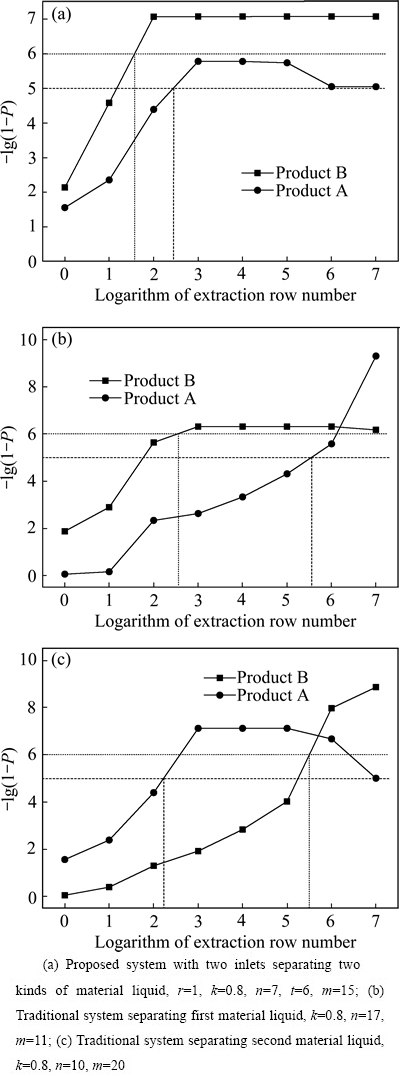
图2 分馏萃取体系的产品纯度变化特性
Fig. 2 Variation of product purities in fractional extraction system
3.2 各组分在各段的积累变化特性
图3所示为组分在分馏萃取体系各段的平均积累变化,横坐标为萃取排数的对数,纵坐标为组分在指定段的平均质量流量。TE、TES和TS分别表示萃取段、萃洗段和洗涤段的平均质量流量。图3反映了随着萃取排数增加组分在各段的积累量的变化特性。从图3可以看出,二进料口分馏萃取体系各组分的积累与相应的二个各自分离一种料液的传统分馏萃取体系有明显不同。由图3(a)可知,在二进料口分馏萃取体系中,当萃取排数为100时,组分A和B在萃取段和洗涤段的积累进入稳态,但是萃洗段的积累则进入假稳态;直到萃取排数为1×106时,组分A和B在萃洗段才真正进入稳态。由图3(b)可知,在单独分离第一种料液的传统分馏萃取体系中,当萃取排数为100时,组分A和B在萃取段的积累进入稳态;但是直到萃取排数为1×107时,组分A和B在洗涤段的积累似乎还未进入稳态。由图3(c)可知,在单独分离第二种料液的传统分馏萃取体系中,当萃取排数为100时,组分A和B在洗涤段的积累进入亚稳态;但是直到萃取排数为1×107时,组分A和B在萃取段和洗涤段的积累似乎尚未进入稳态。因此,人工分液漏斗法的分馏萃取实验不可能达到真正的稳态。如果欲系统地研究制备高纯产品的分馏萃取体系的特性和规律,藉此看来计算机模拟的确是唯一方法[32]。
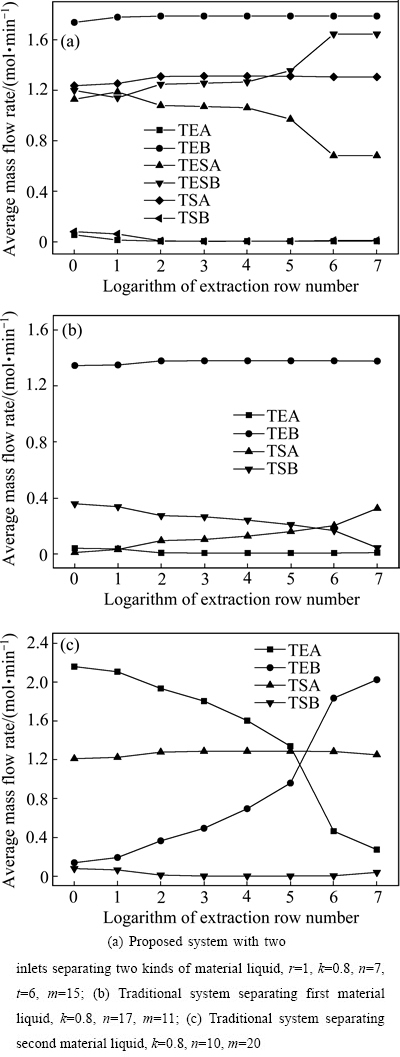
图3 组分在分馏萃取体系各段中的积累变化
Fig. 3 Variation of component accumulation in each section of fractional extraction system
3.3 稳态的级样分布特性
图4所示为分馏萃取体系进入稳态后各级水相和有机相的级样分布图,横坐标为萃取体系的级序,纵坐标为组分在水相的摩尔分数x和在有机相的摩尔分数y。图4中,二进料口分馏萃取体系的萃取级数为28级;单独分离第一种料液的传统分馏萃取体系的萃取级数为28级;单独分离第二种料液的传统分馏萃取体系的萃取级数为30级。仅仅通过萃取级数的比较,也可以反映出二进料口分馏萃取体系的优势。从图4可以看出,A组分在水相的摩尔分数xA和有机相的摩尔分数yA均随着级序的增大而增加(递增规则)[32, 36],而B组分在水相的摩尔分数xB和有机相的摩尔分数yB均随着级序的增大而减小(递减规则)[32, 36];而且3个分馏萃取体系的分布曲线均在进料级附近出现突变区。粗略看来,二进料口分馏萃取稳态体系的分布曲线的变化趋势、形状和位置像是2个传统分馏萃取的分布曲线的叠加再平均而成。但是,分布曲线转折点的位置和数目各不相同。图4(a)中,二进料口分馏萃取稳态体系的每条分布曲线均有4个转折点,其中2个转折点分别出现在在2个进料级(第7级和第13级)。图4(b)中,每条曲线有2个转折点,曲线xA和xB的1个转折点出现在进料级。图4(c)中,水相分布曲线xA和xB各有3个转折点,而有机相分布曲线yA和yB则各有2个转折点;而且在进料级每条分布曲线均有1个转折点。实际分馏萃取工艺体系的在线监测点,应设置于转折点的平台区一侧且适当远离进料级,以利于监控萃取分离工艺的异常波动。

图4 分馏萃取体系的级样分布(萃取排数为1×107)
Fig. 4 Component accumulation in each section of fractional extraction system (extraction row number is 1×107)
4 结论
1) 随着进料比的增大,二进料口的洗酸消耗量和皂化碱消耗量均有所下降。但是,不能随意增大进料比,因为进料比受到分离系数、料液组成和萃取参数的制约。
2) 二进料口分馏萃取体系中水相出口产品纯度的变化,与第一种料液单独分馏萃取体系中水相产品纯度变化相似;二进料口分馏萃取体系中有机相出口产品纯度随萃取排数的变化,与第二种料液单独分馏萃取的有机相产品纯度变化相似。
3) 与单独分离一种料液的传统分馏萃取体系相比较,二进料口分馏萃取体系的2个产品均能够较快地达到纯度设计指标,因此更容易同时获得2个高纯产品;而且,组分的收率也更高。
4) 在二进料口分馏萃取体系中,组分在萃取段和洗涤段的积累只需较少的萃取排数即可直接进入稳态;但是,组分在萃洗段的积累则先进入假稳态,较高萃取排数时才真正进入稳态。
5) 与传统分馏萃取体系一样,二进料口分馏萃取稳态体系的级样分布也遵循递增规则和递减规则,而且每条分布曲线在每个进料级均各有一个转折点。
REFERENCES
[1] YAN Chun-hua, JIA Jiang-tao, LIAO Chun-sheng, WU Sheng, XU Guang-xian. Rare earth separation in China[J]. Tsinghua Science and Technology, 2006, 11(2): 241-247.
[2] JIA Jiang-tao, WU Sheng, LIAO Chun-sheng, YAN Chun-hua. Research and application progress in countercurrent solvent extraction[J]. Journal of Rare Earth, 2004, 22(5): 576-581.
[3] WANG Yan-liang, LIAO Wu-ping, LI De-qian. A solvent extraction process with mixture of CA12 and Cyanex272 for the preparation of high purity yttrium oxide from rare earth ores[J]. Separation and Purification Technology, 2011, 82(2): 197-201.
[4] LI Wei, WANG Xiang-lan, MENG Shu-lan, LI De-qian, XIONG Ying. Extraction and separation of yttrium from the rare earths with sec-octylphenoxy acetic acid in chloride media[J]. Separation and Purification Technology, 2007, 54(2): 164-169.
[5] XIE Feng, ZHANG Ting-an, DREISINGER D, DOYLE F. A critical review on solvent extraction of rare earths from aqueous solutions[J]. Minerals Engineering, 2014, 56(1): 10-28.
[6] BAN Y, HOTOKU S, MORITA Y. Application of N, N-di(2-ethylhexyl) butanamide for mutual separation of U(Ⅵ) and Pu(Ⅳ) by continuous counter-current extraction with mixer-settler extractors[J]. Journal of Nuclear Science and Technology, 2012, 49(6): 588-594.
[7] ANSARI S A, GUJAR R B, PRABHU D R, PATHAK P N, MOHAPATRA P K. Counter-current extraction of neptunium from simulated pressurized heavy water reactor high level waste using N,N,N',N'-tetraoctyl diglycolamide [J]. Solvent Extraction and Ion Exchange, 2012, 30(5): 457-468.
[8] ALI A M I, DAOUD J A, ZEID M M, ALY H F. Counter- current extraction process for recovery of U(IV) from phosphoric acid using octylphenol acid phosphate (OPAP) extractant[J]. Journal of Radioanalytical and Nuclear Chemistry, 2002, 254(2): 263-269.
[9] GOTFRYD L, COX M. The selective recovery of cadmium (Ⅱ) from sulfate solutions by a counter-current extraction–stripping process using a mixture of diisopropylsalicylic acid and Cyanex 471X[J]. Hydrometallurgy, 2006, 81(3): 226-233.
[10] FEATHER A, BOUWER W, SWARTS A, NAGEL V. Pilot- plant solvent extraction of cobalt and nickel for Avmin’s Nkomati project[J]. The Journal of the South African Institute of Mining and Metallurgy, 2002, 102(8): 457-462.
[11] 徐光宪. 串级萃取理论Ⅰ: 最优化方程及其应用[J]. 北京大学学报(自然科学版), 1978(1): 51-66.
XU Guang-xian. Theory of counter current extraction Ⅰ: Equations of optimization and their applications[J]. Acta Scientiarum Naturalium Universitatis Pekinensis, 1978(1): 51-66.
[12] 徐光宪. 串级萃取理论Ⅱ: 纯度对数求解法[J]. 北京大学学报(自然科学版), 1978(1): 67-75.
XU Guang-xian. Theory of counter current extraction Ⅱ: Determination of the number of stages by the method of Log purity plot[J]. Acta Scientiarum Naturalium Universitatis Pekinensis, 1978(1): 67-75.
[13] 李标国, 徐献瑜, 徐光宪. 串级萃取理论Ⅲ: 逆流萃取动态平衡的数学模型[J]. 北京大学学报(自然科学版), 1980(2): 66-84.
LI Biao-guo, XU Xian-yu, XU Guang-xian. Theory of countercurrent extraction Ⅲ: A mathematical model for the dynamic equilibrium of counter current extraction [J]. Acta Scientiarum Naturalium Universitatis Pekinensis, 1980(2): 66-84.
[14] 李标国, 李俊然, 严纯华, 徐光宪. 串级萃取理论Ⅵ: 两组份串级萃取体系动态平衡的研究[J]. 稀有金属, 1985, 4(3): 17-23.
LI Biao-guo, LI Jun-ran, YAN Chun-hua, XU Guang-xian. Theory of countercurrent extraction Ⅵ: In two-component counter-current extraction system[J]. Rare Metals, 1985, 4(3): 17-23.
[15] 李标国, 严纯华, 乔书平, 徐光宪. 串级萃取理论Ⅶ: 三组份体系动态过程的研究[J]. 中国稀土学报, 1985, 3(3): 20-26.
LI Biao-guo, YAN Chun-hua, QIAO Shu-ping, XU Guang-xian. Theory of countercurrent extraction Ⅶ: Dynamic equilibrium in three component system[J]. Journal of Chinese Rare Earth Society, 1985, 3(3): 20-26.
[16] 李标国, 严纯华, 乔书平, 徐光宪. 串级萃取理论Ⅷ: 两组分串级萃取体系回流过程的研究[J]. 中国稀土学报, 1986, 4(2): 1-7.
LI Biao-guo, YAN Chun-hua, QIAO Shu-ping, XU Guang-xian. Theory of countercurrent extraction Ⅷ: Reflux method for starting a cascade of two component system[J]. Journal of the Chinese Society of Rare Earth, 1986, 4(2): 1-7.
[17] 胡启阳. 等效分离系数及多组分稀土串级萃取分离工艺设计[J]. 稀有金属与硬质合金, 1999(4): 1-4.
HU Qi-yang. Equivalent separation coefficient and process design for cascade extraction separation of multi-component rare earth[J]. Rare Metals andCemented Carbides, 1999(4): 1-4.
[18] 吴 声, 廖春生, 贾江涛, 严纯华. 多组分多出口稀土串级萃取静态优化设计研究(Ⅰ): 静态设计算法[J]. 中国稀土学报, 2004, 22(1): 17-21.
WU Sheng, LIAO Chun-sheng, JIA Jiang-tao, YAN Chun-hua. Static design for multiple components and multiple outlets rare earth countercurrent extraction (Ⅰ): Algorithm of static design[J]. Journal of the Chinese Rare Earth Society, 2004, 22(1): 17-21.
[19] 吴 声, 廖春生, 贾江涛, 严纯华. 多组分多出口稀土串级萃取静态优化设计研究(Ⅱ): 静态程序设计及动态仿真验证[J]. 中国稀土学报, 2004, 22(2): 171-176.
WU Sheng, LIAO Chun-sheng, JIA Jiang-tao, YAN Chun-hua. Static design for multicomponent and multi-outlet rare earth countercurrent extraction (Ⅱ): Static design and its verification [J]. Journal of the Chinese Rare Earth Society, 2004, 22(2): 171-176.
[20] 钟学明. 多组分稀土串级萃取有效分离系数的理论分析[J]. 中国有色金属学报, 2008, 18(10): 1926-1930.
ZHONG Xue-ming. Theoretical analysis of effective separation factor for multi-component rare earths in countercurrent extraction[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(10): 1926-1930.
[21] 高新华, 吴文远, 涂赣峰. 四组分体系“组合式”萃取分离工艺[J]. 有色矿冶, 2000, 16(2): 21-25.
GAO Xin-hua, WU Wen-yuan, TU Gun-feng. A separating process of “assemble program” extraction for four-element system[J]. Non-ferrous Mining and Metallurgy, 2000, 16(2): 21-25.
[22] 钟学明. 稀土萃取分离流程的优化选择[J]. 化学研究与应用, 2003, 15(2): 235-236.
ZHONG Xue-ming. Optimization of the extraction processes for the separation of rare earth elements[J]. Chemical Research and Application, 2003, 15(2): 235-236.
[23] LIAO Chun-sheng, WU Sheng, CHENG Fu-xiang, WANG Song-ling, LIU Yan, ZHANG Bo, YAN Chun-hua. Clean separation technologies of rare earth resources in China[J]. Journal of Rare Earth, 2013, 31(4): 331-336.
[24] CHENG Fu-xiang, WU Sheng, ZHANG Bo, LIU Yan, WANG Song-ling, LIAO Chun-sheng, YAN Chun-hua. Minimum amount of extracting solvent of AB/BC countercurrent extraction separation using organic feed[J]. Journal of Rare Earth, 2014, 32(5): 439-444.
[25] 赵治华, 姜晓丽, 胡 刚, 郑 健, 李俊林, 王新宇. 白云鄂博稀土矿联动萃取分离流程的经济技术指标比较[J]. 中国稀土学报, 2016, 34(1): 71-76.
ZHAO Zhi-hua, JIANG Xiao-li, HU Gang, ZHENG Jian, LI Jun-lin, WANG Xin-yu. Economic technological indexes comparison of hyperlinked separation process for Baiyunebo rare earth mine[J]. Journal of the Chinese Society of Rare Earth, 2016, 34(1): 71-76.
[26] 白 彦, 李德谦. 稀土串级萃取过程中“无效区”现象的探究[J]. 稀土, 2009, 30(6): 1-7.
BAI Yan, LI De-qian. Studies on the ineffective separation zone in the extraction cascade for separating rare earths[J]. Chinese Rare Earths, 2009, 30(6): 1-17.
[27] CHENG Fu-xiang, WU Sheng, LIAO Chun-sheng, YAN Chun-hua. Adjacent stage impurity ratio in rare earth countercurrent extraction process[J]. Journal of Rare Earth, 2013, 31(2): 169-173.
[28] WU Sheng, CHENG Fu-xiang, LIAO Chun-sheng, YAN Chun-hua. Impact of backmixing of the aqueous phase on two-component rare earth separation process[J]. Journal of Rare Earth, 2013, 31(5): 517-521.
[29] 吴 声, 廖春生, 严纯华. 含不同价态的多组份体系萃取平衡算法研究[J]. 中国稀土学报, 2012, 30(2): 163-167.
WU Sheng, LIAO Chun-sheng, YAN Chun-hua. Extraction equilibrium algorithm for multi-component system with different-valance ions[J]. Journal of the Chinese Society of Rare Earth, 2012, 30(2): 163-167.
[30] 钟学明, 吴少林, 吴跃辉. 二进料口分馏萃取分离稀土的工艺方法: 中国专利, CN103421965B[P]. 2014-10-01.
ZHONG Xue-ming, WU Shao-lin, WU Yue-hui. Process method of fractional extraction with two inlets for the separation of rear earths: Chinese Patent, CN103421965B[P]. 2014-10-01.
[31] 钟学明. 二进料口满载分馏萃取分离稀土的工艺方法: 中国专利, CN104561546A[P]. 2015-04-29.
ZHONG Xue-ming. Process method for the separation of rare earth by full load fractional extraction with two inlets: Chinese Patent, CN104561546A[P]. 2015-04-29.
[32] 徐光宪. 稀土[M]. 北京: 冶金工业出版社, 1995: 655-689.
XU Guang-xian. Rare earth[M]. Beijing: Metallurgical Industry Press, 1995: 614-689.
[33] 李标国, 徐光宪. 串级萃取理论动态平衡的计算和程序设计[J]. 稀有金属, 1980(1): 1-12.
LI Biao-guo, XU Guang-xian. The dynamic equilibrium calculation and program design for theory of countercurrent extraction[J]. Rare Metals, 1980(1): 1-12.
[34] 徐光宪, 李标国, 严纯华. 串级萃取理论的进展及其在稀土工艺中的应用[J]. 稀土, 1985, 6(1): 56-67.
XU Guang-xian, LI Biao-guo, Yan Chun-hua. Development of theory of countercurrent extraction and its applications in rare earth technology[J]. Chinese Rare Earths, 1985, 6(1): 56-67.
[35] 钟学明. 满载分馏萃取分离稀土的工艺方法: 中国专利, CN104532022A[P]. 2015-04-22.
ZHONG Xue-ming. Process method for the separation of rare earth by full load fractional extraction: Chinese Patent, CN104532022A[P]. 2015-04-22.
[36] 钟学明. 水相进料理想分馏萃取的模拟[J]. 稀土, 2010, 31(5): 48-51.
ZHONG Xue-ming. Simulation of ideal fractional extraction feeding with aqueous material [J]. Chinese Rare Earths, 2010, 31(5): 48-51.
Theory and dynamic characteristics of fractional extraction system with two inlets
ZHONG Xue-ming
(School of Environmental & Chemical Engineering, Nanchang Hangkong University, Nanchang 330063, China)
Abstract: The essential contents of the proposed theory including the calculation of extraction stage numbers of the fractional extraction system with two inlets were introduced. Compared with traditional fractional extraction, the chemical consumption of the novel fractional extraction with two inlets decreases significantly, and the decreasing amplitude amplifies as the feed ratio increases. For example, when the feed ratio is 1, the scrubbing acid consumption drops by 50%. The variation of product purities, component accumulation in each section and component distribution in each extraction stage were investigated by dynamic simulation on a computer. The results indicate that the two products attain respective desired purities using fractional extraction with two inlets are much faster than those using traditional fractional extraction. Hence, the novel extraction system is a superior technology to prepare two high purity products at same time. In a fractional extraction system with two inlets, the component accumulation both in extraction section and scrubbing section enter into steady state directly with less extraction row number. In addition, the component accumulation in extract-scrubbing section access into pseudo steady state with less extraction row number first and then into genuine steady state with much more extraction row number. The component distribution in each extraction stage of the fractional extraction system with two inlets follows the increasing rule and decreasing rule. Moreover, two turning points appear in each distribution curve at the two feed stages, respectively.
Key words: fractional extraction; two inlets; dynamic characteristics; computer simulation
Foundation item: Project(21461016) supported by the National Natural Science Foundation of China; Project (20132BBE50007) supported by the Science and Technology Support Program of Jiangxi Province, China
Received date: 2015-11-10; Accepted date: 2016-04-12
Corresponding author: ZHONG Xue-ming; Tel: +86-791-83953373; E-mail: xuemingzhong@sina.com
(编辑 王 超)
基金项目:国家自然科学基金资助项目(21461016);江西省科技支撑计划资助项目(20132BBE50007)
收稿日期:2015-11-10;修订日期:2016-04-12
通信作者:钟学明,教授;电话:0791-83953373;E-mail: xuemingzhong@sina.com
摘 要:介绍二进料口分馏萃取理论的核心内容,包括各段级数的计算方法等。与传统分馏萃取相比较,二进料口的化工试剂消耗下降且其降幅随进料比增加而增大,当进料比为1时,洗酸的消耗下降了50%。采用动态模拟法研究二进料口分馏萃取体系的产品纯度变化、各段积累量和级样分布的特性。研究表明:二进料口分馏萃取体系的2个产品均能够较快地达到纯度设计指标,即更容易同时获得2个高纯产品,是制备高纯产品的良好技术方法。在二进料口分馏萃取体系中,组分在萃取段和洗涤段的积累只需较少的萃取排数即可直接进入稳态;但是,当萃取排数较低时组分在萃洗段的积累则先进入假稳态,当萃取排数较高时才真正进入稳态。二进料口分馏萃取稳态的级样分布遵循递增规则和递减规则,而且每条分布曲线分别在每个进料级均各有一个转折点。
[11] 徐光宪. 串级萃取理论Ⅰ: 最优化方程及其应用[J]. 北京大学学报(自然科学版), 1978(1): 51-66.
[12] 徐光宪. 串级萃取理论Ⅱ: 纯度对数求解法[J]. 北京大学学报(自然科学版), 1978(1): 67-75.
[13] 李标国, 徐献瑜, 徐光宪. 串级萃取理论Ⅲ: 逆流萃取动态平衡的数学模型[J]. 北京大学学报(自然科学版), 1980(2): 66-84.
[14] 李标国, 李俊然, 严纯华, 徐光宪. 串级萃取理论Ⅵ: 两组份串级萃取体系动态平衡的研究[J]. 稀有金属, 1985, 4(3): 17-23.
[15] 李标国, 严纯华, 乔书平, 徐光宪. 串级萃取理论Ⅶ: 三组份体系动态过程的研究[J]. 中国稀土学报, 1985, 3(3): 20-26.
[16] 李标国, 严纯华, 乔书平, 徐光宪. 串级萃取理论Ⅷ: 两组分串级萃取体系回流过程的研究[J]. 中国稀土学报, 1986, 4(2): 1-7.
[17] 胡启阳. 等效分离系数及多组分稀土串级萃取分离工艺设计[J]. 稀有金属与硬质合金, 1999(4): 1-4.
[18] 吴 声, 廖春生, 贾江涛, 严纯华. 多组分多出口稀土串级萃取静态优化设计研究(Ⅰ): 静态设计算法[J]. 中国稀土学报, 2004, 22(1): 17-21.
[19] 吴 声, 廖春生, 贾江涛, 严纯华. 多组分多出口稀土串级萃取静态优化设计研究(Ⅱ): 静态程序设计及动态仿真验证[J]. 中国稀土学报, 2004, 22(2): 171-176.
[20] 钟学明. 多组分稀土串级萃取有效分离系数的理论分析[J]. 中国有色金属学报, 2008, 18(10): 1926-1930.
[21] 高新华, 吴文远, 涂赣峰. 四组分体系“组合式”萃取分离工艺[J]. 有色矿冶, 2000, 16(2): 21-25.
[22] 钟学明. 稀土萃取分离流程的优化选择[J]. 化学研究与应用, 2003, 15(2): 235-236.
[25] 赵治华, 姜晓丽, 胡 刚, 郑 健, 李俊林, 王新宇. 白云鄂博稀土矿联动萃取分离流程的经济技术指标比较[J]. 中国稀土学报, 2016, 34(1): 71-76.
[26] 白 彦, 李德谦. 稀土串级萃取过程中“无效区”现象的探究[J]. 稀土, 2009, 30(6): 1-7.
[29] 吴 声, 廖春生, 严纯华. 含不同价态的多组份体系萃取平衡算法研究[J]. 中国稀土学报, 2012, 30(2): 163-167.
[30] 钟学明, 吴少林, 吴跃辉. 二进料口分馏萃取分离稀土的工艺方法: 中国专利, CN103421965B[P]. 2014-10-01.
[31] 钟学明. 二进料口满载分馏萃取分离稀土的工艺方法: 中国专利, CN104561546A[P]. 2015-04-29.
[32] 徐光宪. 稀土[M]. 北京: 冶金工业出版社, 1995: 655-689.
XU Guang-xian. Rare earth[M]. Beijing: Metallurgical Industry Press, 1995: 614-689.
[33] 李标国, 徐光宪. 串级萃取理论动态平衡的计算和程序设计[J]. 稀有金属, 1980(1): 1-12.
[34] 徐光宪, 李标国, 严纯华. 串级萃取理论的进展及其在稀土工艺中的应用[J]. 稀土, 1985, 6(1): 56-67.
[35] 钟学明. 满载分馏萃取分离稀土的工艺方法: 中国专利, CN104532022A[P]. 2015-04-22.


