
Electrolyte transfer separation of hollow fiber composite nanofiltration membrane
LIU Jiu-qing(刘久清)
School of Metallurgy Science and Engineering, Central South University, Changsha 410083, China
Received 6 July 2009; accepted 30 December 2009
_____________________________________________________________________________________________________
Abstract:
Using Donnan Steric Partitioning Model (DSPM), the data for the rejection of four salts having common co-ion (LiCl, NaCl, KCl, Na2SO4) were obtained and they show the characters of the polyethersulfone (PES) nanofiltration (NF) membrane in terms of three parameters: an effective pore radius (rp), the ratio of effective thickness over porosity (λ/Ak) and an effective charge density (X). Good agreement between experimental data and prediction data using the three parameters mentioned above was obtained. A theoretical model was developed to predict the transport performance of electrolyte through the hollow fiber composite NF membrane. The model prediction is in good agreement with experimental results based on the method by modern numerical solution.
Key words:
electrolytes; transfer separation; hollow fiber membrane; nanofiltration;
_____________________________________________________________________________________________________
1 Introduction
Nanofiltration (NF) membranes, which exhibit separation characteristics in the intermediate range between reverse osmosis (RO) and ultrafiltration, are gaining interest worldwide because of advantages such as low-operation pressure, high-permeation flux, and high retention of multivalent ion salts[1-5]. The NF process has been used in many applications such as wastewater reclamation, industrial water production, water softening and separation of compounds having different relative molecular mass[6-9].
One of the most widely adopted method for modeling the transportation of electrolyte through the nanofiltration membrane is the Donnan Steric Partitioning Model (DSPM), which is based on the Extended Nernst Planck Equation and the characterization of nanofiltration membranes with three parameters: the pore size, the charge density, and the membrane thickness[10-13]. In this work, the electrolyte transfer mechanism of the hollow fiber nanofiltration membrane is researched to uncover the relationship between the performance and configuration of the membrane by establishing the corresponding mathematical model and predicting the configuration and separation performance of the membrane. A basic frame of references, theories, guidelines, techniques and data for the preparation, design and analysis of hollow fiber nanofiltration membrane is provided.
2 Experimental2.1 Materials
Hollow fiber membrane samples of nanofiltration membranes from University of Science and Technology of China were used. Some characteristics of the nanofiltration membrane used in the experiment are given as follows: membrane material(polyethersulfone, PES), relative molecular mass cut off (MWCO) 600-800, negative charge (at neutral pH). Sodium chloride (NaCl), lithium chloride (LiCl), potassium chloride (KCl) and sodium sulfate (Na2SO4) (chemical grade) were purchased from Shanghai Chemical Factory, China.
2.2 Equipment
Experiments were carried out in a laboratory-scale test cell. A schematic diagram of the apparatus was presented in Ref.[1]. All experiments were carried out at a 15 m3/h feed flow rate and at a constant temperature of 25 ℃. The external and internal diameters of hollow fiber membranes were measured by means of an optical microscope. In one module, there were four 40 cm hollow fiber nanofiltration membranes which have an average external diameter of 1 mm. The total membrane area of one module was about 50 cm2. The permeation flow direction of the membrane module is operated outside-in by means of a suction.
RO water was used to measure the pure water flux at different transmembrane pressures to determine the pure water permeability of the membranes.
The rejection of single salt solutions (NaCl, KCl, LiCl and Na2SO4) was determined as a function of the permeation flux at 2 g/L feed concentrations. Feed and permeation samples were analyzed by conductivity measurements. All solutes were prepared using RO water and analytical grade salts. The experiments were carried out at pH 5.5-6.
Pure water permeation fluxes (PWP, Jw) of membranes are obtained as:
![]() (1)
(1)
where Jw represents the pure water permeation flux of membrane for solution, Q represents the volumetric flow rate of solution (L/h), A represents the membrane surface area.
The membrane rejection (R) is defined as:
![]() (2)
(2)
where Cf and Cp represent the feed and permeation concentrations, respectively.
Each membrane was subjected to pressure at 0.2 MPa for 1 h before the permeation experiment. The flux was equilibrated for the passage of the first 20 mL permeation whilst the following 10 mL permeation was collected for concentration analysis. All the results presented were an average data obtained from three membrane samples with a variation of ±10%.
3 Theoretical background
DSPM was adopted in this simulation, based on SCPM (Space-Charge Porous Model), by substituting the gradient distribution of radial concentration and charge density with the average concentration and charge density inside the membrane. This is reasonable as the calculation was made known[13]. With the pore size less than 2 nm, the following radial transformation of the concentration and charge density inside the membrane can be ignored. At the same time, DSPM has taken the steric hindrance effect on the ion diffusing through the membrane layers into account.
DSPM based on the extended Nernst-Planck equation is expressed as:
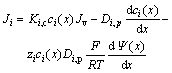 (3)
(3)
where Ki, c denotes the convection steric coefficient, ci(x) denotes the ion concentration within the membrane, Jv represents the volume flux (based on membrane area), Ji refers the ion flux (L/(m2?h)), zi represents the ion valence stale, Ψ(x) represents the Donnan’s electrical potential (V), Di,p represents the ion diffusion coefficient of infinite diluted solution.
The standard deviation is calculated using
![]() (4)
(4)
where n denotes the number of experiment; Rexp represents the rejection data from the experiment; Rcalc refers the rejection data from the modulation calculation.
4 Results and discussion4.1 Simulation of experimental data on inorganic salt solution
This particular experiment is based on DSPM, using the experimental data of the rejection of mono-valence and bivalence inorganic salt to simulate the membrane’s characteristic parameters of polyethersulfone hollow fiber composite nanofiltration membrane. Figs.1-4 show the relationship between the flux and operation pressure of nanofiltration process on LiCl, NaCl, KCl and Na2SO4 solution, respectively. The discrete points in the figures are obtained through the experiments, and the full lines are calculated by Matlab software that simulate many pairs of experimental data of four different types of salts.
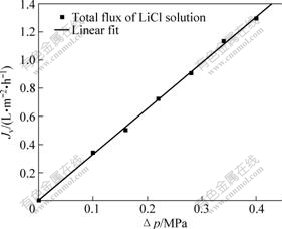
Fig.1 Relationship between operation pressure and solution flux during LiCl solution’s separation
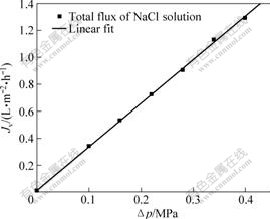
Fig.2 Relationship between operation pressure and solution flux during NaCl solution’s separation
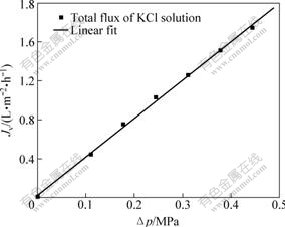
Fig.3 Relationship between operation pressure and solution flux during KCl solution’s separation
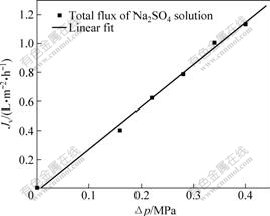
Fig.4 Relationship between operation pressure and solution flux during Na2SO4 solution’s separation
From Figs.1-4, it can be shown that the salt solution flux increases linearly with the operation pressure increasing. At the same time, the hollow fiber membrane feature parameters of effective pore radius rp, effective charge density X and effective thickness λ are calculated from Fig.4 and the values are 0,24 nm, 106.50 mol/m3 and 4 μm, respectively. It can be calculated that the simulation calculation curve tallies with the experimental data, and have a standard deviation (σy) of 0.006 183.
4.2 Predicted effect for separation of inorganic salt
Due to the confirmation of characteristic equation and membrane parameters during the separation process, the prediction of membrane separation can be carried out, in which the separation of composition serves as a function to predict and control that ultimate result process of the separation experiment, thus minimizes the time carrying out the experiment and the cost of it. Fig.5-8 show the separation performance of Na2SO4, K2SO4, KCl and NaCl solutions on the polyethersulfone nanofiltration membrane under different concentrations, respectively.
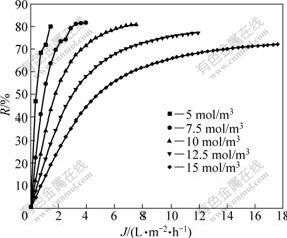
Fig.5 Separation performance of PES nanofiltration membrane on Na2SO4 solution
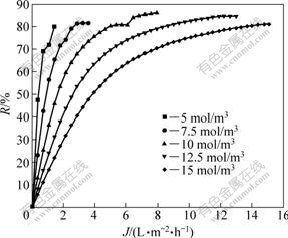
Fig.6 Separation performance of PES nanofiltration membrane on K2SO4 solution
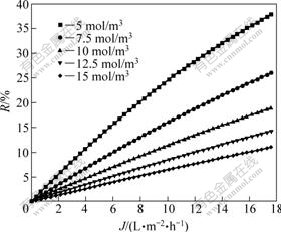
Fig.7 Separation performance of PES nanofiltration membrane on KCl solution
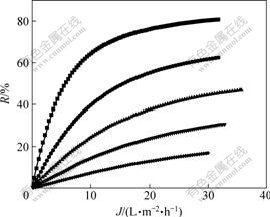
Fig.8 Separation performance of PES nanofiltration membrane on NaCl solution
5 Conclusions1) Integrating the characteristics of nanofiltration membrane with the fundaments of DSPM, the electrolyte transfer mechanism model of nanofiltration membrane is established, which uncovers the relationship between the membrane’s performance and its configuration and provides references of guidelines and techniques for the preparation, design and analysis of nanofiltration membrane.
2) There are three types of configuration parameters (effective pore radius, the ratio of effective thickness to porosity and effective charge density) in this model, which can be predicted after knowing the configuration parameters which ensure the relationship between electrolyte rejection and the flux of the membrane.
3) The membrane configuration parameters are estimated from the rejection experiment. The results from the rejection experiment prove that the model is applicable.
References[1] LIU Jiu-qing, XU Zheng-liang, LI Xin-hai, ZHANG Yao, ZHOU Ying, WANG Zhi-xing, WANG Xue-jun. An improved method to prepare high separation performance PA/PVDF hollow fiber composite NF membranes [J]. Separ Purif Tech, 2007, 58(1): 53-60.
[2] BANVOLGYI S, KISS I, BEKASSY-MOLNAR E, VATAI G. Concentration of red wine by nanofiltration [J]. Desalination, 2006, 198(1/3): 8-15.
[3] BOUSSU K, KINDTS C, VANDECASTEELE C, van DER BRUGGEN B. Applicability of nanofiltration in the carwash industry [J]. J Separ Purif Tech, 2007, 54(2): 139-146.
[4] KO?UTI? K, DOLAR D, A?PERGER D, KUNST B. Removal of antibiotics from a model wastewater by RO/NF membranes [J]. J Separ Purif Tech, 2007, 53(3): 244-249.
[5] SUAREZ E, LOBO A, ?LVAREZ S, RICARDO F A, ?LVERZA R. Partial demineralization of whey and milk ultrafiltration permeate by nanofiltration at pilot-plant scale [J]. Desalination, 2006, 198(1/3): 274-281.
[6] BODZEK M, DUDZIAK M. Elimination of steroidal sex hormones by conventional water treatment and membrane processes [J]. Desalination, 2006, 198(1/3): 24-32.
[7] BLANCO J F, SUBLET J, NGUYEN Q T, SCHAETZEL P. Formation and morphology studies of different polysulfones-based membranes made by wet phase inversion process [J]. J Membr Sci, 2006, 283(1/2): 27-37.
[8] TANNINEN J, M?NTT?RI M, NYSTR?M M. Effect of salt mixture concentration on fractionation with NF membranes [J]. J Membr Sci, 2006, 283(1/2): 57-64.
[9] SZYMCZYK A, FATIN-ROUGE N, FIEVET P, RAMSEYER C, VIDONNE A. Identification of dielectric effects in nanofiltration of metallic salts [J]. J Membr Sci, 2007, 287(1): 102-110.
[10] WANG Da-xin, WU Ling, LIAO Zhuo-dan, TOMI Y, ANDO M, SHINTANI T. Modeling the separation performance of nanofiltration membranes for the mixed salts solution with Mg2+ and Ca2+ [J]. J Membr Sci, 2006, 284(1/2): 384-392.
[11] HUSSAIN A A, NATARAJ S K, ABASHAR M E E, AL-MUTAZ I S, AMINABHAVI T M. Prediction of physical properties of nanofiltration membranes using experiment and theoretical models [J]. J Membr Sci, 2008, 310(1/2): 321-336.
[12] AMOUDI A A, WILLIAMS P, HOBAIB A S, LOVITT R W. Cleaning results of new and fouled nanofiltration membrane characterized by contact angle, updated DSPM, flux and salts rejection [J]. Appli Surf Sci, 2008, 254(13): 3983-3992.
[13] LANTERI Y, FIEVET P, SZYMCZYK A. Evaluation of the steric, electric, and dielectric exclusion model on the basis of salt rejection rate and membrane potential measurements [J]. J Collo Inter Sci, 2009, 331(1): 148-155.
______________________
Foundation item: Project(20806094) supported by the National Natural Science Foundation of China; Project(2008SK1001) supported by Energy-saving and Emission-reducing Major Special Projects of Department of Science & Technology of Hunan Province, China; Projects(K0901082-11, K0902123-11) supported by Plan on Science and technology Bureau of Changsha, China
Corresponding author: LIU Jiu-qing; Tel: +86-731-88883633; E-mail: jiuqing_liu@163.com
(Edited by LIU Hua-sen)


