
J. Cent. South Univ. (2021) 28: 2022-2036
DOI: https://doi.org/10.1007/s11771-021-4750-6
Variation in energy metabolism structure of microbial community during bioleaching chalcopyrites with different iron-sulfur ratios
YANG Yu(杨宇)1, 2, ZHU Zhen-yu(朱振宇)1, HU Ting-ting(胡婷婷)1,ZHANG Meng-jun(张梦君)1, QIU Guan-zhou(邱冠周)1, 2
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Biometallurgy of Ministry of Education, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Abstract:
The energy metabolism structure of microbial community plays an important role in the process of biohydrometallurgy. In this article, an artificial microbial community composed of three strains (Acidithiobacillus ferrooxidans, Leptospirillum ferriphilum and Acidithiobacillus thiooxidans) was used to leach three kinds of chalcopyrites with different iron-sulfur ratios. After 36 d of leaching, the chalcopyrite with iron-sulfur ratio of about 1:1 achieved the highest copper extraction (69.62%). In the early stage, iron oxidizing bacteria predominated, and the expression of rus and rio was 8 times higher than that in the late stage. In the late stage, sulfur oxidizing bacteria predominated, and the expression of tetH and HdrAB was 4 times higher than that in the early stage. Furthermore, the three bioleaching systems above were added with elemental sulfur (3 g/L); the chalcopyrite with iron-sulfur ratio of about 2:1 achieved the highest copper extraction (80.63%). The results suggest that the energy metabolism structure of the microbial community could be changed by changing the iron-sulfur ratio during the leaching process for improving the leaching efficiency of chalcopyrite.
Key words:
energy metabolism structure; microbial community; bioleaching; chalcopyrite; iron-sulfur ratio;
Cite this article as:
YANG Yu, ZHU Zhen-yu, HU Ting-ting, ZHANG Meng-jun, QIU Guan-zhou. Variation in energy metabolism structure of microbial community during bioleaching chalcopyrites with different iron-sulfur ratios [J]. Journal of Central South University, 2021, 28(7): 2022-2036.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-021-4750-61 Introduction
The bioleaching of minerals is essentially the process of microorganisms such as bacteria oxidizing sulfide minerals to obtain the energy for their growth. Meanwhile, the bacteria-mineral interaction with the microbial growth and metabolism can solubilize minerals and release metal ions. Similarly, acid mine drainage (AMD) derived from acidophilic microorganism oxidizing metal sulfide minerals and releasing high amounts of metals, sulfate, and protons [1, 2]. Unique microorganisms from AMD environments are the major contributors in bioleaching of low-grade ores, which have significant potentials in acid mine drainage bioremediation. Thus, the ecological environment, material, and energy cycle of bioleaching process as well as acid mine drainage have gradually become the focus of research [3-5].
However, the current research mainly focuses on the study of related community structure changes macroeconomically, which results in limited number of studies focused on the energy metabolism structures in biohydrometallurgy and AMD ecology [6-10]. Although the shifting patterns of microbial community under specific conditions have been identified [11-14], these community-based researches did not address factors causing the shifts in microbial communities and the specific roles of different components of minerals variating in iron-sulfur ratios causing these microbial communities to change [15-17]. Since the information provided by previous studies is limited, the site-based laws developed for one specific mine cannot be effectively applied to another, as well as situations within the same mine under various conditions. Thus, the limitation of the proposed methods leads to the limitations of the research results. The underlying cause of this situation includes the rich diversity of microbial community structures, the complexity of the mining environment, and mineral characteristics [18].
Due to the limited information presented from previous publications, it is essential for us to find a solution for one of the most urgent issues and study the mechanism of microbial metabolism, microbial interaction, and tolerance to heavy metals in AMD. In this study, three acidophiles containing different iron and sulfur energy metabolism pathway were applied for construction of an artificial community [19, 20], namely Acidithiobacillus ferrooxidans (A. ferrooxidans) with metabolism pathway involved in the oxidation of ferrous iron (Fe (II)) and reduced inorganic sulfur compounds (RISCs) [21, 23], Leptospirillum ferriphilum (L. ferriphilum) with the similar metabolism pathway of Fe(II) oxidation to Acidithiobacillus ferrooxidans [24, 25], Acidithiobacillus thiooxidans (A. thiooxidans) with a RISC oxidation pathway different from Acidithiobacillus ferrooxidans [26]. We speculated that the variation in energy metabolism structure of microbial community during bioleaching chalcopyrites with different iron-sulfur ratios should be different. Optimizing the energy metabolism structure of microbial community by adjusting the iron-sulfur ratio may be the key to improving the efficiency of the bioleaching system [27]. Three kinds of chalcopyrites with different iron-sulfur ratios were bioleached by the same artificial microbial community. Furthermore, the same chalcopyrite bioleaching systems mentioned above were investigated under the conditions of adding elemental sulfur, which can effectively boost the development speed of microbial communities [28]. The succession of microbial communities and the variation in the energy metabolism structure were characterized by the richness of characteristic microorganisms and the expression of typical genes associated with energy metabolism. We will attempt to illustrate the differences in energy metabolism structures, the ecological niches of iron-oxidizing bacteria and sulfur-oxidizing bacteria in different stages during the bioleaching process of chalcopyrite with different iron-sulfur ratios. The findings of this study can be applied to the industry of biohydrometallurgy and microbial ecological research of AMD.
2 Materials and methods
2.1 Source and composition of minerals
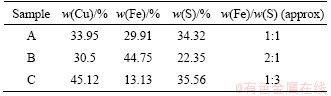
The copper sulfide ores were obtained from Dexing porphyry copper ore mine located in Jiangxi Province, China. The element composition of three types of chalcopyrite was analyzed by XRF (X-ray fluorescence spectrometry). The copper sulfide ores were mixed in different proportions into three leaching samples with different copper, iron and sulfur contents (Table 1), named as samples A, B and C.
Table 1 Major element content of three categories
2.2 Preparation of artificial microbial community
The acidophil microorganisms A. ferrooxidans ATCC23270, L. ferriphilum ML-04 and A. thiooxidans ATCC19377 were obtained from Key Laboratory of Biometallurgy of Ministry of Education, Central South University, Hunan, China. The isolates of A. ferrooxidans and L. ferriphilum were grown in 9K medium (0.15 g/L (NH4)2SO4, 0.05 g/L KH2PO4, 0.05 g/L KCl, 0.5 g/L MgSO4·7H2O, 0.01 g/L Ca(NO3)2·4H2O, pH 2.0) with 44.7 g/L FeSO4. The isolate of A. thiooxidans was grown in 9K medium with 10 g/L elemental sulfur. A. ferrooxidans ATCC23270 could simultaneously perform iron oxidation and sulfur oxidation metabolism. L. ferriphilum ML-04 could only carry out iron oxidative metabolism. A. thiooxidans ATCC19377 could only carry out sulfur oxidation metabolism, using low valence sulfur as energy source. We used these three strains which had unique metabolic functions to construct an artificial microbial community for bioleaching experiments.
2.3 Bioleaching experiments
Three leaching systems with three different iron-sulfur ratios of chalcopyrite were built. Each leaching system was leached at 30 °C under normal aeration conditions and continuously leached for 36 d. The initial cell concentration of each strain was approximately equal (about 4×106 cell/mL) in all three systems with pH 2.0. The number of bacteria, pH value, and the concentrations of Cu2+, Fe2+ and Fe3+ were analyzed every 3 d.
2.4 Determination of metal ions in solution
The concentrations of copper ion, ferrous ion and ferric ion in solution were determined by spectrophotometry. Copper ion could react with sodium diethyldithiocarbamate to form a yellow brown complex, of which absorbance could be measured at 452 nm. Ferrous ion could react with 1,10-phenanthroline monohydrate to form orange red complex, of which absorbance could be measured at 510 nm. Using cytochrome c to reduce the ferric iron in the solution, the total iron concentration could be determined by the same method, and the ferric ion concentration could be obtained by total iron concentration minus ferrous iron concentration.
2.5 Analysis of microbial community structure
Genomic DNA was extracted from synthetic microbial community system using the Genomic DNA Purification Kit (JetFlex, Invitrogen) in accordance with the protocol provided by manufacturer and stored at -80 °C until analysis. The 515F/806R primer set (V4 region) was used to amplify the target gene fragment. Amplification, barcoding, and high-throughput sequencing were performed on the Illumina MiSeq platform at Ecogene Biotech (Shanghai, China). The composition of the community at each time point was determined three times.
2.6 Method of energy metabolism structure analysis
The energy metabolism structure was analyzed by the expression of key energy metabolism genes. Real-time quantitative PCR was used to determine the expression level of key energy metabolism genes. Real-time quantitative PCR analysis was performed on a CFX96 optical real-time detection system (Bio-Rad, Laboratories Inc., Hercules, CA, USA) to determine the copy numbers of the key genes in the DNA gradient fractions. The primers of these key genes were described in detail in supplementary materials (Table S1-S3). The reactions were performed in a 20-μL mixture containing 10.0 μL of SYBR Premix Ex Taq (TaKaRa), each primer at 0.5 μmol/L, and 2 μL of DNA template. For qPCR of the key genes, 40 cycles of 30 s at 95°C, 30 s at 55°C and 45 s at 72°C were applied. Standard curves were obtained using 10-fold serial dilutions of linearized recombinant plasmids containing every key gene with known copy numbers. The amplification efficiencies were 92%-100%, with R2 >0.99.
3 Results and discussion
3.1 Leaching behavior of chalcopyrites with different iron-sulfur ratios
The results of the leaching behavior after 36 d of bioleaching are shown in Figure 1. Sample A had the highest copper leaching rate (69.62%), whose pH had decreased in two stages. The decrease of pH corresponds to the decrease in ferrous ion concentration in early stage due to the possibility of high level of free hydrogen ions generated during the metabolic process of iron oxidizing bacteria. Sample B had a copper leaching rate of 67.25% with the highest microbial abundance among three samples, indicating that the development of microbial community could be promoted in the early stage under the condition of high iron-sulfur ratio. The copper leaching rate of sample C was only 56.35%, whose pH reduction was the least (only reduced to 1.29 in 36 d) among three samples, indicating that the lower iron-sulfur ratio could make the pH higher in the early stage of leaching and be not conducive to the development of microbial community. Furthermore, the development of microbial community had been greatly improved and the pH had also been reduced greatly in the system with elemental sulfur as additional energy substance (shown in Figure 2), which promoted the acid leaching process of minerals and ensured that the iron-oxidizing bacteria and sulfur-oxidizing bacteria had sufficient energy metabolism activity in sample B especially, with the highest leaching rate (80.63%).
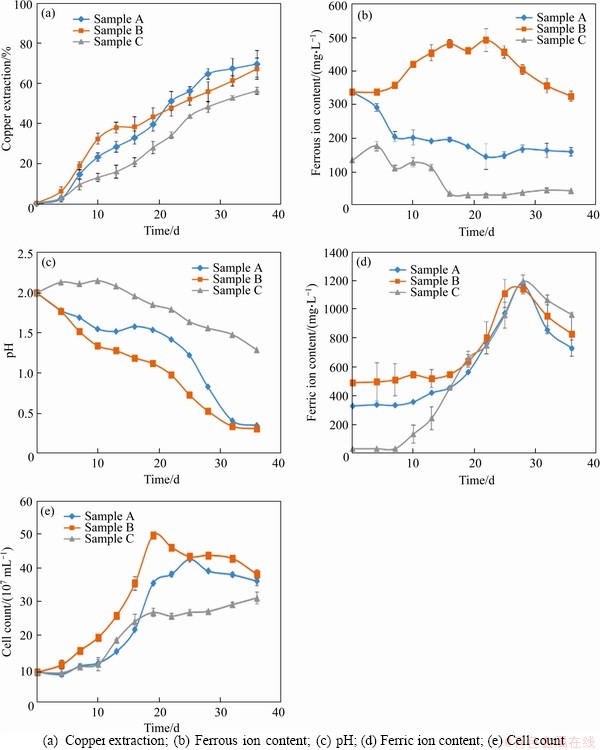
Figure 1 Analysis of bioleaching behavior of three chalcopyrite systems:
3.2 Succession of artificial microbial community in bioleaching of minerals with different iron sulfur ratios
In the initial stage of leaching, A.thiooxidans was dominant after 19 d in sample C with the minimum iron sulfur ratio (shown in Figure 3). Even after 22 d, the proportion of its population was more than the sum of the other two strains. However, sample C had the slowest decline rate of pH and the lowest leaching rate of copper ion (only 56.35%). In contrast, A.ferrooxidans and L.ferriphilum were dominant in sample B with the maximum iron sulfur ratio, due to the high concentration of ferrous ion (shown in Figure 1(b)). This result indicates that oxidative metabolism of ferrous iron could produce a large number of hydrogen ions, which resulted in a rapid decrease of pH value in the culture system and promoted the dissolution of chalcopyrite in the initial stage of leaching (the leaching rate reached 30% after 10 d). However, the dominance of L.ferriphilum was gradually replaced by A.ferrooxidans over time.
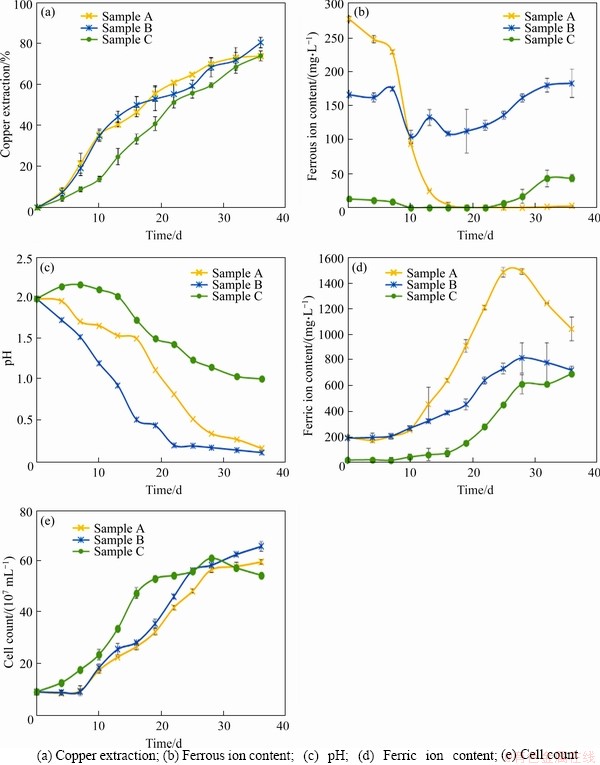
Figure 2 Analysis of bioleaching behavior of three chalcopyrite systems with additional sulfur:
In the middle stage of leaching, iron precipitates could cover the surface of minerals, which made it difficult to digest the crystal structure of minerals, and the advantage of a higher proportion of iron oxidizing bacteria in the early stage was more prominent (shown in Figure 3(a)). However, lower pH could inhibit the formation of iron precipitates and make mineral leaching easier.
In the final stage of leaching, A.thiooxidans and A.ferrooxidans played a vital role in inhibiting and eliminating the passivation layer and improving the acid solubility of chalcopyrite. Especially, A.thiooxidans had a higher proportion, which could metabolize complex sulfur sources and further promote the acidification of the leaching system in a complicated and harsh environment, helping to improve the leaching efficiency.
In the experimental group with elemental sulfur added, the pH decreased significantly, but the number of cells and the copper ion leaching rate increased. There were more microbial cells in sample C with elemental sulfur. Although the leaching rate was still low in the early stage, the more A.thiooxidans (shown in Figure 3(b)) ensured higher leaching rate at the final stage (the final leaching rate reached 74.17%, shown in Figure 2(a)). The growth rate of microorganism in sample B with elemental sulfur was faster in the early stage. A.ferrooxidans (shown in Figure 3(b)) occupied the ecological status quickly with the decrease of pH value and the increase of mineral dissolution rate (the leaching rate reached 35% after 10 d, shown in Figure 2(a)). However, the rapid growth of A.ferrooxidans caused a sharp drop in concentration of ferrous ion (shown in Figure 2(b)), and a large amount of iron precipitation led to the bottleneck of leaching rate (from the 13th to the 22nd day). Subsequently, A.thiooxidans began to dominate, which could promote the further dissolution of chalcopyrite (the final leaching rate reached 80.63%, shown in Figure 2(a)).
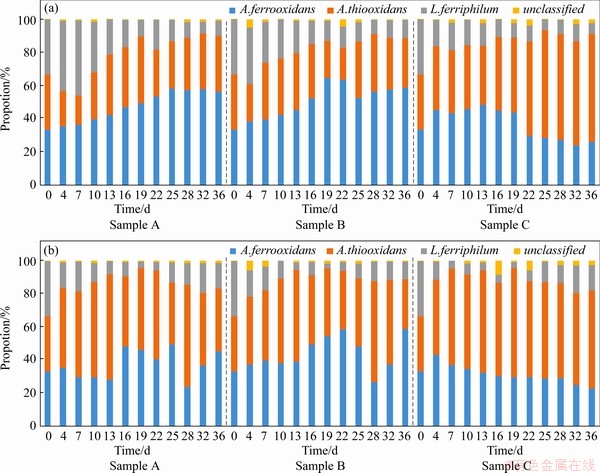
Figure 3 A histogram of microbial community structure over time during leaching of three samples without (a) and with (b) elemental sulfur added
3.3 Construction of artificial microbial community and simulation of energy metabolism structure
An energy metabolism structure model (shown in Figure 4) in an bioleaching system was constructed by analyzing the genome-wide information of the three strains (A.ferrooxidans ATCC23270, L.ferriphilum ML-04 and A.thiooxidans ATCC19377) [29, 30]. The expression of key genes in the metabolism of the three strains was analyzed by real-time quantitative PCR (shown in Figure 5).
In A.ferrooxidans, the electrons generated from the oxidation of elemental sulfur and/or reduced inorganic sulfur compounds (by HdrABC, tetH, sor, etc.) would be transferred via the quinone pool (QH2) in the inner membrane directly to terminal oxidases or a periplasmic high potential iron-sulfur protein (HiPIP) or other pathway [31, 32]. Thereafter, sulfur would enter the sulfur cycle in the cell until it is consumed as a raw material to participate in amino acid metabolism and so on. For the iron oxidation,rusticyanin A (rus), a key enzyme in the iron respiratory chain, would be utilized to oxidate ferrous ion in A.ferrooxidans. Afterwards, electrons would be transferred via periplasmic cyc1 either to an inner membrane cbb3-type terminal oxidase that reduces oxygen or to the NADH dehydrogenase complex via QH2. Instead of rus (shown in Table S1), the outer membrane cytochrome572 (Cyt572) would be utilized to transfer electrons from Fe(II) to the periplasmic cytochrome579 (Cyt579) in L.ferriphilum [33, 34]. The subsequent electron transfer pathway was similar to that of A.ferrooxidans. The expression of genes related to the first two steps of iron oxidation was received with concern (shown in Table S2 and Table S3).
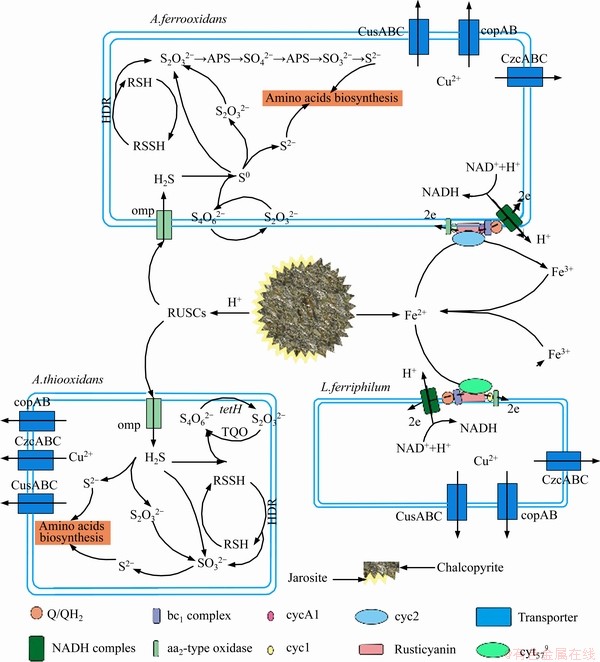
Figure 4 Schematic showing major metabolic pathways and adaptive mechanisms of autotrophic acidophiles widely found in extremely acidic environments
Similar but not identical to A.ferrooxidans, A.thiooxidans had a greater potential for sulfur metabolism. A.thiooxidans could oxidize a variety of sulfur compounds [35], including thiosulfate, sulfur, sulfite and sulfide. The electron transfer chain associated with sulfur oxidation in A.thiooxidans was thought as follows: electrons derived from TQO, SQR, and HDR would be transferred via QH2 either to the terminal oxidases to generate a proton gradient, or to NADH complex I to produce reducing capacity [36, 37]. The gene expression associated with the first step of sulfur oxidation was received with concern (shown in Table S4).
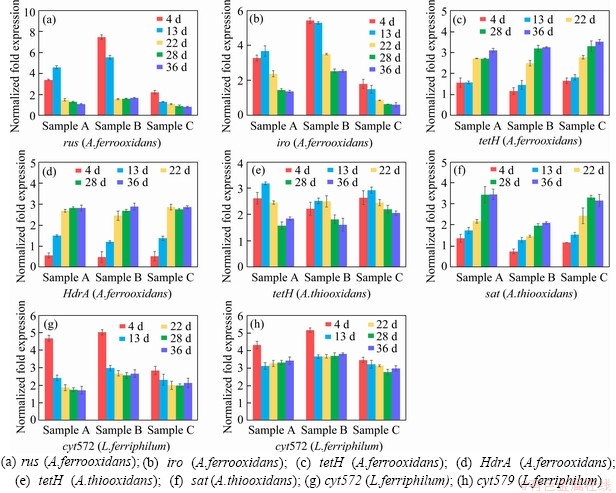
Figure 5 Key metabolic pathway gene expression of bioleaching behavior of three samples:
3.4 Energy metabolism structure of microbial community in different leaching systems
Samples were collected at certain key time points, and the metabolic activities of the three strains were determined by gene expression. In this study, eight key energy metabolism genes of three strains were selected for qPCR verification, and the data of gene expression are shown in Figure 5. Furthermore, the data of seven other genes expression were detailed in the supplementary materials (shown in Figure S1-S3). These results shown that A.ferrooxidans could oxidize ferrous ion to acquire electrons in sample A with higher ferrous ion concentration in early stage (Figure 1(b)). While the concentration of ferrous ion decreases sharply (less than 200 mg/L), it will reduce the expression of genes related to iron oxidation and turn on genes related to sulfur oxidation (shown in Figure 1(b)). Previous studies have shown that some iron metabolism genes of A.ferrooxidans, such as fur, can inhibit the expression of sulfur metabolism genes, which was similar to an intracellular iron homeostasis response regulation system. The expression level of iron oxidation genes such as rus and iro decreased by 4 times in the late stage of leaching (from the 22nd to the 39th day), and the expression level of sulfur-oxidation genes such as HdrA and tetH increased by 3 times (shown in Figure 5). These results indicated that A.ferrooxidans should have different energy metabolism pathways at different stages of leaching and play different roles in the leaching system.
L.ferriphilum could oxidize a large amount of acid-leached ferrous ions by expressing iron-oxidation-related genes in the early stage of bioleaching (from the 4th to the 13rd day), which prompted a rapid decrease in pH value and contributed to high leaching rate. However, the expression level of iron-oxidizing genes significantly decreased by 2 times and the proportion of L.ferriphilum gradually decreased after 13 d [38, 39] (shown in Figure 5). Therefore, in the environment with lower iron ion concentration in the final stage of leaching, L.ferriphilum was less dominant in microbial community than A.ferrooxidans.
Unlike ferrous iron, which was consumed rapidly, sulfur-containing substances are energy sources with a slower metabolism, so that A.thiooxidans could become dominant in the final stage. The expression level of HdrABC and sat increased by about 2 times in the final stage, while the expression level of soxABZY and tetH decreased by about 3 times (shown in Figure 5). It was speculated that the metabolic pathway of A.thiooxidans could change with the change of the form of sulfur source, which changed from simple sulfur substances (like S0, S2O32- and S2-) to complex sulfur substances over time. It showed that A.thiooxidans could play a very important role in the final stage of bioleaching, which could change the metabolic pathway to adapt to the complex and harsh leaching environment [40, 41], including reducing the adsorption of jarosite on the surface of pyrite and improving the dissolution efficiency again [42]. These results were consistent with the conclusions of the microbial community structure experiment. The expression level experiment and the change of the microbial community structure confirmed each other, indicating that the high metabolic activity strains could guarantee the dominant position in different development stages of the microbial community.
Compared with sample C, sample B with higher iron-sulfur ratio promoted the rapid growth of A.ferrooxidans and L.ferriphilum, and the expression of iron-oxidation-related genes increased by about 2 times in the early stage (shown in Figure 5). The higher expression of iron metabolism gene could make A.ferrooxidans and L.ferriphilum grow and develop rapidly, and reduce the pH value of leaching system rapidly, which could ensure the higher leaching rate in the early stage. Compared with sample B, sample A with lower iron-sulfur ratio promoted the rapid dominance of A.ferrooxidans and A.thiooxidans in the final stage, in which the expression of sulfur-oxidation-related genes increased by about 2 times (shown in Figure 5). The higher expression of sulfur metabolism genes could enable A.ferrooxidans and A.thiooxidans to promote the further dissolution of chalcopyrite and ensure a higher leaching rate in the final stage.
The iron oxidation pathway was simpler and faster than the sulfur oxidation pathway, so iron oxidizing bacteria could dominate faster in the leaching system. The higher iron concentration ensured the iron metabolism activity of A.ferrooxidans and L.ferriphilum in the early stage of microecological development, and promoted the pH reduction and acid dissolution of the mineral. On the basis of a large amount of mineral dissolution, the more sulfur substances should be exposed, which could provide a better living environment for sulfur-metabolizing strains, thus ensuring that the leaching system could have a higher leaching rate after environmental changes. Subsequently, A.thiooxidans and A.ferrooxidans could accelerate the acidification of the environment and promote the further dissolution of minerals in the final stage. Moreover, A.ferrooxidans could achieve a stable ecological state by changing its own metabolic pathway, which should play a very important role in the whole process of microecological development.
With the addition of elemental sulfur, the expression level of sulfur oxidation related genes in the experimental group increased in varying degrees (shown in Figure 6). Sample B with elemental sulfur had a higher leaching rate, and the expression of iron oxidation gene in A.ferrooxidans was reduced more significantly, indicating that the sulfur oxidation metabolic pathway could have an inhibitory effect on the iron oxidation metabolic pathway in A.ferrooxidans. These results suggested that there should be some energy metabolism regulation mechanisms that could regulate the balance between different energy metabolism pathways [43].
4 Conclusions
In the bioleaching process, the synthetic microbial community composed of three strains with three typical energy metabolism pathways had a regular dynamic succession process. The dynamic succession of synthetic microbial community in the mineral leaching process with different iron-sulfur ratios was different. The results showed that the processes of microbial community succession and energy metabolism were closely related to the leaching behavior of minerals, and could be regulated by mineral composition and element ratio. The iron-sulfur ratio of the bioleaching system could be artificially changed at different stages of chalcopyrite, leaching to the highest leaching efficiency under the energy metabolism structure of the microbial community. In our opinion, the above research findings could have certain guiding significance for the development of the biohydrometallurgical industry.
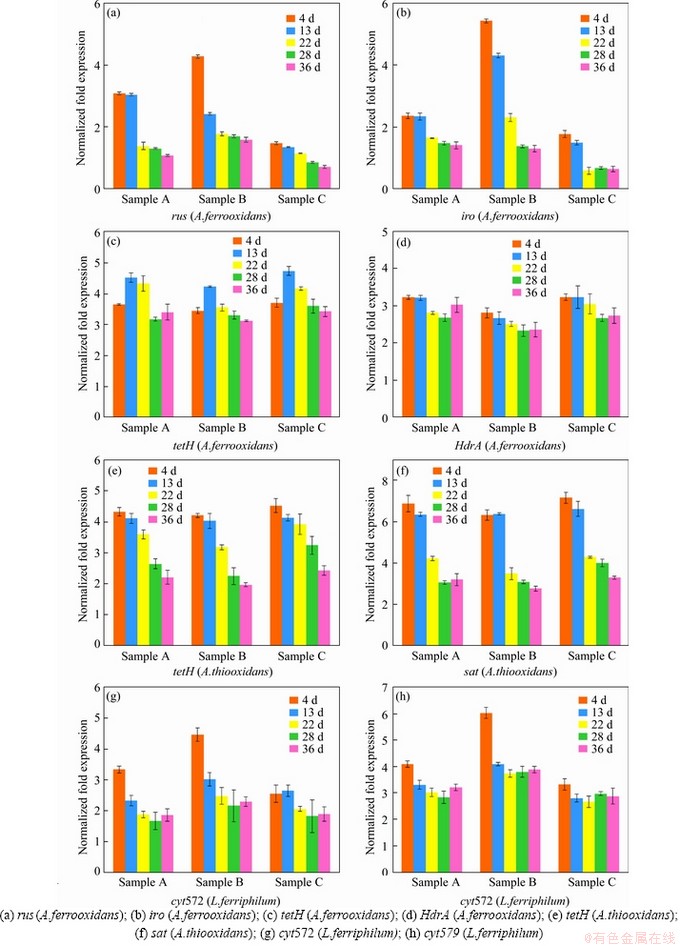
Figure 6 Key metabolic pathway gene expression of bioleaching behavior of three samples with additional sulfur:
Contributors
YANG Yu contributed to conceptualization (lead), funding acquisition (lead), writing-review and editing (equal). ZHU Zhen-yu contributed to conceptualization (equal), data curation (equal), formal analysis (equal), methodology (lead), writing-original draft (lead) and writing-review and editing (lead). HU Ting-ting contributed to data curation (supporting), validation (equal) and visualization (equal). ZHANG Meng-jun contributed to project administration (supporting), validation (supporting), visualization (supporting), writing-review and editing (supporting). QIU Guan-zhou contributed to funding acquisition (equal), project administration (equal), esources (equal).
Conflict of interest
The authors declare that they have no conflicts of interest.
Supplementary materials
Table S1 Key metabolic pathway gene of A.ferrooxidans
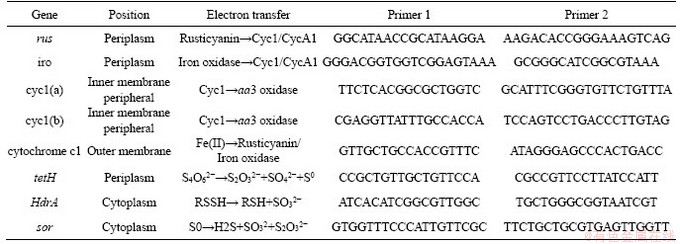
Table S2 Key metabolic pathway gene of A.thiooxidans
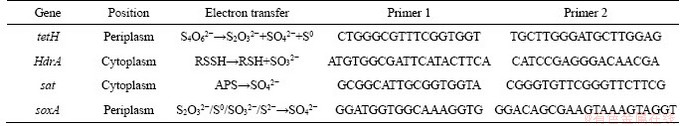
Table S3 Key metabolic pathway gene of L.ferriphilum
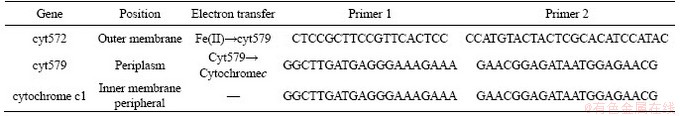
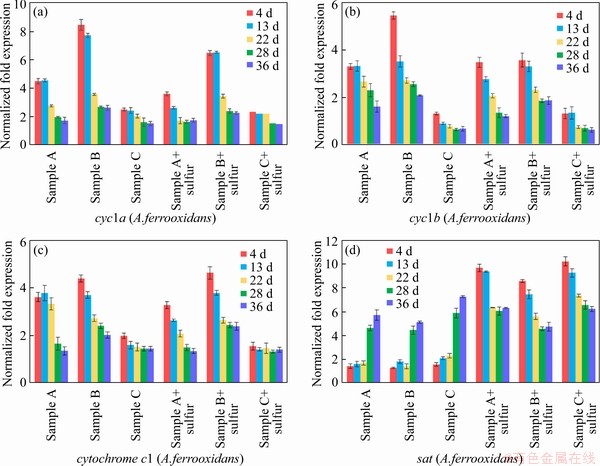
Figure S1 Expression of key metabolic pathway gene of A.ferrooxidans during bioleaching
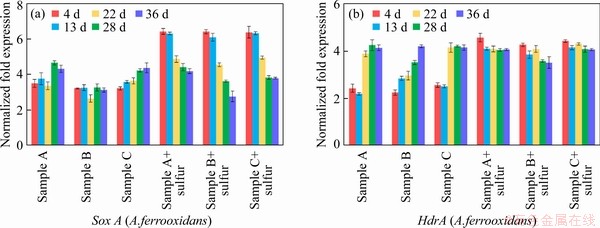
Figure S2 Expression of key metabolic pathway gene of A.thiooxidans during bioleaching
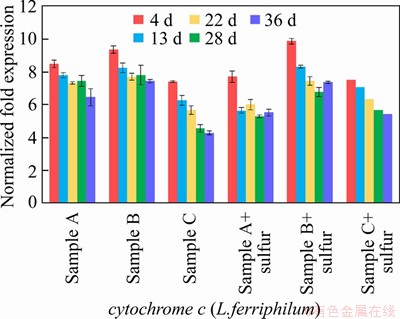
Figure S3 Expression of key metabolic pathway gene of L.ferriphilum during bioleaching
References
[1] DRUSCHEL G K, BAKER B J, GIHRING T M, BANFIELD J F. Acid mine drainage biogeochemistry at iron mountain, California [J]. Geochemical Transactions, 2004, 5(2): 13-32. DOI: 10.1186/1467-4866-5-13.
[2] YOON Y J, LEE J E, BANG S J, BAEK Y D, KIM Y. Behaviors of trace elements caused by the precipitation of minerals in acid mine drainage [J]. Journal of the Mineralogical Society of Korea, 2018, 31(3): 173-182. DOI: 10.9727/jmsk.2018.31.3.173.
[3] DENEF V J, MUELLER R S, BANFIELD J F. AMD biofilms: Using model communities to study microbial evolution and ecological complexity in nature [J]. ISME Journal Multidisciplinary Journal of Microbial Ecology, 2010, 4(5): 599-610. DOI: 10.1038/ismej.2009.158.
[4] BAKER-AUSTIN C, DOPSON M. Life in acid: pH homeostasis in acidophiles [J]. Trends in Microbiology, 2007, 15(4): 165-71. DOI: 10.1016/j.tim.2007.02.005.
[5] RAWLINGS D E, D BARRIE J. The microbiology of biomining: Development and optimization of mineral-oxidizing microbial consortia [J]. Microbiology, 2007, 153(2): 315-324. DOI: 10.1099/mic.0.2006/001206-0.
[6] CELIA M, ANA I P, VICTORIA M, JESUS S, OLGA V G, MANUEL F. Microbial diversity and metabolic networks in acid mine drainage habitats [J]. Front Microbiol, 2015, 6: 475. DOI: 10.3389/fmicb.2015.00475.
[7] CHEN L X, HUANG L N, MéNDEZ-GARCíA C, KUANG J L, HUA Z S, LIU J, SHU W S. Microbial communities, processes and functions in acid mine drainage ecosystems [J]. Curr Opin Biotechnol, 2016, 38: 150-158. DOI: 10.1016/ j.copbio.2016.01.013.
[8] HUANG L N, KUANG J L, SHU W S. Microbial ecology and evolution in the acid mine drainage model system [J]. Trends in Microbiology, 2016, 24(7): 581-593. DOI: 10.1016/ j.tim.2016.03.004.
[9] HAO X D, LIANG Y L, YIN H Q, LIU H W, ZENG W M, LIU X D. Thin-layer heap bioleaching of copper flotation tailings containing high levels of fine grains and microbial community succession analysis [J]. International Journal of Minerals, Metallurgy, and Materials, 2017, 24(4): 360-368. DOI: 10.1007/s12613-017-1415-4.
[10] MA L, WANG X, LIU X, WANG S, WANG H. Intensified bioleaching of chalcopyrite by communities with enriched ferrous or sulfur oxidizers [J]. Bioresource Technology, 2018, 268: 415-423. DOI: 10.1016/j.biortech.2018.08.019.
[11] LIU J, HUA Z S, CHEN L X, KUANG J L, LI S J, SHU W S, HUANG L N. Correlating microbial diversity patterns with geochemistry in an extreme and heterogeneous environment of mine tailings [J]. Appl Environ Microbiol, 2014, 80(12): 3677-3686. DOI: 10.1128/AEM.00294-14.
[12] HUA Z S, HAN Y J, CHEN L X, LIU J, HU M, LI S J, KUANG J L, CHAIN P S, HUANG L N, SHU W S. Ecological roles of dominant and rare prokaryotes in acid mine drainage revealed by metagenomics and metatranscriptomics [J]. The ISME Journal, 2015, 9(6): 1280-1294. DOI: 10.1038/ismej.2014.212.
[13] KUANG J, HUANG L, HE Z, CHEN L, HUA Z, JIA P, LI S, LIU J, LI J, ZHOU J, SHU W. Predicting taxonomic and functional structure of microbial communities in acid mine drainage [J]. The ISME Journal, 2016, 10(6): 1527-1539. DOI: 10.1038/ismej.2015.201.
[14] KUANG J, HUANG L, CHEN L, HUA Z, LI S, HU M, LI J, SHU W. Contemporary environmental variation determines microbial diversity patterns in acid mine drainage [J]. The ISME Journal, 2013, 7(5): 1038-1050. DOI: 10.1038/ ismej.2012.139.
[15] MA L, WANG X, FENG X, LIANG Y, XIAO Y, HAO X, YIN H, LIU H, LIU X. Co-culture microorganisms with different initial proportions reveal the mechanism of chalcopyrite bioleaching coupling with microbial community succession [J]. Bioresource Technology, 2017, 223: 121-130. DOI: 10.1016/j.biortech.2016.10.056.
[16] KAKSONEN A H, BOXALL N J, GUMULYA Y, NAHREEN K H, CHRISTINA M, TSING B, CHENG K Y, KAYLEY U, AINO-MAIJA L. Recent progress in biohydrometallurgy and microbial characterisation [J]. Hydrometallurgy, 2018, 180: 7-25. DOI: 10.1016/j.hydromet.2018.06.018.
[17] WANG Y, CHEN X, ZHOU H. Disentangling effects of temperature on microbial community and copper extraction in column bioleaching of low grade copper sulfide [J]. Bioresource Technology, 2018, 268: 480-487. DOI: 10.1016/ j.biortech.2018.08.031.
[18] YIN S, WANG L, EUGIE K, XUN C, YAN R, AN K, ZHANG L, WU A. Copper bioleaching in China: Review and prospect [J]. Minerals, 2018, 8(2): 32. DOI: 10.3390/min8020032.
[19] HALLBERG K, COUPLAND K, S, JOHNSON D. Macroscopic streamer growths in acidic, metal-rich mine waters in north wales consist of novel and remarkably simple bacterial communities [J]. Applied & Environmental Microbiology, 2006, 72(3): 2022-2030. DOI: 10.1128/aem.72. 3.2022-2030.2006.
[20] JOHNSON D B, ROLFE S, HALLBERG K B, IVERSEN E. Isolation and phylogenetic characterization of acidophilic microorganisms indigenous to acidic drainage waters at an abandoned Norwegian copper mine [J]. Environmental Microbiology, 2010, 3(10): 630-637. DOI: 10.1046/j.1462-2920.2001.00234.x.
[21] WILLIAMS K P, KELLY D P. Proposal for a new class within the phylum Proteobacteria, Acidithiobacillia classis nov., with the type order Acidithiobacillales, and emended description of the class Gammaproteobacteria [J]. Int J Syst Evol Microbiol, 2013, 63(9): 2901-2906. DOI: 10.1099/ijs.0.049270-0.
[22] FALAGa N C, JOHNSON D B. Acidithiobacillus ferriphilus sp. nov., a facultatively anaerobic iron- and sulfur-metabolizing extreme acidophile [J]. Int J Syst Evol Microbiol, 2016, 66(1): 206-211. DOI: 10.1099/ijsem.0.000698.
[23] SABRINA H, D BARRIE J. Acidithiobacillus ferridurans sp. nov., an acidophilic iron-, sulfur- and hydrogen-metabolizing chemolithotrophic gammaproteobacterium [J]. Int J Syst Evol Microbiol, 2013, 63(11): 4018-4025. DOI: 10.1099/ ijs.0.049759-0.
[24] GOLTSMAN D S A, MAUNA D, THOMAS B C, SHAH M B, VERBERKMOES N C, HETTICH R L, BANFIELD J F. New group in the Leptospirillum clade: cultivation-independent community genomics, proteomics, and transcriptomics of the new species “Leptospirillum group IV UBA BS” [J]. Applied & Environmental Microbiology, 2013, 79(17): 5384-5393. DOI: 10.1128/aem.00202-13.
[25] ZHANG X, LIU X, YANG F, LV C. Pan-genome analysis links the hereditary variation of Leptospirillum ferriphilum with its evolutionary adaptation [J]. Frontiers in Microbiology, 2018, 9: 577. DOI: 10.3389/fmicb.2018.00577.
[26] BACELAR-NICOLAU P, JOHNSON D B. Leaching of pyrite by acidophilic heterotrophic iron-oxidizing bacteria in pure and mixed cultures [J]. Applied & Environmental Microbiology, 1999, 65(2): 585-590. DOI: 10.1089/oli. 1.1999.9.105.
[27] FENG S, YANG H, WANG W. Improved chalcopyrite bioleaching by Acidithiobacillus sp. via direct step-wise regulation of microbial community structure [J]. Bioresource technology, 2015, 192: 75-82. DOI: 10.1016/j.biortech. 2015.05.055.
[28] HE J, CAI Z, ZHANG Y, XUE N, ZHENG Q. Effects of energy source on bioleaching of vanadium-bearing shale by Acidithiobacillus ferrooxidans [J]. Biochemical Engineering Journal, 2019, 151: 107355. DOI: 10.1016/j.bej.2019.107355.
[29] ZHANG X, LIU X, LIANG Y, FAN F, ZHANG X, YIN H. Metabolic diversity and adaptive mechanisms of iron- and/or sulfur-oxidizing autotrophic acidophiles in extremely acidic environments [J]. Environ Microbiol Rep, 2016, 8(5): 738-751. DOI: 10.1111/1758-2229.12435.
[30] ZHAN Y, YANG M, ZHANG S, ZHAO D, DUAN J, WANG W, YAN L. Iron and sulfur oxidation pathways of Acidithiobacillus ferrooxidans [J]. World Journal of Microbiology and Biotechnology, 2019, 35(4): 60. DOI: 10.1007/s11274-019-2632-y.
[31] AMOURIC A, BROCHIERARMANET C, JOHNSON D B, BONNEFOY V, HALLBERG K B. Phylogenetic and genetic variation among Fe(II)-oxidizing acidithiobacilli supports the view that these comprise multiple species with different ferrous iron oxidation pathways [J]. Microbiology, 2011, 157(1): 111-122. DOI: 10.1099/mic.0.044537-0.
[32] ZHANG X, YIN H Q, LIANG Y L, QIU Q Z, LIU X D. Theoretical model of the structure and the reaction mechanisms of sulfur oxygenase reductase in Acidithiobacillus thiooxidans [J]. Advanced Materials Research, 2015, 1130: 67-70. DOI: 10.4028/www.scientific. net/AMR.1130.67.
[33] MANGOLD S, VALDéS J, HOLMES D S, DOPSON M. Sulfur metabolism in the extreme acidophile Acidithiobacillus Caldus [J]. Frontiers in Microbiology, 2011, 2(1): 17. DOI: 10.3389/fmicb.2011.00017.
[34] BONNEFOY V, HOLMES D S. Genomic insights into microbial iron oxidation and iron uptake strategies in extremely acidic environments [J]. Environmental Microbiology, 2012, 14(7): 1597-1611. DOI: 10.1111/j.1462-2920.2011.02626.x.
[35] YIN Z, FENG S, TONG Y, YANG H. Adaptive mechanism of Acidithiobacillus thiooxidans CCTCC M 2012104 under stress during bioleaching of low-grade chalcopyrite based on physiological and comparative transcriptomic analysis [J]. Journal of Industrial Microbiology & Biotechnology, 2019, 46(12): 1643-1656. DOI: 10.1007/s10295-019-02224-z.
[36] TALLA E, HEDRICH S, MANGENOT S, JI B, JOHNSON D B, BARBE V, BONNEFOY V. Insights into the pathways of iron- and sulfur-oxidation, and biofilm formation from the chemolithotrophic acidophile Acidithiobacillus ferrivorans CF27 [J]. Research in Microbiology, 2014, 165(9): 753-760. DOI: 10.1016/j.resmic.2014.08.002.
[37] MEI K, NOGAMI S, KANAO T, TAKADA J, KAMIMURA K. Tetrathionate-forming thiosulfate dehydrogenase from the acidophilic, chemolithoautotrophic bacterium Acidithiobacillus ferrooxidans [J]. Applied & Environmental Microbiology, 2013, 79(1): 113-120. DOI: 10.1128/AEM. 02251-12.
[38] EDWARD C J, KOTSIOPOULOS A, HARRISON S T L. Low-level thiocyanate concentrations impact on iron oxidation activity and growth of Leptospirillum ferriphilum through inhibition and adaptation [J]. Research in microbiology, 2018, 169(10): 576-781. DOI: 10.1016/ j.resmic.2018.10.003.
[39] KHACHATRYAN A C. Influence of Fe2+ and Fe3+ on the growth of Leptospirillum ferriphilum CC and oxidation of Fe2+ [J]. Biological Journal of Armenia, 2019, 71(3): 83-88.
[40] CORTES M P, ACUA V, TRAVISANY D, SIEGEL A, LATORRE M. Integration of biological networks for Acidithiobacillus thiooxidans describes a modular gene regulatory organization of bioleaching pathways [J]. Frontiers in Molecular Biosciences, 2020, 6: 155. DOI: 10.3389/fmolb.2019.00155.
[41] ZHANG X, LIU Z, WEI G, FEI Y, LIU X. In silico genome-wide analysis reveals the potential links between core genome of Acidithiobacillus thiooxidans and its autotrophic lifestyle [J]. Frontiers in Microbiology, 2018, 9(1255. DOI: 10.3389/fmicb.2018.01255.
[42] MéNDEZ-TOVAR M, GARCíA-MEZA J V, GONZáLEZ I. Electrochemical monitoring of Acidithiobacillus thiooxidans biofilm formation on graphite surface with elemental sulfur [J]. Bioelectrochemistry, 2019, 128: 30-38. DOI: 10.1016/ j.bioelechem.2019.03.004.
[43] LIU H, LU X, ZHANG L, XIANG W, ZHU X, LI J, WANG X, LU J, WANG R. Collaborative effects of Acidithiobacillus ferrooxidans and ferrous ions on the oxidation of chalcopyrite [J]. Chemical Geology, 2018, 493: 109-120. DOI: 10.1016/ j.chemgeo.2018.05.032.
(Edited by YANG Hua)
中文导读
不同铁硫比的黄铜矿浸出过程中微生物群落能量代谢结构的变化
摘要:微生物群落的能量代谢结构在生物湿法冶金过程中起着重要作用。本文采用由嗜酸氧化亚铁硫杆菌、嗜铁钩端螺旋菌和嗜酸氧化硫硫杆菌三种菌株组成的人工微生物群落对三种不同铁硫比的黄铜矿进行浸出。经过 36 d的浸出,铁硫比约为 1:1的黄铜矿的铜浸出率最高(69.62%)。前期以铁氧化菌为主,rus和rio的表达量比后期高8倍。后期以硫氧化菌为主,tetH和HdrAB的表达量比前期高4倍。此外,上述三个生物浸出系统添加元素硫(3 g/L),且铁硫比约为2:1的黄铜矿的铜浸出率最高(80.63%)。结果表明,在浸出过程中改变铁硫比可以改变微生物群落的能量代谢结构,从而提高黄铜矿的浸出效率。
关键词:能量代谢结构;微生物群落;生物浸出;黄铜矿;铁硫比
Foundation item: Project(2017zzts382) supported by Central South University Postgraduate Independent Exploration and Innovation, China; Project(2014jpkc003) supported by Central South University Graduate Excellent Course, China; Project(2015JJ2165) supported by Hunan Provincial Natural Science Foundation of China; Project (165611031) supported by Central South University Fundamental Research Funds Special Funding, China
Received date: 2019-05-20; Accepted date: 2021-03-07
Corresponding author: YANG Yu, PhD, Professor; E-mail: csuyangyu@csu.edu.cn; ORCID: https://orcid.org/0000-0002-2112-7014
Abstract: The energy metabolism structure of microbial community plays an important role in the process of biohydrometallurgy. In this article, an artificial microbial community composed of three strains (Acidithiobacillus ferrooxidans, Leptospirillum ferriphilum and Acidithiobacillus thiooxidans) was used to leach three kinds of chalcopyrites with different iron-sulfur ratios. After 36 d of leaching, the chalcopyrite with iron-sulfur ratio of about 1:1 achieved the highest copper extraction (69.62%). In the early stage, iron oxidizing bacteria predominated, and the expression of rus and rio was 8 times higher than that in the late stage. In the late stage, sulfur oxidizing bacteria predominated, and the expression of tetH and HdrAB was 4 times higher than that in the early stage. Furthermore, the three bioleaching systems above were added with elemental sulfur (3 g/L); the chalcopyrite with iron-sulfur ratio of about 2:1 achieved the highest copper extraction (80.63%). The results suggest that the energy metabolism structure of the microbial community could be changed by changing the iron-sulfur ratio during the leaching process for improving the leaching efficiency of chalcopyrite.


