Trans. Nonferrous Met. Soc. China 24(2014) 2427-2439
Research progress of magnesium anodes and their applications in chemical power sources
Nai-guang WANG1, Ri-chu WANG2, Chao-qun PENG2, Cheng-wang HU2, Yan FENG2, Bing PENG1
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. School of Materials Science and Engineering, Central South University, Changsha 410083, China
Received 21 October 2013; accepted 14 July 2014
Abstract:
Magnesium is a promising metal used as anodes for chemical power sources. This metal could theoretically provide negative discharge potential and exhibit large capacity during the discharge process. However, when the magnesium anode is adopted for practical applications, several issues, such as the discharge products adhered to the electrode surface, the self-discharge occurring on the anode material, and the detachment of metallic particles, adversely affect its inherently good discharge performance. In this work, the types of chemical power sources using magnesium as anodes were elaborated, and the approaches to enhance its anode performance were analyzed.
Key words:
magnesium anode; discharge activity; anodic efficiency; activation mechanism; electrolyte;
1 Introduction
It is well known that chemical power source normally adopts active metal as anode to deliver electrons for current generation. During the discharge process, the anode loses electrons and dissolves into the electrolyte in the form of metallic ions. Meanwhile, the electrons are sent through external circuit to produce current for energy supply. Thus, the performance of the power source is mainly affected by the metal anode, which plays a vital role in determining the cell voltage, energy density and battery capacity [1,2]. Magnesium is promising anode material due to its inherently good discharge performance.
Firstly, magnesium has negative standard electrode potential of -2.37 V (vs SHE) [3], which is more negative than those of aluminum (-2.31 V (vs SHE)) and zinc (-1.25 V (vs SHE)) [4,5]. Thus, magnesium anode could theoretically exhibit high discharge activity and possess strong ability to deliver electrons for power generation.
Secondly, magnesium has high Faradic capacity of 2.205 A·h/g [3,4], which is lower than those of lithium (3.862 A·h/g) and aluminum (2.980 A·h/g) [4], but significantly higher than that of zinc (0.820 A·h/g) [5]. As a consequence, magnesium anode could theoretically offer a large number of electrons per unit mass to produce electric current.
Thirdly, magnesium has low density of 1.74 g/cm3, which is lower than those of aluminum (2.70 g/cm3) and zinc (7.14 g/cm3). The low density of the anode favors mass reduction of the battery system, thus leading to an achievement of high output energy density.
Based on the above three advantages, magnesium has been widely employed as ideal anode materials for many chemical power sources. The types of these power sources are summarized below and the aim of this work is to clarify the property of magnesium employed as anodes and present the approaches to enhance its discharge performance.
2 Applications of magnesium anodes in chemical power sources
As prospective anode material used in chemical power sources, magnesium possesses many excellent properties such as high discharge activity, wide voltage range, high energy density, large current capacity, and less environmental contamination [6-8]. Accordingly, this metal has been successfully used as anodes for a wide range of power sources, such as seawater activated battery, dissolved-oxygen seawater battery, air battery, hydrogen peroxide semi-fuel battery, primary battery, and secondary battery [9-13]. When these batteries are put into use, the magnesium anode is normally discharged in neutral electrolytes containing aggressive ions (e.g., Cl- and ClO4-) [14,15], which exert important effect on dissolving the discharge products, i.e., Mg(OH)2 film, adhered to the electrode surface. Thus, the anode material can be quickly activated and the lagging voltage effect for the battery system is effectively inhibited. The types of chemical power sources adopting magnesium as anodes are summarized as follows.
2.1 Seawater activated battery
Seawater activated battery was first developed in 1940s to meet the requirement of military applications [8,9]. This battery system normally includes two indispensible parts, i.e., active metal anode (e.g., magnesium) and metal chloride cathode (e.g., AgCl, CuCl, Cu2I2, and PbCl2). Since the battery is constructed in dry and stored in the dry condition, it usually has a long shelf life. The structure of the basic seawater activated battery is shown in Fig. 1 [9]. In the course of discharge, seawater acting as the electrolyte is poured into the battery system and the magnesium anode is activated to deliver electrons for power generation. At the cathode, the metal chloride receives the circulating electrons in the form of reduction reaction and the overall cell reaction is established. Seawater activated battery can be used in a wide range of short-term high-power undersea devices, e.g., detection devices, electric torpedoes, ocean buoys, air-sea rescue equipment, and sonobuoys [8,9,15,16].
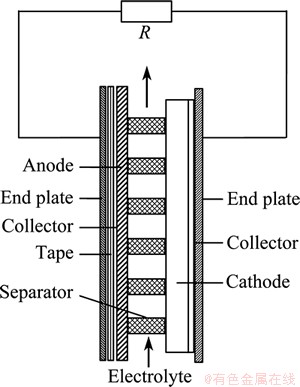
Fig. 1 Structure of basic seawater activated battery [9]
The sorts of seawater activated batteries include Mg/AgCl battery, Mg/CuCl battery, Mg/Cu2I2 battery, and Mg/PbCl2 battery. Among these batteries, Mg/AgCl battery was developed by Bell Telephone Laboratories as the power source for electric torpedoes. The design of this battery promotes the development of other small high-energy density batteries, which is also adaptable for serving as the power sources for undersea devices [9]. The current-producing and principal overall reactions for Mg/AgCl battery are as follows:
Anode: Mg→Mg2++2e (1)
Cathode: 2AgCl+2e→2Ag+2Cl- (2)
Overall: Mg+2AgCl→MgCl2+2Ag (3)
Mg/AgCl battery is able to operate at large current density and provide high energy density of 88 W·h/kg [17,18]. Moreover, this battery can be used in a wide temperature range and stored in the dry condition for more than 5 years. However, Mg/AgCl battery is costly due to the use of AgCl.
Mg/CuCl battery was developed by the former Soviet Union and became commercially available in 1949 [9]. In contrast with Mg/AgCl battery, this battery is significantly less expensive because the costly cathode material (i.e., AgCl) is replaced by the relatively cheap CuCl cathode. However, Mg/CuCl battery exhibits lower energy density and smaller capability in comparison with Mg/AgCl battery. In addition, Mg/CuCl battery cannot be stored at high humidity, and SnCl2 or argon are needed to avoid the oxidation of CuCl cathode. The major application of Mg/CuCl battery is in airborne meteorological equipment, in which the use of the more expensive Mg/AgCl system is not warranted. The current-producing and principal overall reactions for Mg/CuCl battery are as follows:
Anode: Mg→Mg2++2e (4)
Cathode: 2CuCl+2e→2Cu+2Cl- (5)
Overall: Mg+2CuCl→MgCl2+2Cu (6)
Except for the electrochemical reactions listed above, the magnesium anode used for seawater activated battery also suffers sever self-discharge occurring at the electrode/electrolyte interface. This self-discharge is side reaction, which promotes the evolution of hydrogen and leads to heat release during the discharge process. It can be expressed as follows:
Mg+2H2O→Mg(OH)2+H2 (7)
The self-discharge reduces the anodic efficiency and actual capacity of magnesium anode, thus the metal cannot be completely used to generate current. However, the evolved hydrogen caused by self-discharge stirs the electrolyte near the electrode surface, thus accelerating the self-peeling of the discharge products and sustaining relatively large active electrode area. Moreover, the heat released in the course of self-discharge plays an important role in activating the battery system, hence giving the battery good low-temperature performance.
2.2 Dissolved oxygen seawater battery
Dissolved oxygen seawater battery also adopts magnesium as the anode material and seawater as the electrolyte. An inert electrode, e.g., carbon fiber or graphite electrode [19,20], serves as the cathode, and the reaction taking place at this cathode is the reduction of dissolved oxygen in seawater [21]. The cathode does not deplete and it plays two roles during the discharge: 1) Serving as place for the cathode reaction; 2) Acting as catalyzer to promote the reduction of oxygen. The current-producing and principal overall reactions for the dissolved oxygen seawater battery are as follows:
Anode: Mg→Mg2++2e (8)
Cathode: O2+2H2O+4e→4OH- (9)
Overall: 2Mg+O2+2H2O→2Mg(OH)2 (10)
The magnesium anode used for dissolved oxygen seawater battery cannot be used in warm seawater because of the severe self-discharge of magnesium [19], even though this side reaction can be minimized by reducing the surface area of electrode.
Dissolved oxygen seawater battery can provide cell voltage of about 1 V [19]. However, the cathode reaction is restricted by the low solubility of oxygen in seawater [10,19]. Thus, the cathode current is relatively low and the battery cannot provide high energy density to meet the requirements of the high-power undersea devices. Although this defect can be compensated by increasing the velocity of seawater flowing through the battery system, dissolved oxygen seawater battery is mainly used in several long-duration and low-power undersea vehicles [10,20].
HASVOLD et al [20] invented undersea vehicle using dissolved oxygen seawater battery as the power source. When the single cell of this battery operated at load of 133 W, the endurance of the vehicle was 504 h with hydrodynamic loss of 24 W and 430 h with hydrodynamic loss of 17 W. This vehicle can travel for 2963.2 km at speed of 2 m/s when it was 600 m under the sea level. The top view of this battery is shown in Fig. 2 [20]. It can be seen that the magnesium rods and carbon fiber cathodes are placed in parallel in the cell. Seawater acting as the electrolyte enters the battery system in one end and leaves in the other. On the way of seawater flowing through the cell, the concentration of oxygen decreases whereas that of discharge products increases. Moreover, these changes in the seawater chemistry increase with the length of the cell and decrease with increasing flow velocity. Therefore, the cell voltage normally increases with increasing flow, as indeed observed. However, since the hydrodynamic work also increases with the increase of flow, there is optimum flow velocity at which the output power of the battery achieves the maximum value. In addition, there also exists minimum flow, under which the battery clogs with the discharge products and thus the discharge performance is weakened.
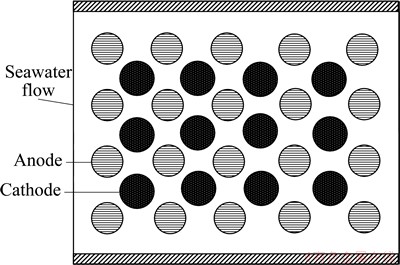
Fig. 2 Top view of structure of dissolved oxygen battery [20]
2.3 Air battery
Air battery is a special fuel cell which has the ability to provide high energy density and stable cell voltage [11]. This battery adopts magnesium as the anode and air diffusion electrode as the cathode. A neutral solution containing aggressive salt (such as NaCl) is used as the electrolyte. The structure of magnesium/air battery is shown in Fig. 3. In the course of discharge, the magnesium anode is used to deliver electrons for current generation and the atmospheric oxygen is catalytically reduced to OH- ions at the cathode. Thus, the reactant at the cathode is inexhaustible so long as the air diffusion electrode is well running. Since magnesium is the only active material in the battery system, the energy density and actual capacity are mainly determined by the anode [11]. However, the performance of air battery is significantly affected by the outside conditions and the battery can only work well in a narrow range of temperature [11,22].
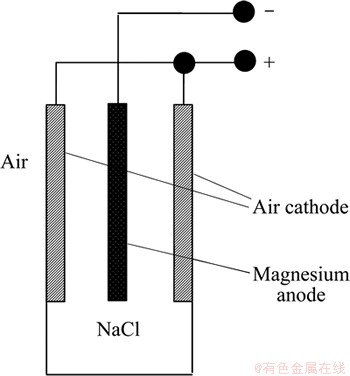
Fig. 3 Structure of air battery [11]
The current-producing and principal overall reactions for the air battery are as follows:
Anode: Mg→Mg2++2e (11)
Cathode: O2+2H2O+4e→4OH- (12)
Overall: 2Mg+O2+2H2O→2Mg(OH)2 (13)
Although magnesium/air battery could theoretically provide voltage of 3.1 V, the actual cell voltage for this battery is only 1.6 V [11]. This phenomenon is mainly attributed to the Mg(OH)2 film formed on the surface of magnesium, thus decreasing the reaction surface area and positively shifting the discharge potential. A common approach to overcome this disadvantage is using magnesium alloys to displace pure magnesium as the anode. Moreover, selecting suitable electrolytes is also important to enhance the battery performance.
KHOO et al [23] invented new electrolyte based on a phosphonium chloride ionic liquid/water mixture. They demonstrated that this electrolyte is promising candidate for magnesium/air battery because it promotes the formation of amorphous gel-like film on magnesium surface, thus resulting in a level of passivation when the battery is at open circuit. However, during the discharge process, this interfacial film becomes sufficiently conductive to allow stable discharge of magnesium for long periods. In addition, the film appears to recover its high resistance upon resting at open circuit. This phenomenon favors the longevity of actual magnesium/ air batteries because the high reactivity of magnesium is suppressed when the battery is not operating. Furthermore, KHOO et al [23] also found that water plays an important role in the formation of protective surface film on the surface of magnesium when the battery is operated at high discharge current. When the content of water reaches 8%, the battery can provide voltage of 1.6 V at the current density of 1 mA/cm2.
2.4 Hydrogen peroxide semi-fuel battery
Magnesium/hydrogen peroxide semi-fuel battery uses carbon paper electrode doped with palladium and iridium as the cathode and hydrogen peroxide (H2O2) as the cathode active material [3,4]. This battery also adopts seawater as the anolyte whereas the catholyte consists of NaCl, H2SO4, and H2O2. The anode and cathode in the battery system are separated by a conductive membrane [4]. The structure of magnesium/hydrogen peroxide semi-fuel battery is shown in Fig. 4. During the discharge process, magnesium or magnesium alloy serving as the anode delivers electrons for power generation. Meanwhile, hydrogen peroxide at the cathode receives the electrons in the form of reduction reaction and the overall cell reaction is established. The cathode of hydrogen peroxide semi-fuel battery does not consume during the discharge process, it mainly serves as place for cathodic reaction and acts as catalyzer to accelerate the reduction of hydrogen peroxide.
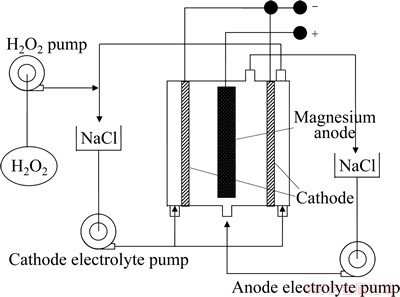
Fig. 4 Structure of magnesium/hydrogen peroxide semi-fuel battery [11]
Magnesium/hydrogen peroxide semi-fuel battery could theoretically provide voltage of 3.25 V in neutral electrolyte [3,24], which is higher than that of the magnesium/dissolved oxygen seawater battery and magnesium/air battery. This result is mainly attributed to the cathode active material, i.e., H2O2, which has stronger oxidizing activity in contrast with oxygen. The current-producing and principal overall reactions for magnesium/hydrogen peroxide semi-fuel battery in neutral electrolyte are as follows:
Anode: Mg→Mg2++2e (14)
Cathode: HO2-+H2O+2e→3OH- (15)
Overall: Mg+HO2-+H2O→Mg(OH)2+OH- (16)
In addition, this battery also suffers several side reactions in neutral electrolyte as follows.
Decomposition of hydrogen peroxide:
2H2O2→2H2O+O2 (17)
Self-discharge of magnesium anode:
Mg+2H2O→Mg(OH)2+H2 (18)
Deposition reactions:
Mg2++2OH-→Mg(OH)2 (19)
Mg2++CO32-→MgCO3 (20)
These side reactions not only promote the evolution of hydrogen, but also accelerate the deposition of MgCO3 and Mg(OH)2 in the battery system, hence decreasing the actual cell voltage and anodic efficiency. Sulphuric acid is usually added into the catholyte to dissolve the sediments (MgCO3 and Mg(OH)2), thus enlarging the active electrode area and enhancing the cell voltage. The theoretical voltage of this battery reaches 4.14 V in acid electrolyte [3,24], and the electrochemical reactions of magnesium/hydrogen peroxide semi-fuel battery in acid electrolyte are as follows:
Anode: Mg→Mg2++2e (21)
Cathode: H2O2+2H++2e→2H2O (22)
Overall: Mg+H2O2+2H+→Mg2++2H2O (23)
So far, magnesium/hydrogen peroxide semi-fuel battery is mainly used as an energetic system for low rate, long endurance undersea vehicle [12,25,26]. MEDEIROS et al [25] investigated the factors that control the performance of magnesium/hydrogen peroxide semi-fuel battery. They found that the battery performance was mainly determined by the flow rates of anolyte and catholyte, the concentration of hydrogen peroxide, the working current density, and the temperature of battery system. In addition, MEDEIROS et al [26] also investigated the impact of different cathode materials on the discharge performance of magnesium/hydrogen peroxide semi-fuel battery. The results indicated that the battery can provide voltage of 1.3 V at 25 mA/cm2 when electrocatalyst of nickel foil catalyzed with palladium and iridium was utilized, whereas this voltage reached 1.5 V when electrocatalyst of planar carbon catalyzed with palladium and iridium was tested.
2.5 Primary battery and secondary battery
Primary battery can only be used once and is unable to return to its initial state via charging. Magnesium primary battery uses magnesium or magnesium alloys as the anode and manganese dioxide (MnO2) as the cathode. Magnesium perchlorate (Mg(ClO4)2) usually serves as the electrolyte for this battery [14]. The structure of magnesium primary battery is shown in Fig. 5. It can be observed that the battery has shape of column with carbon rod locating in the central of the column. This carbon rod is connected with the cup wall to shorten the path of the battery. In addition, magnesium anode is surrounded by the mixture of cathode materials, which has good contact with magnesium anode, carbon rod, and cup wall. Thus, the reaction surface area is enlarged. During the discharge process, the magnesium anode is activated in the magnesium perchlorate to deliver electrons for power generation. At the cathode, the manganese dioxide receives the circulating electrons via reduction reaction and the overall cell reaction is established. The current-producing and principal overall reactions for magnesium/manganese dioxide primary battery are as follows:
Anode: Mg+2OH-→Mg(OH)2+2e (24)
Cathode: 2MnO2+H2O+2e→Mn2O3+2OH- (25)
Overall: Mg+2MnO2+H2O→Mn2O3+Mg(OH)2 (26)
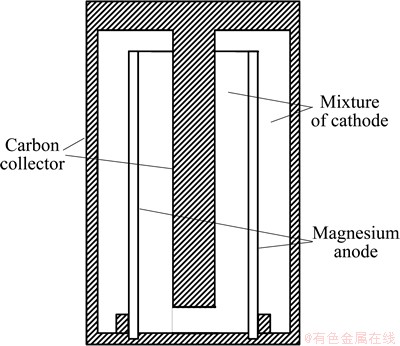
Fig. 5 Structure of magnesium primary battery [11]
The theoretical voltage provided by magnesium/ manganese dioxide primary battery is 2.8 V [11]. When the battery is put into use, the surface of magnesium is normally covered by Mg(OH)2 or MgO film, which reduces the active electrode area and makes the voltage decrease to 1.9-2.0 V. In addition, this film induces the lagging voltage effect but protects the magnesium anode and inhibits the self-discharge occurring in the course of storage. However, the protective film is not stable and will break in the course of discharge. It is hard for the fractured film to recover itself and thus the magnesium anode suffers severe self-discharge when it is used intermittently, resulting in the decrease of the anodic efficiency and the accumulation of evolved hydrogen in the battery system. Adding corrosion inhibitor (such as BaCrO4 and Li2CrO4) into the electrolyte is effective approach to inhibit the self-discharge and thus enhance the intermittent performance [11]. The anodic efficiency of the magnesium anode during the process of successive discharge is 60%-70%, whereas that in the course of intermittent discharge or discharge at low current density is 40%-50%. The average voltage of the magnesium/ manganese dioxide primary battery is 1.6-1.8 V, which is higher than that of the zinc/manganese dioxide primary battery. Moreover, it is found that the magnesium/manganese dioxide primary battery has good low temperature property and is able to operate at low temperature of -20 °C [11].
Secondary battery is able to return to its initial state via charging after discharge. Magnesium secondary battery uses magnesium or magnesium alloys as the anode, and uses transition-metal sulfides, oxides, or organic compounds as the cathode. Organic aprotic polar solvents usually serve as the electrolyte. One of the key technologies for developing practical rechargeable batteries is establishing reversible redox (deposition and dissolution) process of magnesium [13]. However, because magnesium is easily oxidized by water (moisture) and other protic-solvents to form passivation film, it is generally difficult to establish reversible process of electrochemical deposition and dissolution of magnesium, not only in aqueous but also in organic media. One possible option to realize reversible process of magnesium deposition/dissolution is to use Grignard Reagents as the electrolytes, which consists of ether solution and organo- magnesium complex [13]. It has been generally accepted that no compact passivating film covers the surface of magnesium and then reversible deposition and dissolution of magnesium occur with low overvoltage in such electrolyte system. In addition, ionic liquids are also good candidates for the electrolytes used in magnesium secondary battery [13]. This electrolyte is composed of ions without solvents and thus it provides great range of properties.
The charge-discharge performance of magnesium secondary battery is also controlled by the cathode material, in which the Mg2+ ions diffuse slowly [27,28]. Hence, it is of crucial importance to select suitable embedded cathode material for the secondary battery. ZHENG et al [29] prepared the MgCoSiO4 cathode material by high-temperature solid-state reaction, molten salt method, and mixed solvothermal approach. The mesoporous MgCoSiO4 was achieved using the non-surfactant mixed solvothermal approach. The electrochemical data indicated that the mesoporous MgCoSiO4 had higher peak current, larger discharge capacity, and higher flat plateau compared with the corresponding bulk materials. This phenomenon is mainly due to the fact that the mesoporous MgCoSiO4 possesses larger surface area in contact with the electrolyte and thus provides more active sites for electrochemical reactions to occur. In addition, the thin pore walls also shorten the transportation/diffusion path for both electrons and ions. Accordingly, using the cathode material with mesoporous structure is new approach to enhance its reaction activity in rechargeable magnesium batteries.
3 Factors adversely affecting performance of magnesium anode
It is mentioned above that magnesium possesses negative standard electrode potential of -2.37 V (vs SHE). Thermodynamically, this metal is able to provide negative discharge potential when connected with load. However, the electrode surface of magnesium is normally covered by the discharge products (i.e., Mg(OH)2 film) in the course of discharge [3,4,30,31], thus hindering the electrode surface from contact with the electrolyte. As a result, the discharge potential shifts positively with the increase of discharge time when magnesium is put into practical applications.
The Mg(OH)2 film adhered to the electrode surface is attributed to the dissolution/deposition reactions of magnesium [32]. The magnesium anode dissolves into the electrolyte in the form of Mg2+ ions in the course of discharge and these ions will deposit on the electrode surface in the form of Mg(OH)2 when the concentration of Mg2+ ions is higher than the solubility limit [32]. This process is depicted as follows.
Dissolution of magnesium:
Mg→Mg2++2e (27)
Deposition of magnesium hydroxide:
Mg2++2H2O→Mg(OH)2+2H+ (28)
It is also elaborated previously that magnesium has high Faradic capacity of 2.205 A·h/g. Thus, the magnesium anode could theoretically deliver large number of electrons per unit mass to generate current. However, the magnesium anode is prone to suffer severe self-discharge or side hydrogen evolution reaction in aqueous electrolyte [3,4,33,34], especially several impurities with electrode potentials more positive than that of magnesium exist in the anode material [35,36]. These impurities act as local cathode to promote the evolution of hydrogen, resulting in decrease of anodic efficiency and loss of actual capacity. Moreover, many metallic particles are detached from the electrode surface in the course of discharge. These detached particles cannot be used to generate current and therefore also result in loss of anodic efficiency.
The self-discharge of magnesium is due to its inherently negative difference effect (NDE) [35,37,38], which means the increase of hydrogen evolution by increasing the applied current density. SONG et al [37] investigated the electrochemical and corrosion behavior of pure magnesium in 1 mol/L NaCl solution and summarized a model that clearly revealed the NDE of magnesium.
1) The surface of magnesium in 1 mol/L NaCl solution is normally covered by partially protective film, which cannot effectively protect magnesium in the course of anodic polarization.
2) The dissolution of magnesium during anodic polarization includes two steps:
Mg→Mg++e (29)
2Mg++2H+→2Mg2++H2 (30)
Only the first step delivers electrons for current generation, whereas the electrons of Mg+ ions are grabbed by the hydrated proton for hydrogen evolution in the second step.
3) In the course of magnesium dissolution, the formation of magnesium hydride (MgH2) is observed and this phenomenon also results in loss of magnesium used for current generation:
Mg+2H++2e→MgH2 (31)
Magnesium hydride is not stable and will decompose.
MgH2+2H2O→Mg2++2OH-+2H2 (32)
4) Many metallic particles are locally detached in the course of anodic polarization. These detached particles promote the evolution of hydrogen via chemical dissolution and thus cannot be used to generate current.
4 Approaches to enhance discharge performance of magnesium anode
The magnesium anode possessing good discharge performance is required to exhibit several characters summarized below.
1) Negative discharge potential, which means that the magnesium anode retains strong ability to deliver electrons for energy supply.
2) Short activation time, which signifies that the discharge potential can quickly come into the steady state in the course of discharge.
3) High anodic efficiency, which implies that the per unit mass of magnesium is able to offer more electrons to generate current.
Until recently, several approaches have been adopted to enhance the discharge performance of magnesium as follows.
1) Adding alloying elements, such as Ce, Zn, Mn, Pb, Tl, Sn, Hg, and Ga [1,3,4,8,16,39-41], into magnesium. This approach, usually known as alloying, accelerates the self-peeling of the discharge products and inhibits the side hydrogen evolution reaction.
2) Using heat treatment, e.g., homogenization annealing, solid solution and aging, to modify the distributions of alloying elements and secondary phases in the magnesium matrix [42,43], thus improving the electrochemical and corrosion performances of magnesium.
3) Adopting plastic deformation (such as rolling and extrusion) to modify the microstructure of magnesium [1,15,39,44], thus leading to an enhancement of the discharge performance.
Moreover, the discharge performance of magnesium is also affected by the electrolyte [14,44,45]. Accordingly, adding a small quantity of additives into the electrolyte favors the self-peeling of the discharge products and suppresses the side reactions if the battery possesses closed system [45,46].
The approaches mentioned above are elaborated below.
4. 1 Alloying
Alloying is an effective approach to enhance the discharge performance of magnesium [47]. The alloying elements added into magnesium, on one hand, accelerate the self-peeling of the discharge products, on the other hand, minimize the self-discharge. Several alloying elements even possess both the two functions. According to the alloying elements, the magnesium anodes can be classified into different categories.
4.1.1 Mg-Al-Zn series alloys
These alloys include AZ31 (Mg-3%Al-1%Zn), AZ61 (Mg-6%Al-1%Zn), AZ63 (Mg-6%Al-3%Zn), and AZ91 (Mg-9%Al-1%Zn) (hereafter in mass fraction, except for special illustration). The common feature of these magnesium alloys is low discharge activity but good corrosion resistance. They are mainly used as anodes in chemical power sources to supply energy for long-term low-power undersea applications. In Mg-Al-Zn series alloys, aluminum plays important role in inhibiting the self-discharge, whereas zinc decreases the lagging effect of voltage induced by aluminum and favors uniform dissolution of magnesium.
The effect of aluminum on the corrosion behavior of magnesium has been well investigated. In Mg-Al-Zn series alloys, aluminum mainly exists in two forms: 1) solid solution in magnesium matrix; 2) β-Mg17Al12 phase within the grains and along the grain boundaries [32,48]. SONG et al [49] investigated the corrosion behavior of as-cast AZ91D magnesium alloy in 1 mol/L NaCl solution. They found that the β-Mg17Al12 phase plays double role in determining the corrosion behavior of AZ91D magnesium alloy. When the fraction of this phase is high and the phase distributes continuously along the grain boundaries, the β-Mg17Al12 phase mainly serves as corrosion barrier to hinder the corrosion. In contrast, accelerated corrosion is expected when the fraction of the β-Mg17Al12 phase is low and this phase distributes discontinuously along the grain boundaries. PARDO et al [48] studied the corrosion behavior of AZ31, AZ80, and AZ91D magnesium alloys in 3.5% NaCl solution. The results indicated that the corrosion degrees of these magnesium alloys were determined by the content of aluminum and the microstructures of magnesium alloys. The increase in the content of aluminum was valuable to enhance the corrosion resistance of magnesium alloy.
The discharge behavior of Mg-Al-Zn series alloys used as anodes has been reported in several references. BALASUBRAMANIAN et al [45] investigated the discharge performance of Mg/AgCl battery using AZ31 as anode in de-ionized water and 3.3% NaCl solution, and clarified the effects of electrolyte concentration, temperature and current density on the battery performance. The results indicated that the activation time for the battery to reach voltage of 2 V under 2 Ω load was 1500 and 400 ms in de-ionized water and 3.3% NaCl solution, respectively. The discharge potential of AZ31 alloy exhibited negative shift with the increase of NaCl concentration at each current density. In addition, the discharge potential displayed positive shift with the increase of current density, and this phenomenon was inhibited in NaCl solution with high concentration (0.5 mol/L) because the electrolyte conductivity increased with the increase of the electrolyte concentration. Furthermore, the electrolyte temperature also affected the cell performance. The cell voltage increased as the electrolyte temperature increased. When the temperature reached 30 °C, the cell voltage was close to 1.5 V at 400 mA.
HIROI [17] investigated the effect of pressure on the discharge behavior of Mg/AgCl seawater activated battery using AZ31 and AZ61 magnesium alloys as the anodes. The results indicated that the cell voltages of the two batteries at high pressure were 20-30 mV higher than those at low pressure. The voltage of the battery using AZ61 as the anode was higher and more stable than that adopting AZ31 as the anode, attributed to the nature of the discharge products covered on the anode surfaces. The observations of the cells after discharge showed clogging between the plates in the case of AZ31. The sludge was rather dense and adhesive. Grayish-white product also formed on the surface of AZ61, but it was granular, rapidly settling sludge, and not so adherent to the plate. Thus, AZ61 is more suitable to serve as the anode for seawater activated battery in comparison with AZ31.
4.1.2 Mg-Li series alloys
Mg-Li series alloys with higher discharge activity than Mg-Al-Zn series alloys are employed as the anodes for metal/hydrogen peroxide semi-fuel batteries, which mainly serve as the power sources for undersea vehicles [3,4,24,50]. The addition of lithium into magnesium exerts important effect on enhancing the discharge activity and theoretical capacity of magnesium due to the high activity and large Faradic capacity of lithium. In addition, the β phase with body centered cubic structure forms in the Mg-Li series alloys when the addition of lithium exceeds 5.7%, which improves the plastic deformability and is valuable to obtain the magnesium electrode with different shapes.
CAO et al [4] investigated the electrochemical behavior of Mg-Li, Mg-Li-Al, and Mg-Li-Al-Ce alloys in 0.7 mol/L NaCl solution. The results indicated that the discharge products of these alloys were loosely adhered to the alloy surfaces and thus were partially responsible for the high discharge activity. The discharge activities and anodic efficiencies increased in the order: Mg-Li < Mg-Li-Al < Mg-Li-Al-Ce, whereas the polarization resistances decreased in the order: Mg-Li > Mg-Li-Al > Mg-Li-Al-Ce. The Mg-Li-Al-Ce alloy exhibited the best performance in term of discharge activity, anodic efficiency and activation time. Thus, the alloying elements, i.e., aluminum and germanium, played vital role in enhancing the discharge performance of Mg-Li series alloys.
CAO et al [3] also prepared the Mg-Li-Al-Ce-Zn and Mg-Li-Al-Ce-Zn-Mn alloys using an induction melting and studied their electrochemical behavior in 0.7 mol/L NaCl solution. The results showed that the Mg-Li-Al-Ce-Zn-Mn alloy gave better performance than Mg-Li-Al-Ce-Zn alloy. The discharge products of Mg-Li-Al-Ce-Zn-Mn alloy exhibited as less dense and less homogeneous surface layer and thus was partially responsible for the high discharge activity. The anodic efficiency of the Mg-Li-Al-Ce-Zn-Mn alloy was more than 80% at typical discharge potentials. The magnesium/hydrogen peroxide semi-fuel cell using Mg-Li-Al-Ce-Zn-Mn as the anode displayed maximum power density of 91 mW/cm2 at room temperature.
 et al [24] investigated the electrochemical performances of magnesium/hydrogen peroxide semi-fuel cells with Mg, Mg-Li-Al-Ce and Mg-Li-Al-Ce-Y as anodes. They found that pure Mg and Mg-Li-Al-Ce-Y were more anti-corrosive than Mg-Li-Al-Ce when immersed in 0.7 mol/L NaCl solution. However, Mg-Li-Al-Ce-Y showed higher discharge activity than pure Mg and Mg-Li-Al-Ce in the course of potentiostatic discharge. The discharge current density of Mg-Li-Al-Ce-Y at -1.0 V in 0.7 mol/L NaCl solution was about 43 mA/cm2. Moreover, the maximum peak power density of magnesium/ hydrogen peroxide semi-fuel cells using Mg, Mg-Li-Al-Ce and Mg-Li-Al-Ce-Y as anodes reached 83.4, 91.3 and 110.0 mW/cm2, respectively.
et al [24] investigated the electrochemical performances of magnesium/hydrogen peroxide semi-fuel cells with Mg, Mg-Li-Al-Ce and Mg-Li-Al-Ce-Y as anodes. They found that pure Mg and Mg-Li-Al-Ce-Y were more anti-corrosive than Mg-Li-Al-Ce when immersed in 0.7 mol/L NaCl solution. However, Mg-Li-Al-Ce-Y showed higher discharge activity than pure Mg and Mg-Li-Al-Ce in the course of potentiostatic discharge. The discharge current density of Mg-Li-Al-Ce-Y at -1.0 V in 0.7 mol/L NaCl solution was about 43 mA/cm2. Moreover, the maximum peak power density of magnesium/ hydrogen peroxide semi-fuel cells using Mg, Mg-Li-Al-Ce and Mg-Li-Al-Ce-Y as anodes reached 83.4, 91.3 and 110.0 mW/cm2, respectively.
4.1.3 Mg-Hg and Mg-Hg-Ga series alloys
The Mg-Hg and Mg-Hg-Ga series alloys were developed by the former Soviet Union and have been utilized by the Russian army until recently. In these alloy, the mercury possesses high over-potential for hydrogen evolution reaction and thus plays important role in enhancing the anodic efficiency [51]. Because mercury is not environment friendly, gallium which also has high over-potential for the evolution of hydrogen is adopted to partially displace mercury. The Mg-Hg and Mg-Ga-Hg series alloys exhibit higher discharge activities and shorter activation time than other magnesium alloys, and mainly serve as anodes for Mg/CuCl seawater activated batteries which are used as power sources for electrical torpedoes [9].
FENG et al [6,16,40] investigated the electrochemical and corrosion behavior of Mg-Hg-Ga series alloys in 3.5% NaCl solution. The results indicated that three secondary phases, i.e., Mg3Hg, Mg5Ga2 and Mg21Ga5Hg3, existed in these alloys. The Mg3Hg phase with large volume greatly enhanced the discharge activity of magnesium but decreased the corrosion resistance. The alloy containing Mg3Hg phase provided discharge potential of -1.989 V (vs SCE) at 100 mA/cm2. The Mg21Ga5Hg3 phase presented as small particles and was able to enhance the corrosion resistance of magnesium. The alloy containing Mg21Ga5Hg3 phase had a corrosion current density of 1.19 mA/cm2. In addition, the corrosion resistance of Mg-Hg-Ga series alloy dramatically decreased when the secondary phases distributed along the grain boundaries in the form of eutectic, whereas the corrosion resistance and the comprehensive discharge performance were improved when the secondary phases had small sizes and distributed homogeneously in the magnesium matrix. Furthermore, FENG et al [6] analyzed the activation mechanism of Mg-Hg-Ga series alloy and clarified that the activation mechanism was dissolution-deposition. The addition of gallium accelerated the self-peeling of corrosion film and favored the formation of magnesium amalgams, thus promoting the discharge activity.
4.1.4 Mg-Al-Tl series alloys
Mg-Al-Tl series alloys were developed by British Magnesium Electronics Company. These alloys possess high discharge activity and have the ability to provide high cell voltage for seawater activated batteries [9]. In Mg-Al-Tl series alloys, thallium exhibits high over-potential for hydrogen evolution and thus plays an important role in enhancing the anodic efficiency. In addition, the dissolved Tl3+ ions during the discharge process are reduced by magnesium and deposit on the electrode surface in the form of thallium, which exerts effect on promoting the self-peeling of the discharge products and activating the anode material. Until recently, the Mg-Al-Tl series alloys which have been successfully used include AT61 (Mg-6%Al-1%Tl) and AT75 (Mg-7%Al-5%Tl) [9]. AT61 with shorter activation time than AZ61 is mainly used as anode for short-term and high-power seawater activated battery. The discharge products of AT75 presents in fine black flaky form that washes out of the battery system with flowing electrolyte. In static battery, thicker but porous brown black film may accumulate. This does not affect the anode performance but may require special design consideration. The seawater activated battery with AT75 anode and AgCl cathode provides stable voltage higher than 1.75 V at 12.5 mA/cm2. This voltage decreases with the increase of discharge time at 310 mA/cm2, and it is close to 1.3 V after discharge for 4 min. Thus, Mg-Al-Tl series alloys exhibit good discharge performance in contrast with Mg-Al-Zn series alloys. However, thallium is poisonous and there exists the possibility of accumulation of thallium in the body by skin absorption from continuous unprotected handing. All handing should therefore be carried out using non-absorbent type gloves.
4.1.5 Mg-Al-Pb series alloys
Mg-Al-Pb series alloys which were also developed by British Magnesium Electronics Company possess lower discharge activity in comparison with Mg-Al-Tl series alloys [9]. However, these alloys still exhibit higher discharge activity than Mg-Al-Zn series alloys. One of Mg-Al-Pb series alloys is AP65 (Mg-6%Al- 5%Pb), which has been used as the anode for high-power seawater activated battery. In AP65 magnesium alloy, lead has high over-potential for hydrogen evolution and thus is valuable to enhance the anodic efficiency.
UDHAYAN and BHATT [14] investigated the electrochemical behavior of pure magnesium, AZ31, AZ61, and AP65 magnesium alloys in aqueous magnesium perchlorate solution. The results indicated that the electrode/electrolyte interfacial mechanism was activation-controlled. In addition, the dissolution rate is the greatest in the case of AP65 alloy and hence its charge-transfer resistance is lower than that for either AZ31 or AZ61 alloy. Furthermore, the exchange current and double-layer capacitance follow the following sequence: pure magnesium>AP65>AZ61>AZ31.
WANG et al [8] investigated the activation mechanism of AP65 in 3.5% NaCl solution and found that aluminum and lead cannot significantly enhance the discharge activity of magnesium when only one of the alloying elements existed. However, the magnesium electrode can be dramatically activated when both of the alloying elements were added. The activation mechanism for aluminum and lead to magnesium was dissolution-deposition, and there was synergistic effect between aluminum and lead: the dissolved Pb2+ ions during the discharge process can easily precipitate on magnesium surface in the form of lead oxides, and this process facilitated the deposition of the dissolved Al3+ ions in the form of Al(OH)3, which detached the precipitated Mg(OH)2 film via 2Mg(OH)2·Al(OH)3 and promoted the self-peeling of the discharge products, thus enhancing the discharge activity of magnesium.
4. 2 Heat treatment
Heat treatment modifies the number and distribution of secondary phases in magnesium alloys, and favors the compositional homogeneity of magnesium matrix, thus leading to the improvement of discharge performance. The secondary phase in magnesium alloy normally has more positive electrode potential than magnesium matrix and hence serves as local cathode to accelerate the corrosion of magnesium. Heat treatment promotes the dissolution of secondary phase or makes the secondary phase distribute homogeneously in the magnesium matrix, therefore controlling the electrochemical behavior of magnesium. The sorts of heat treatment used for magnesium alloys include homogenization annealing, solid solution treatment, aging and subsequent annealing after plastic deformation.
ANDREI et al [36] investigated the homogenization annealing on microstructure evolution and electrochemical behavior of AZ63 magnesium alloy. They found that equiaxed arrangement with eutectic precipitation of β-Mg17Al12 phase located homogeneously along the grain boundaries and inside the grains of the as-cast AZ63 alloy. After homogenization annealing at 385 °C for 10 h, the β phase almost dissolved, only some particles with small sizes remained in the magnesium matrix. However, the homogenization annealing did not improve the electrochemical performance of the as-cast AZ63 alloy. The homogenized alloy exhibited lower anodic efficiency and actual capacity compared with the as-cast one. The results demonstrated that the β phase played an important role in protecting the as-cast AZ63 alloy.
FENG et al [43] reported the effect of precipitate morphology evolution on the electrochemical and corrosion properties of aged Mg-4.8%Hg-8%Ga alloys. The results indicated that dispersed Mg21Ga5Hg3 phase with tetragonal structure precipitated at 423 K. Slab and massive Mg5Ga2 phases with orthogonal structure precipitated at 439 K and dissolved in magnesium matrix at 506 K. The number densities of the dispersed Mg21Ga5Hg3 and slab and massive Mg5Ga2 precipitates increased when the aging time increased to 96 h and decreased when the aging time increased to 160 h in the alloys aged at 473 K. The size and number density of the dispersed Mg21Ga5Hg3 increased when the aging temperature increased from 423 to 523 K in the alloys aged for 8 h. The larger number densities of the precipitates phases promoted the activation dissolution of magnesium matrix according to the dissolving-deposition mechanism. The most negative stable potential of -1.935 V (vs SCE) occurred in the alloy aged at 473 K for 96 h. Bad electrochemical properties occurred in the alloy aged at 473 K for 160 h due to gathering and growing-up of Mg21Ga5Hg3 and Mg5Ga2 precipitates. Larger sizes and number densities of the dispersed Mg21Ga5Hg3 and slab and massive Mg5Ga2 precipitates in the alloys led to larger galvanic corrosion driving-force and worse corrosion resistance. The best produced alloy for application in seawater battery was Mg-4.8%Hg-8%Ga alloys aged at 473 K for 8 h.
4. 3 Plastic deformation
Plastic deformation distinctly modifies the microstructure of magnesium alloy and thus plays vital role in determining its discharge performance. Magnesium alloys normally exhibit poor deformability owing to its hexagonal close packed (HCP) structure. Accordingly, these alloys are usually subjected to hot working (such as hot rolling and hot extrusion) to obtain electrodes with different shapes.
Until recently, the research about magnesium anode mainly focused on the roles of alloying elements and heat treatment, whereas the effect of plastic deformation on the anode performance is yet to be properly understood. ZHAO et al [44] prepared the AZ31B magnesium alloy strip with thickness of 1.5 mm using hot extrusion. This strip was then homogenized at 400 °C for 24 h followed by multi-pass hot rolling to obtain the magnesium plates with different thicknesses. The results indicated that the hot extrusion refined the grains of AZ31B magnesium alloy and changed the distribution of β-Mg17Al12 phase. The multi-pass hot rolling further refined the grains and made the β-Mg17Al12 phase distributed homogeneously in the matrix. The electrochemical data implied that the fine grains and uniform grain boundaries were valuable to improve the discharge current of AZ31B magnesium alloy. The hot rolled alloy after subsequent annealing for 1 h exhibited higher discharge current density but shorter life span, attributed to the change of the grain size and the distribution of β-Mg17Al12 phase. The β-Mg17Al12 phase dissolved into the magnesium matrix as the annealing time increases, thus weakening the discharge performance.
ZHAO et al [1] reported the discharge behavior of Mg-4%Ga-2%Hg alloy and found that this alloy displayed different discharge behavior in as-cast, homogenized, hot rolled, and annealed states. The as-cast alloy possessed dendritic structure morphology with many coarse intermetallic compounds distributed both on the grain boundary and in the matrix. After homogenization annealing at 698 K for 16 h, only the large Mg3Hg phase with block shape and the dispersed Mg21Ga5Hg3 phase with point shape existed in the alloy. A hot rolling at 673 K fractured the Mg3Hg phase and made it distribute along the rolling direction. The subsequent annealing at 533 K for 2 h favored homogeneous distribution of the secondary phases. The electrochemical data indicated that hot rolling and annealing shifted the corrosion potential to more negative value and increased the corrosion current density of Mg-4%Ga-2%Hg alloy. The annealed alloy exhibited shorter activation time and more negative discharge potential than the other alloys at each current density. The seawater activated battery using the annealed alloy as anode provided voltage of 1.451 V and mass energy density of 147 W·h/kg.
WANG et al [15] investigated the effect of hot rolling and subsequent annealing on discharge behavior of AP65 magnesium alloy in 3.5% NaCl solution. The results indicated that the microstructure of AP65 alloy played vital role in determining the discharge performance. The β-Mg17Al12 phase in the as-cast alloy hindered the discharge process but enhanced the anodic efficiency. The homogenized alloy possessed higher discharge activity than the as-cast one, attributed to the disappearance of β-Mg17Al12 phase and the existence of Al-Mn particles. These Al-Mn particles served as strong local cathodes to promote the discharge process. The hot rolling refined the grains of AP65 alloy and favored the compositional homogeneity of the magnesium matrix, thus leading to an enhancement of the utilization efficiency. The subsequent annealing at 150 °C for 4 h reduced the high density of dislocations caused by hot rolling without increasing the grain size, and thus the annealed alloy at 150 °C exhibited higher discharge activity and anodic efficiency than other alloys. This result revealed that hot rolling and subsequent annealing at proper temperature is effective approach to boosted the discharge performance of AP65 alloy. The subsequent annealing at 350 °C for 4 h enlarged the fine grains produced by hot rolling and thus debased the anode performance.
4. 4 Electrolyte modification
The electrode process of magnesium usually includes the following steps: 1) diffusion of hydrated protons and corrosive ions (such as Cl-) to magnesium surface; 2) dissolution of magnesium which leads to the formation of Mg(OH)2 and promotes the side hydrogen evolution reaction; 3) detachment of Mg(OH)2 from magnesium surface. Generally, the second step is the rate-determining step and therefore the electrode process of magnesium is mainly activation-controlled. This means that the discharge behavior of magnesium is greatly determined by the property of electrolyte. Thus, modifying the electrolyte is an effective approach to enhance the anode performance.
UDHAYAN et al [14] investigated the electrochemical behavior of pure magnesium, AZ31, AZ61, and AP65 magnesium alloys in magnesium perchlorate with different concentrations. They found that the exchange current densities were greatly affected by the concentration of magnesium perchlorate. All these exchange current densities increased with the increase of electrolyte concentration when the concentration was lower than 2.0 mol/L, whereas the exchange current densities tend to be constant when the concentration is higher than 2.0 mol/L. Hence, the magnesium perchlorate with concentration of 2.0 mol/L was suitable to serve as the electrolyte for magnesium battery.
ZHAO et al [44] studied the electrochemical behavior of the hot rolled AZ31B magnesium alloy in sodium chloride solutions with different concentrations. The results indicated that the discharge current increased with the increase of electrolyte concentration during the process of potentiostatic discharge. However, the increase in electrolyte concentration decreased the life span and actual capacity. Thus, selecting the sodium chloride solution with proper concentration is an effective approach to improve the discharge performance of magnesium.
CAO et al [4] investigated the electrochemical behavior of Mg-Li, Mg-Li-Al, and Mg-Li-Al-Ce alloys in 0.7 mol/L NaCl solution with and without the addition of 5×10-5 mol/L Ga2O3. They found that the addition of Ga2O3 promoted the discharge activities and enhanced the anodic efficiencies of all these magnesium alloys. The anodic efficiency of Mg-Li-Al-Ce alloy in 0.7 mol/L NaCl solution was 81.8%, whereas that in the solution added with Ga2O3 reached 87.6%. Therefore, adding additives into the electrolyte is valuable to enhance the discharge performance when the battery system is closed.
5 Conclusions
1) Magnesium has been widely used as the anodes for different kinds of chemical power sources. The magnesium anode possessing good discharge performance is specially required to exhibit high anodic efficiency and provide negative discharge potential with short activation time for the potential to reach the steady value.
2) The approaches to achieve this performance include alloying, heat treatment, plastic deformation and electrolyte modification. These approaches not only accelerate the self-peeling of the discharge products but also inhibit the evolution of hydrogen in the course of discharge, thus leading to an enhancement of the discharge performance.
3) Because of the abundant reserve and good discharge performance, it is believed that magnesium anode possesses good application prospect in the future and has the potential to displace lithium as the anodes for chemical power sources.
References
[1] ZHAO J, YU K, HU Y, LI S, TAN X, CHEN F, YU Z. Discharge behavior of Mg-4wt%Ga-2wt%Hg alloy as anode for seawater activated battery [J]. Electrochimica Acta, 2011, 56(24): 8224-8231.
[2] UDHAYAN R, MUNIYANDI N, MATHUR P B. Studies on magnesium and its alloys in battery electrolytes [J]. British Corrosion Journal, 1992, 27(1): 68-71.
[3] CAO D, WU L, WANG G, LV Y. Electrochemical oxidation behavior of Mg-Li-Al-Ce-Zn and Mg-Li-Al-Ce-Zn-Mn in sodium chloride solution [J]. Journal of Power Sources, 2008, 183(2): 799-804.
[4] CAO D, WU L, SUN Y, WANG G,  Y. Electrochemical behavior of Mg-Li, Mg-Li-Al and Mg-Li-Al-Ce in sodium chloride solution [J]. Journal of Power Sources, 2008, 177(2): 624-630.
Y. Electrochemical behavior of Mg-Li, Mg-Li-Al and Mg-Li-Al-Ce in sodium chloride solution [J]. Journal of Power Sources, 2008, 177(2): 624-630.
[5] SURESH KANNAN A R, MURALIDHARAN S, SARANGAPANI K B, BALARAMACHANDRAN V, KAPALI V. Corrosion and anodic behaviour of zinc and its ternary alloys in alkaline battery electrolytes [J]. Journal of Power Sources, 1995, 57(1): 93-98.
[6] FENG Yan, WANG Ri-chu, YU Kun, PENG Chao-qun, ZHANG Jia-pei, ZHANG Chun. Activation of Mg-Hg anodes by Ga in NaCl solution [J]. Journal of Alloys and Compounds, 2009, 473(1): 215-219.
[7] WANG Nai-guang, WANG Ri-chu, PENG Chao-qun, FENG Yan, ZHANG Xiang-yu. Corrosion behavior of Mg-Al-Pb and Mg-Al-Pb-Zn-Mn alloys in 3.5% NaCl solution [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(10): 1936-1943.
[8] WANG Nai-guang, WANG Ri-chu, PENG Chao-qun, FENG Yan, ZHANG Xiang-yu. Influence of aluminium and lead on activation of magnesium as anode [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(8): 1403-1411.
[9] KOONTZ R F, LUCERO R D. Handbook of batteries [M]. New York: McGraw-Hill, 2002.
[10] HASVOLD  ,
,  N. Electrochemical power sources for unmanned underwater vehicles used in deep sea survey operations [J]. Journal of Power Sources, 2001, 96(1): 252-258.
N. Electrochemical power sources for unmanned underwater vehicles used in deep sea survey operations [J]. Journal of Power Sources, 2001, 96(1): 252-258.
[11] LINDEN D, REDDY T B. Handbook of batteries (3rd ed) [M]. New York: McGraw-Hill, 2002.
[12] LV Yan-zhuo, LIU Min, XU Yan, CAO Dian-xue, FENG Jing. The electrochemical behaviors of Mg-8Li-3Al-0.5Zn and Mg-8Li- 3Al-1.0Zn in sodium chloride solution [J]. Journal of Power Sources, 2012, 225: 124-128.
[13] KAKIBE T, HISHII J, YOSHIMOTO N, EGASHIRA M, MORITA M. Binary ionic liquid electrolytes containing organo-magnesium complex for rechargeable magnesium batteries [J]. Journal of Power Sources, 2012, 203: 195-200.
[14] UDHAYAN R, BHATT D P. On the corrosion behaviour of magnesium and its alloys using electrochemical techniques [J]. Journal of Power Sources, 1996, 63(1): 103-107.
[15] WANG Nai-guang, WANG Ri-chu, PENG Chao-qun, FENG Yan, CHEN Bin. Effect of hot rolling and subsequent annealing on electrochemical discharge behavior of AP65 magnesium alloy as anode for seawater activated battery [J]. Corrosion Science, 2012, 64: 17-27.
[16] FENG Yan, WANG Ri-chu, YU Kun, PENG Chao-qun, LI Wen-xian. Influence of Ga and Hg on microstructure and electrochemical corrosion behavior of Mg alloy anode materials [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(6): 1363-1366.
[17] HIROI M. Pressure effects on the performance and the e.m.f. of the Mg-AgCl seawater battery [J]. Journal of Applied Electrochemistry, 1980, 10(2): 203-211.
[18] SAMMOURA F, LEE K B, LIN L. Water-activated disposable and long shelf life microbatteries [J]. Sensors and Actuators A: Physical, 2004, 111(1): 79-86.
[19] WILCOCK W S D, KAUFFMAN P C. Development of a seawater battery for deep-water applications [J]. Journal of Power Sources, 1997, 66(1): 71-75.
[20] HASVOLD  , LIAN T, HAAKAAS E,
, LIAN T, HAAKAAS E,  N, PERELMAN O, CORDIER S. A long-range, autonomous underwater vehicle using magnesium fuel and oxygen from the sea [J]. Journal of Power Sources, 2004, 136(2): 232-239.
N, PERELMAN O, CORDIER S. A long-range, autonomous underwater vehicle using magnesium fuel and oxygen from the sea [J]. Journal of Power Sources, 2004, 136(2): 232-239.
[21] HASVOLD  , HENRIKSEN H, CITI G, JOHANSEN B
, HENRIKSEN H, CITI G, JOHANSEN B  ,
,  T, GALETTI R. Sea-water battery for subsea control systems [J]. Journal of Power Sources, 1997, 65(1): 253-261.
T, GALETTI R. Sea-water battery for subsea control systems [J]. Journal of Power Sources, 1997, 65(1): 253-261.
[22] MA Yi-bin, LI Ning, LI De-yu, ZHANG Mi-lin, HUANG Xiao-mei. Performance of Mg-14Li-1Al-0.1Ce as anode for Mg-air battery [J]. Journal of Power Sources, 2011, 196(4): 2346-2350.
[23] KHOO T, HOWLETT P C, TSAGOURIA M, MACFARLANE D R, FORSYTH M. The potential for ionic liquid electrolytes to stabilise the magnesium interface for magnesium/air batteries [J]. Electrochimica Acta, 2011, 58: 583-588.
[24]  Yan-zhuo, XU Yan, CAO Dian-xue. The electrochemical behaviors of Mg, Mg-Li-Al-Ce and Mg-Li-Al-Ce-Y in sodium chloride solution [J]. Journal of Power Sources, 2011, 196(20): 8809-8814.
Yan-zhuo, XU Yan, CAO Dian-xue. The electrochemical behaviors of Mg, Mg-Li-Al-Ce and Mg-Li-Al-Ce-Y in sodium chloride solution [J]. Journal of Power Sources, 2011, 196(20): 8809-8814.
[25] MEDEIROS M G, BESSETTE R R, DESCHENES C M, ATWATER D W. Optimization of the magnesium-solution phase catholyte semi-fuel cell for long duration testing [J]. Journal of Power Sources, 2001, 96(1): 236-239.
[26] MEDEIROS M G, DOW E G. Magnesium-solution phase catholyte seawater electrochemical system [J]. Journal of Power Sources, 1999, 80(1): 78-82.
[27] SHARMA J, HASHMI S A. Magnesium ion transport in poly (ethylene oxide)-based polymer electrolyte containing plastic- crystalline succinonitrile [J]. Journal of Solid State Electrochemistry, 2013, 17(8): 2283-2291.
[28] CHENG Gang, XU Qiang, ZHAO Xi, DING Fei, ZHANG Jing, LIU Xing-jiang, CAO Dian-xue. Electrochemical discharging performance of 3D porous magnesium electrode in organic electrolyte [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(5): 1367-1374.
[29] ZHENG Y, NULI Y, CHEN Q, WANG Y, YANG J, WANG J. Magnesium cobalt silicate materials for reversible magnesium ion storage [J]. Electrochimica Acta, 2012, 66: 75-81.
[30] QUACH N C, UGGOWITZER P J, SCHMUTZ P. Corrosion behaviour of an Mg-Y-RE alloy used in biomedical applications studied by electrochemical techniques [J]. Comptes Rendus Chimie, 2008, 11(9): 1043-1054.
[31] LIU M, SCHMUTZ P, ZANNA S, SEYEUX A, ARDELEAN H, SONG G, ATRENS A, MARCUS P. Electrochemical reactivity, surface composition and corrosion mechanisms of the complex metallic alloy Al3Mg2 [J]. Corrosion Science, 2010, 52(2): 562-578.
[32] ZHAO M C, SCHMUTZ P, BRUNNER S, LIU M, SONG G, ATRENS A. An exploratory study of the corrosion of Mg alloys during interrupted salt spray testing [J]. Corrosion Science, 2009, 51(6): 1277-1292.
[33] HUANG D B, HU J Y, SONG G L, GUO X P. Self-corrosion, galvanic corrosion and inhibition of GW103 and AZ91D Mg alloys in ethylene glycol solution [J]. Corrosion Engineering, Science and Technology, 2013, 48(2): 155-160.
[34] HUANG D, HU J, SONG G L, GUO X. Galvanic corrosion and inhibition of GW103 and AZ91D Mg alloys coupled to an Al alloy in an ethylene glycol solution at ambient and elevated temperatures [J]. Corrosion, 2012, 68(6): 475-488.
[35] LIU L J, SCHLESINGER M. Corrosion of magnesium and its alloys [J]. Corrosion Science, 2009, 51(8): 1733-1737.
[36] ANDREI M, DI GABRIELE F, BONORA P L, SCANTLEBURY D. Corrosion behaviour of magnesium sacrificial anodes in tap water [J]. Materials and Corrosion, 2003, 54(1): 5-11.
[37] SONG G, ATRENS A, STJOHN D, NAIRN J, LI Y. The electrochemical corrosion of pure magnesium in 1 N NaCl [J]. Corrosion Science, 1997, 39(5): 855-875.
[38] BALASUBRAMANIAN R, VELUCHAMY A, VENKATAKRISHNAN N. Gasometric corrosion-rate studies of magnesium alloy in magnesium batteries [J]. Journal of Power Sources, 1994, 52(2): 305-308.
[39] YU K, TAN X, HU Y, CHEN F, LI S. Microstructure effects on the electrochemical corrosion properties of Mg-4.1%Ga-2.2%Hg alloy as the anode for seawater-activated batteries [J]. Corrosion Science, 2011, 53(5): 2035-2040.
[40] FENG Yan, WANG Ri-chu, PENG Chao-qun, WANG Nai-guang. Influence of Mg21Ga5Hg3 compound on electrochemical properties of Mg-5%Hg-5%Ga alloy [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(1): 154-159.
[41] WANG Ping, LI Jian-ping, GUO Yong-chun, YANG Zhong, XIA Feng, WANG Jian-li. Effect of Sn on microstructure and electrochemical properties of Mg alloy anode materials [J]. Rare Metal Materials and Engineering, 2012, 41(12): 2095-2099. (in Chiense)
[42] FENG Y, WANG R, PENG C. Influence of aging treatments on microstructure and electrochemical properties in Mg-8.8Hg-8Ga (wt%) alloy [J]. Intermetallics, 2012, 33: 120-125.
[43] FENG Yan, WANG Ri-chu, PENG Chao-qun, QIU Ke, WANG Nai-guang, ZHANG Chun, ZHANG Jia-pei. Aging behaviour and electrochemical properties in Mg-4.8Hg-8Ga (wt.%) alloy [J]. Corrosion Science, 2010, 52(10): 3474-3480.
[44] ZHAO H, BIAN P, JU D. Electrochemical performance of magnesium alloy and its application on the sea water battery [J]. Journal of Environmental Science, 2009, 21: s88-s91.
[45] BALASUBRAMANIAN R, VELUCHAMY A, VENKATAKRISHNAN N, GANGADHARAN R. Electrochemical characterization of magnesium/silver chloride battery [J]. Journal of Power Sources, 1995, 56(2): 197-199.
[46] SONG G, ATRENS A, JOHN D S, WU X, NAIRN J. The anodic dissolution of magnesium in chloride and sulphate solutions [J]. Corrosion Science, 1997, 39(10): 1981-2004.
[47] MALYI O I, TAN T L, MANZHOS S. In search of high performance anode materials for Mg batteries: Computational studies of Mg in Ge, Si, and Sn [J]. Journal of Power Sources, 2013, 233: 341-345.
[48] PARDO A, MERINO M C, COY A E, VIEJO F, ARRABAL R, FELI S. Influence of microstructure and composition on the corrosion behaviour of Mg/Al alloys in chloride media [J]. Electrochimica Acta, 2008, 53(27): 7890-7902.
[49] SONG G, ATRENS A, DARGUSCH M. Influence of microstructure on the corrosion of diecast AZ91D [J]. Corrosion Science, 1999, 41(2): 249-273.
[50]  Yan-zhuo, LIU Min, XU Yan, CAO Dian-xue, FENG Jing, WU Rui-zhi, ZHANG Mi-lin. The electrochemical behaviors of Mg-8Li-0.5Y and Mg-8Li-1Y alloys in sodium chloride solution [J]. Journal of Power Sources, 2013, 239(1): 265-268.
Yan-zhuo, LIU Min, XU Yan, CAO Dian-xue, FENG Jing, WU Rui-zhi, ZHANG Mi-lin. The electrochemical behaviors of Mg-8Li-0.5Y and Mg-8Li-1Y alloys in sodium chloride solution [J]. Journal of Power Sources, 2013, 239(1): 265-268.
[51] YU Kun, HUANG Qiao, ZHAO Jun, DAI Yu-long. Electrochemical properties of magnesium alloy anodes discharged in seawater [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(9): 2184-2190.
镁阳极的研究进展及其在化学电源中的应用
王乃光1,王日初2,彭超群2,胡程旺2,冯 艳2,彭 兵1
1. 中南大学 冶金与环境学院,长沙 410083;
2. 中南大学 材料科学与工程学院,长沙 410083
摘 要:镁是一种极有应用前景的化学电源阳极材料。理论上,金属镁在放电过程中能提供较负的放电电位和较大的比容量。但在实际使用过程中,一些因素严重影响镁与生俱来的优异放电性能,如覆盖在阳极表面的放电产物、析氢副反应和金属颗粒的脱落等。阐述镁作为阳极在不同化学电源中的应用,并分析提高镁放电性能的途径。
关键词:镁阳极;放电活性;阳极效率;活化机制;电解液
(Edited by Chao WANG)
Foundation item: Project supported by the Postdoctoral Science Foundation of Central South University; Project (2014M552151) supported by the China Postdoctoral Science Foundation; Project (51101171) supported by the National Natural Science Foundation of China
Corresponding author: Nai-guang WANG; Tel: +86-1331960772; Fax: +86-731-88836638; E-mail: wangnaiguang505@163.com
DOI: 10.1016/S1003-6326(14)63367-7
Abstract: Magnesium is a promising metal used as anodes for chemical power sources. This metal could theoretically provide negative discharge potential and exhibit large capacity during the discharge process. However, when the magnesium anode is adopted for practical applications, several issues, such as the discharge products adhered to the electrode surface, the self-discharge occurring on the anode material, and the detachment of metallic particles, adversely affect its inherently good discharge performance. In this work, the types of chemical power sources using magnesium as anodes were elaborated, and the approaches to enhance its anode performance were analyzed.


