Trans. Nonferrous Met. Soc. China 24(2014) 1813-1818
Facile synthesis of tin oxide nanocrystals and their photocatalytic activity
Zhi-qian JIA1, Hui-jie SUN1, Yan WANG1, Tian-li ZHEN1, Qing CHANG1,2
1. College of Chemistry, Beijing Normal University, Beijing 100875, China;
2. Science and Technology on Remanufacturing Laboratory, Academy of Armored Forces Engineering, Beijing 100072, China
Received 26 June 2013; accepted 23 January 2014
Abstract:
Tin oxide nanocrystals with diameters smaller than 10 nm were synthesized using Na2SnO3 and CO2 as reactants and cetyltrimethylammonium bromide (CTAB) as stabilizer under mild conditions. As a mild acidic gas, CO2 is favorable for the accurate adjustment of pH value of Na2SnO3 solution. Stannate salt is stable, cheap and easy in operation. The effects of Na2SnO3 concentration, CTAB concentration, aging temperature, and aging time on the nanocrystals were studied. It was found that, with the increasing Na2SnO3 concentration, aging temperature and aging time, SnO2 nanocrystals size decreases. The formation of SnO2 nanocrystals can be interpreted by electrostatic-interaction mechanism. SnO2 nanocrystals show high photocatalytic activities in the degradation of Rhodamine B solution. The catalytic activity of small nanocrystals is higher than that of large ones.
Key words:
tin oxide nanocrystals; facile synthesis; photocatalytic activity;
1 Introduction
As a wide band-gap n-type semiconductor (Eg = 3.6 eV at bulk state), tin oxide has drawn considerable attention owing to its various applications such as catalysts for oxidation of organic compounds [1], solid-state gas sensors for reducing gases [2], rechargeable Li-batteries [3,4], optical electronic devices [5], photocatalysts [6]. The size of tin oxide particles has been shown to drastically affect their properties due to surface and/or spacial confinement effects. Various methods have been developed for the synthesis of tin oxide nanocrystals, including thermolysis of organometallic precursors, sol-gel [7], hydrolysis of SnCl2 [8,9] or SnF2 [10], sonochemistry, and hydrothermal synthesis [11,12]. However, the fabrication of tin oxide nanocrystals with diameter smaller than 10 nm is still a challenge. Recently, WU et al [13] reported a lysteine-assisted hydrothermal route for generating SnO2 nanocrystals (<10 nm). But the conditions (120-240 °C) are very harsh. JUTTUKONDA et al [14] employed stannate salt, carbon dioxide and fourth-generation dendritic polymers for the synthesis of tin oxide nanocrystals (<10 nm). Nevertheless, the dendritic polymers are very expensive due to the difficulty in synthesis.
In this work, tin oxide nanocrystals with diameters smaller than 10 nm were synthesized using Na2SnO3 and CO2 as reactants and cetyltrimethylammonium bromide (CTAB) as stabilizer under mild conditions. Firstly, Na2SnO3 aqueous solution was carbonated by CO2 to generate stannate acid. Then, CTAB solution was added, which interacted with stannate anions via electrostatic interaction. After aging, filtration, washing, drying and calcinations, tin oxide nanocrystals (<10 nm) were obtained. Compared with strong or moderate acids such as hydrochloric acid, H2SO4 or H3PO4, CO2 is a mild acidic gas and is favorable for the accurate adjustment of pH value of Na2SnO3 solution, as well as for the emission reduction of greenhouse gas [15]. Meanwhile, in contrast with moisture-sensitive reagents (e.g. tin chloride), stannate salt is stable, cheap and easy in operation. In addition, the CTAB stabilizer is much cheaper than most of the other stabilizers. The effects of Na2SnO3 concentration, CTAB concentration, aging temperature, and aging time on the nanocrystals were studied. The photocatalytic activity of the SnO2 nanocrystals in the degradation of Rhodamine B (RhB, Scheme 1) aqueous solution was evaluated.
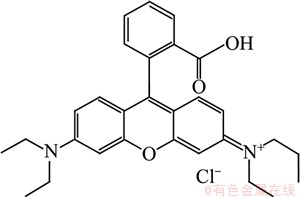
Scheme 1 Rhodamine B
2 Experimental
2.1 Materials
The chemical reagents, including Na2SnO3 and CTAB, were in analytical grade and used as-received without further purification. The fresh Na2SnO3 solution was prepared with degassed pure water prior to the reaction.
2.2 Preparation of tin oxide
In a typical synthesis, CO2 gas was bubbled into 250.0 mL of Na2SnO3 solution in a stirred tank at ambient temperature until a certain pH was attained. Then CTAB solution was added to precipitate the stannate acid, and the white turbid suspension was aged at design temperature for a certain time. After centrifugation, the as-obtained precipitate was washed with pure water and alcohol, dried at room temperature and then at 110 °C for 2 h. Last, the white powder was calcinated at 150 °C in Muffle furnace for 2 h and at 400 °C for another 2 h to remove CTAB completely.
2.3 Analysis
The products were observed on a transmission electron microscope (JEM 2010, Japan). In each image, more than one hundred particles were measured to determine the average size. The XRD pattern was analyzed by monochromatized Cu Kα incident radiation (Shimadzu XRD-6000). Nitrogen absorption-desorption isotherms were measured at 77 K by a volumetric technique (V-Sorb 4800P, Beijing), and the surface area was calculated by the Brunaner-Emmett-Teller (BET) method.
2.4 Photocatalytic activity
The photocatalytic activities of the SnO2 nanocrystals in the degradation of RhB aqueous solution were evaluated. First, 50.0 mL of 1×10-5 mol/L RhB solution (containing 50.0 mg of SnO2 sample) was stirred in a vessel (d115 mm) for 30 min at room temperature to attain an adsorption-desorption equilibrium. Then, a 500 W high-pressure mercury lamp (Beijing Tianmai Henghui Co. Ltd) was employed to irradiate the stirred RhB solution, and the distance between the lamp and the solution was 45.0 cm. The concentration of RhB was monitored by an UV-vis spectrometer (Cintra 10e, Australia) during UV irradiation.
3 Results and discussion
3.1 Evolution of pH value during carbonation
Figure 1 shows the pH evolution of Na2SnO3 solution during carbonation. The pH value drops quickly in the initial period (pH>10) and then declines slowly (8<pH<10). In the initial period, the dissolved CO2 reacts with the excess alkaline in Na2SnO3 reagent, resulting in the rapid decline of pH value. The reactions can be expressed as follows [16]:
CO2+OH- =HCO3- (1)
HCO3-+OH- =CO32-+H2O (2)
Then, with the consumption of OH-, Na2SnO3 hydrolyzes and generates stannate acid and OH-, leading to the slow decline of pH values. The hydrolyzation of Na2SnO3 is written as follows:
SnO32-+3H2O=Sn(OH)4+2OH- (3)
2Sn(OH)4=(OH)3Sn—O—Sn(OH)3+H2O (4)
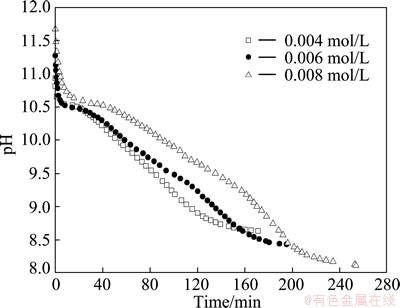
Fig. 1 Evolution of pH values in carbonation for various Na2SnO3 concentrations
3.2 Effects of reaction conditions on SnO2 nanocrystals
The effects of Na2SnO3 concentrations were carried out at carbonation pH of 9.0, 0.0033 mol/L CTAB, aging temperature of 110 °C for 24 h, Na2SnO3 concentrations of 0.004, 0.006 and 0.008 mol/L, respectively. Figures 2(a), (b) and (c) show that with the increasing Na2SnO3 concentration, the SnO2 nanoparticles size decreases from 5.8 nm to 4.9 nm and 4.5 nm, respectively. Electron diffraction (ED) of nanoparticles exhibits polycrystalline rings (Fig. 2(d)). In contrast, the effects of CTAB concentrations (0.0011, 0.0020 and 0.0033 mol/L) on the nanoparticles are found to be not significant.
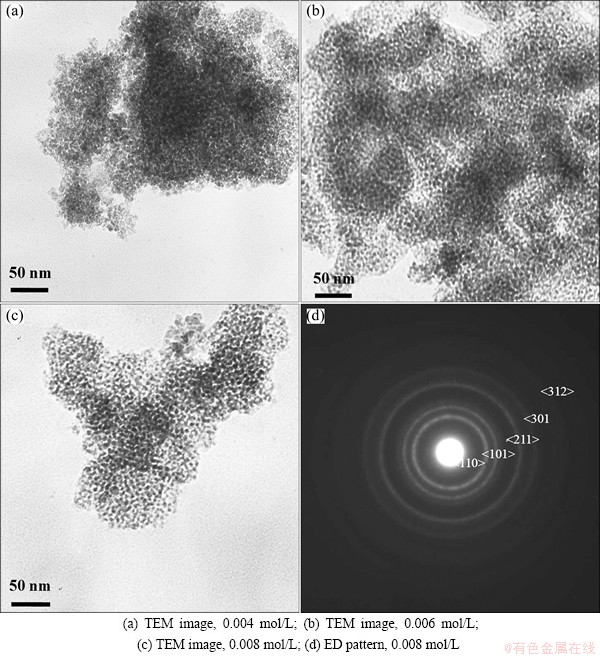
Fig. 2 Effects of Na2SnO3 concentrations on SnO2 nanoparticles
The effects of aging temperature were conducted at carbonation pH of 10.0, 0.0033 mol/L CTAB, 0.008 mol/L Na2SnO3, aging time of 24 h, aging temperature of 25, 70 and 110 °C, respectively. Figure 3 shows that with the increasing aging temperature, the nanoparticles size declines from 16.2 nm to 8.4 nm and 6.7 nm, respectively. The HRTEM analysis (Fig. 3(d)) shows that the interplaner spacing of 1D fringes is 0.335 nm, which is consistent well with the (110) lattice spacing of rutile SnO2. Figure 4 shows the respective XRD patterns of SnO2 nanoparticles with 6.7 nm and 16.2 nm in size. The diffraction peaks are in agreement with those of tetragonal tin oxide with rutile structure (PDF00-001-0657, a=4.7382 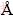 , b=4.7382
, b=4.7382 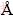 , c=3.1871
, c=3.1871 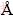 ). The crystallites size is estimated in terms of the Scherrer equation:
). The crystallites size is estimated in terms of the Scherrer equation:
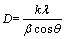 (5)
(5)
where l is wavelength of X–ray (0.154056 nm); k=0.89; β is the full-width-half-maximum (FWHM) of diffraction peak; θ is the diffraction angle. The crystallites sizes are found to be 7.8 nm and 14.2 nm respectively. These values are consistent with the TEM results (6.7 nm and 16.2 nm), indicating that the nanoparticles are single crystals. The BET specific surface areas of the nanocrystals are 480 m2/g (6.7 nm in size) and 149 m2/g (16.2 nm in size).
The effects of aging time were studied at carbonation pH of 10.0, 0.0033 mol/L CTAB, 0.008 mol/L Na2SnO3, aging temperature of 110 °C, and aging time of 12 h, 24 h and 48 h, respectively. It was found that with rising aging time, the particles size declines from 8.5 nm to 6.7 nm and 6.5 nm, respectively.
3.3 Formation mechanism of SnO2 nanocrystals
The plausible formation mechanism of SnO2 nanocrystals is shown in Fig. 5. During carbonation, the solution is alkaline and the as-generated H2SnO3 sol is negative charged (denoted as I-). It interacts with the positively charged head-groups of CTAB (S+) to form I-S+ precipitate. In the subsequent aging period, the H2SnO3 sol protected by the surrounding CTAB molecules further condenses. After washing, drying and calcinations, CTAB is removed and SnO2 nanocrystals are obtained.
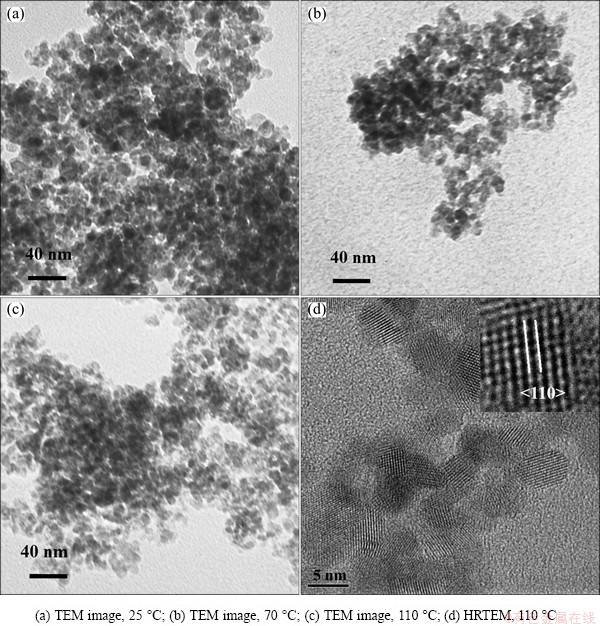
Fig. 3 Effects of aging temperature on SnO2 nanoparticles

Fig. 4 XRD patterns of SnO2 nanoparticles with size of 6.7 nm (a) and 16.2 nm (b)
The experimental results can be well interpreted by the above electrostatic-interaction mechanism. With rising Na2SnO3 concentration, the nucleation rate of H2SnO3 increases, resulting in the reduced particles size. The CTAB concentration in the experiment is sufficient to interact with the H2SnO3 sol so that its effect is negligible. At elevated aging temperature and prolonged aging time, the condensation degree of hydrous SnO2 increases, leading to the reduction of particles size.
3.4 Photocatalytic activity
The as-prepared SnO2 nanocrystals were employed as photocatalysts in the degradation of RhB solution. Figure 6(a) shows that, under UV irradiation and catalysis of SnO2 nanocrystals (6.7 nm in size), the absorption of RhB solution declines with time and disappears completely after 80 min. Figure 6(b) shows the evolution of c/c0 of RhB solution (i.e. the ratio of RhB concentration to its initial concentration). When UV irradiation was not provided, in the presence of SnO2 nanocrystals, the variation of RhB concentration in 80 min can be neglected. When SnO2 nanocrystals were not presented, under UV irradiation, the c/c0 of RhB solution at 40 min was 58%. In the presence of SnO2 nanocrystals and UV irradiation, for nanocrystals of 16.2 nm, 8.4 nm and 6.7 nm in size, the c/c0 values of RhB solution at 40 min were 30%, 20% and 15%, respectively. That is, the catalytic activity of small nanocrystals is higher than that of large ones, which is ascribed to the high surface area of small nanocrystals [17]. For all the nanocrystals, the c/c0 of RhB solution at 80 min is near zero.
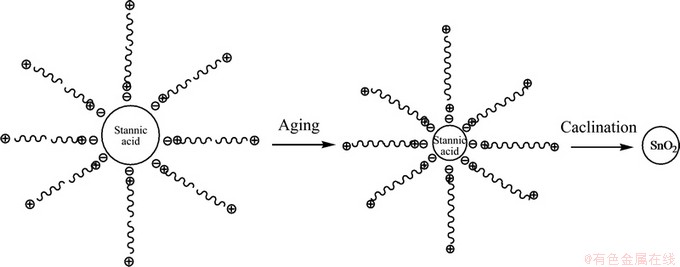
Fig. 5 Formation mechanism of SnO2 nanocrystals
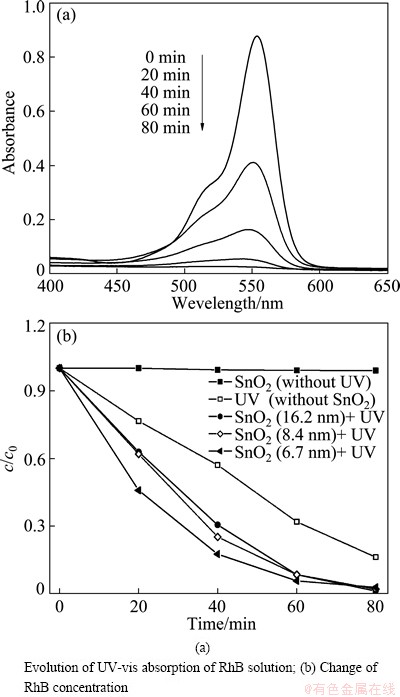
Fig. 6 Photocatalytic degradation of RhB solution
The degradation mechanism of RhB under UV light is described as follows: when the energy (hv) of a photon matches or exceeds the bandgap of SnO2, an electron (e-) is excited from the valence band into the conductive band, generating a hole (h+) [18]; then, oxidation agents (such as ·O2-, ·OH, ·OOH) are formed, which oxidize RhB molecules.
SnO2+hv→e-+h+ (6)
H2O+h+→·OH+H+ (7)
O2+e-→·O2- (8)
·O2-+H+→·OOH (9)
RhB+hv→RhB* (10)
RhB*+O2 (or·O2-, ·OH)→degraded products (11)
The high photodegradation rate of RhB in the presence of SnO2 nanocrystals can be attributed to the generation of oxidative free radicals on the surface of SnO2 nanocrystals.
4 Conclusions
Tin oxide nanocrystals stabilized with CTAB were synthesized by the Na2SnO3-CO2 reaction system. With the increased Na2SnO3 concentration, elevated aging temperature and prolonged aging time, the size of SnO2 nanocrystals decreases. The SnO2 nanocrystals show high photocatalytic activities in the degradation of RhB solution. The catalytic activity of small nanocrystals is higher than that of large ones. A simple, cost-effective and versatile method for the preparation of SnO2 nanocrystals was provided, as well as other weak-acidic metal oxide nanoparticles, such as Al2O3, SiO2 and ZnO.
References
[1] GARRO R, NAVARRO M T, PRIMO J, CORMA A. Lewis acid-containing mesoporous molecular sieves as solid efficient catalysts for solvent-free Mukaiyama-type aldol condensation [J]. J Catal, 2005, 233: 342-350.
[2] LEITE E R, WEBER I T, LONGO E, VARELA J A. A new method to control particle size and particle size distribution of SnO2 nanoparticles for gas sensor application [J]. Adv Mater, 2000, 12: 965-968.
[3] IDOTA Y, KUBOTA T, MATSUFUJI A, MAEKAW Y, MIYASAKA T. Tin-based amorphous oxide: A high-capacity lithium-ion-storage material [J]. Science, 1997, 276: 1395-1397.
[4] ZHANG Yue-lan, LIU Ying, LIU Mei-lin. Nanostructured columnar tin oxide thin film electrode for lithium ion batteries [J]. Chem Mater, 2006, 18: 4643-4646.
[5] CHOPRA K L, MAJOR S, PANDYA D K. Transparent conductors—A status review [J]. Thin Solid Films, 1983, 102: 1-46.
[6] BAYAL N, JEEVANANDAM P. Sol-gel synthesis of SnO2-MgO nanoparticles and their photocatalytic activity towards methylene blue degradation [J]. Materials Research Bulletin, 2013, 48: 3790-3799.
[7] BRIOIS V, BELIN S, CHALACA M Z, SANTOS R H A, SANTILLI C V, PULCINELLI S H. Solid-state and solution structural study of acetylacetone-modified tin(IV) chloride used as a percursor of SnO2 nanoparticles prepared by a sol-gel route [J]. Chem Mater, 2004, 16: 3885-3894.
[8] JIANG Lu-hua, SUN Gong-quan, ZHOU Zhen-hua, SUN Shi-guo, WANG Qi, YAN Shi-you, LI Huan-qiao, TIAN Juan, GUO Jun-song, ZHOU Bing, XIN Qin. Size-controllable synthesis of monodispersed SnO2 nanoparticles and application in electrocatalysts [J]. J Phys Chem B, 2005, 109: 8774-8778.
[9] LEITE E R, GIRALDI T R, PONTES F M, LONGO E, BELTRAN A, ANDRE J. Crystal growth in colloidal tin oxide nanocrystals induced by coalescence at room temperature [J]. Appl Phys Lett, 2003, 83: 1565-1568.
[10] MASUDA Y, OHJI W, KATO K. Highly enhanced surface area of tin oxide nanocrystals [J]. J Am Ceram Soc, 2010, 93: 2140-2143.
[11] EDUARDO J H L, CAUE R, ELSON L, EDSON R L. Growth kinetics of tin oxide nanocrystals in colloidal suspensions under hydrothermal conditions [J]. Chem Phys, 2006, 328: 229-235.
[12] RISTIC M, IVANDA M, POPOVIC S, MUSIC S. Dependence of nanocrystalline SnO2 particle size on synthesis route [J]. J Non-Cryst Solid, 2002, 303: 270-280.
[13] WU Shui-sheng, CAO Hua-qiang, YIN Shuang-feng, LIU Xiang-wen, ZHANG Xin-rong. Amino acid-assisted hydrothermal synthesis and photocatalysis of SnO2 nanocrystals [J]. J Phys Chem C, 2009, 113: 17893-17898.
[14] JUTTUKONDA V, PADDOCK R, RAYMOND J E, DENOMME D, RICHARDSON A E, SLUSHER L E, FAHLMAN B D. Facile synthesis of tin oxide nanoparticles stabilized by dendritic polymers [J]. J Am Chem Soc, 2006, 128: 420-421.
[15] ZEVENHOVEN R, ELONEVA S, TEIR S. Chemical fixation of CO2 in carbonates: Routes to valuable products and long-term storage [J]. Catal Today, 2006, 115: 73-79.
[16] HOLLIDAY A K, HUGHES G, WALKER S M. Carbon in comprehensive inorganic chemistry [M]. Vol. Ⅰ. Oxford: Pergamon Press, 1973.
[17] TALEBIAN N, JAFARINEZHAD F. Morphology-controlled synthesis of SnO2 nanostructures using hydrothermal method and their photocatalytic applications [J]. Ceramics International, 2013, 39: 8311-8317.
[18] LI Jing-yi, MA Wan-hong, CHEN Chong-cheng, ZHAO Jin-cai, ZHU Huai-yong, GAO Xue-ping. Photodegradation of dye pollutants on one-dimensional TiO2 nanoparticles under UV and visible irradiation [J]. J Mol Catal A, 2007, 261: 131-138.
SnO2纳米晶的制备及光催化性能
贾志谦1,孙慧杰1,王 妍1,甄甜丽1,常 青1,2
1. 北京师范大学 化学学院,北京 100875;
2. 装甲兵工程学院 再制造技术重点实验室,北京 100072
摘 要:以Na2SnO3和CO2为反应物,十六烷基三甲基溴化铵(CTAB)为稳定剂,在温和条件下合成粒径小于 10 nm的SnO2纳米晶。CO2作为酸性气体,有利于精确调控Na2SnO3溶液的pH值,Na2SnO3价廉且性质稳定,易于操作。研究Na2SnO3浓度、CTAB浓度、熟化温度和熟化时间等对产物的影响。结果表明,随着Na2SnO3浓度的增加、熟化温度的升高和熟化时间的延长,粒径变小,粒子形成过程可由静电作用机理解释。SnO2 纳米晶具有良好的光催化活性,且随着粒径的减小,其活性增大。
关键词:SnO2纳米晶;合成;光催化
(Edited by Hua YANG)
Foundation item: Projects (20676016, 21076024) supported by the National Natural Science Foundation of China
Corresponding author: Zhi-qian JIA; Tel: +86-10-58802945; E-mail: zhqjia@bnu.edu.cn
DOI: 10.1016/S1003-6326(14)63258-1
Abstract: Tin oxide nanocrystals with diameters smaller than 10 nm were synthesized using Na2SnO3 and CO2 as reactants and cetyltrimethylammonium bromide (CTAB) as stabilizer under mild conditions. As a mild acidic gas, CO2 is favorable for the accurate adjustment of pH value of Na2SnO3 solution. Stannate salt is stable, cheap and easy in operation. The effects of Na2SnO3 concentration, CTAB concentration, aging temperature, and aging time on the nanocrystals were studied. It was found that, with the increasing Na2SnO3 concentration, aging temperature and aging time, SnO2 nanocrystals size decreases. The formation of SnO2 nanocrystals can be interpreted by electrostatic-interaction mechanism. SnO2 nanocrystals show high photocatalytic activities in the degradation of Rhodamine B solution. The catalytic activity of small nanocrystals is higher than that of large ones.


