Trans. Nonferrous Met. Soc. China 23(2013) 855-860
Regeneration of activated carbon adsorbed EDTA by electrochemical method
Xiang-yu YOU1,2,3, Li-yuan CHAI1,2, Yun-yan WANG1,2, Yan-rong SU4, Na ZHAO1,5, Yu-de SHU1
1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. National Engineering Research Center for Pollution Control of Heavy Metals, Changsha 410083, China;
3. Hunan Research Academy of Environmental Sciences, Changsha 410004, China;
4. China Machinery International Engineering Design & Research Institute Co., Ltd., Changsha 410007, China;
5. Changsha Nonferrous Metallurgy Engineering and Research Institute, Changsha 410011, China
Received 20 December 2011; accepted 31 May 2012
Abstract:
Activated carbon after saturated adsorption of EDTA was used as particle electrode in a three-dimensional electrode reactor to treat EDTA-containing wastewater. Electrochemical method was used to regenerate activated carbon after many times of electrolysis. Based on the analysis of infrared spectra of activated carbon after adsorption and repeated electrolysis, EDTA was degraded into glycine, and then non-catalytic activated associated complex was formed with N—H bond on the activated carbon. The catalytic ability of the activated carbon vanished and the EDTA degradation efficiency was dropped. Activated carbon could be effectively regenerated by electrochemical method in the three-dimensional reactor. Effects of electric current, conductivity and pH on activated carbon regeneration were investigated, and the optimum conditions were concluded as follows: 100-300 mA of current intensity, 1.39 mS/cm of electric conductivity, 60 min of electrolysis time and pH 6.0-8.0. Under the optimized conditions, the activity of the activated carbon can be recovered and the residual total organic carbon (TOC) was below 10 mg/L (the initial TOC was 200 mg/L) in the three-dimensional electrode reactor.
Key words:
activated carbon; electrochemical regeneration; three-dimensional electrode; EDTA;
1 Introduction
Activated carbon is produced with nut shell or quality anthracite, and is composed of graphite structure particles material [1]. Due to its high surface and pore volume, activated carbon has been widely used as a common carrier of adsorbent [2] and catalyst [3] in industrial [4] and urban wastewater treatment [5]. In wastewater treatment with electrochemical technology, activated carbon was used as particle electrode. In our previous study, a three-dimensional electrode was used to treat organic water containing EDTA [6]. The results showed that total organic carbon (TOC) significantly dropped from 200 mg/L to 20 mg/L at the beginning, but after 4 times of repeated use, the residual TOC increased to 90 mg/L. It was shown that after being electrolyzed for a certain time, organic removal efficiency dropped quickly due to the weakening of catalytic performance of activated carbon. Thus, it is required to investigate the regeneration of activated carbon.
There were many methods for regeneration of activated carbon, such as thermal method and chemical method [7]. At present, thermal method is the most commonly used method, but it would result in serious mass loss and burned loss as high temperature is involved [8]. Chemical regeneration using alkali and other chemicals has been frequently reported with secondary pollution and some mass loss [9]. How to find a novel method to regenerate activated carbon without pollution is very important for practical application of activated carbon in wastewater treatment.
In this study, electrochemical method for activated carbon regeneration was developed. The effects of current intensity, electric conductivity, electrolysis time and pH on electrochemical regeneration were investigated.
It was very useful for chemical adsorption separation, and will make a great basis for the electrochemical regeneration of activated carbon [10].
2 Experimental
2.1 Experimental equipment
A novel three-dimensional electrode reactor (Fig. 1) was used in this study. It is a batch cuboids cell with a three-dimensional electrode. Both the reactor and micro porous diaphragm were made of PVC plastic. The anode and cathode (feeder electrodes) were made from high purity graphite plates with dimensions of 5 cm×10 cm, situating 11 cm apart from each other. The cathode electrode and the particle electrode were separated by micro porous diaphragm which was 5 cm and 0.5 cm away from anode and cathode, respectively. The activated carbon (GAC) was packed between the anode and micro porous diaphragm as bed electrode with dimensions of 5 cm×5 cm×10 cm. The electric power was supplied with regulated DC power supply (DF1797B-8003, Ningbo, China), whose working voltage is in the range of 0-80 V and output current ranges from 0 to 2.5 A.
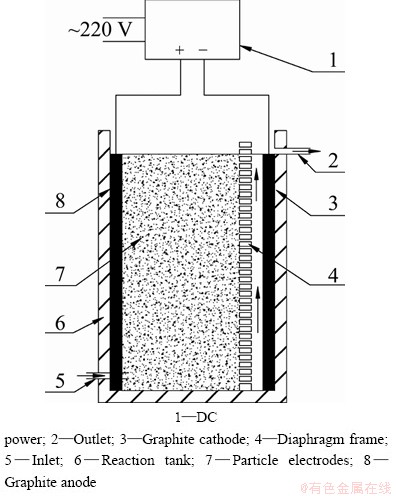
Fig. 1 Sketch of three-dimensional electrode reactor
2.2 Chemicals and analytical method
All the chemicals (Na2SO4, H2SO4 and NaOH) used in this study were of analytical grade. The EDTA and TOC concentrations in the simulated water were 0.6 and 200 mg/L, respectively. Activated carbon was made of coconut shell (Fujian Yuanli Active Carbon Co.,Ltd.) with specific surface area of 831.16 m2/g and average pore diameter of 2.38 nm.
All experiments were carried out in duplicate and analysis of each parameter was done in triplicate. Coefficient of variation obtained was no more than 5%. The pH value of the solution during the experiments was determined with a PHS-3E meter (Shanghai Rex Instrument Factory). TOC was measured by a TOC analyzer (TOC-VCPH, Shimadzu Instrument Factory). Conductivity was determined by a DDS-307 (Shanghai Rex Instrument Factory).
2.3 Activated carbon regeneration experiment
The regenerated activated carbon was used as particle electrode to treat wastewater containing EDTA in the same condition [6]. The three-dimensional electrode method was used to regenerate the activated carbon that was used in the electrolytic experiment. The condition that has the best TOC removal was considered to be the optimum condition for activated carbon regeneration. Before electrolysis, the graphite electrodes were soaked in dilute sulfuric acid and washed repeatedly with deionized water to remove contaminants absorbed on the surface. Then, they were placed in the electrochemical reactor as shown in Fig. 1. Between the anode and the diaphragm plate, 150 g carbon after adsorption saturation was filled in. For batch electrolysis experiments, 300 mL water was placed in 500 mL breaker by adjusting conductivity with Na2SO4, then the water was pumped into the three-dimensional electrode reactor prior to each run using a constant flow pump. The reactor was in real-time control when the DC power was switched on. After regeneration, the three-dimensional electrode was used to treat wastewater containing EDTA. The purified solution was analyzed for TOC.
3 Results and discussion
3.1 Theory of electrochemical regeneration
Infrared spectrogram of EDTA is shown in Fig. 2. The absorption peaks at 3523.93 cm-1 and 3389.87 cm-1 are attributed to the hydroxyl stretching vibration of [11]. A sharp peak at 3026.81 cm-1 is due to the stretching vibration of —CH2—. The peaks at 1475.08 cm-1 and 1396.72 cm-1 associate with the stretching vibration of —COO— and 1656.37 cm-1 is the N—C stretching vibration peak [12]. The results indicated that the main group of EDTA has characteristic peak.
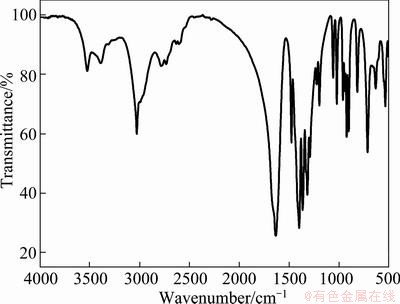
Fig. 2 Infrared spectrum of EDTA
Figure 3 shows the infrared spectrogram of activated carbon after adsorbing EDTA. There were many differences between the spectra in Figs. 2 and 3. In Fig. 3, it is obvious that two peaks at 3500-3300 cm-1 and 3414.88 cm-1 are ascribed to symmetric stretching vibration absorption of N—H [12]. The peaks at 3450 cm-1 and 1650 cm-1 are attributed to respective asymmetric stretching vibration and bend vibration of N—H. It is indicated that the peaks for primary amine appeared on activated carbon after adsorbing EDTA.
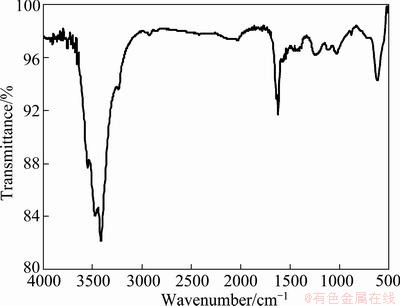
Fig. 3 Infrared spectrum of activated carbon adsorbed EDTA
The peak at 1025.36 cm-1 is due to the C—N stretching vibration, and is assigned to aliphatic amide. A strong peak at 3550.59 cm-1 is attributed to the stretching vibration of hydroxyl in carboxyl [13]. The peak at 1618.94 cm-1 is associated with the symmetric stretching vibration of C—O. This indicates that aliphatic amide was adsorbed by activated carbon.
In the early study, glycine (H2NCH2COOH) was generated when wastewater containing EDTA was treated by ozone [14] or UV photo oxidation [15] at pH 3.0. So it is possible that EDTA adsorbed on activated carbon was degraded to glycine. The reason was that σ-π back bonding was formed after the EDTA adsorption on activated carbon, causing the breakdown of chemical bond of EDTA.
Figure 4 shows the infrared spectrum of activated carbon used as particle electrode in the three-dimensional electrode. It had been used to treat EDTA-containing wastewater many times. The absorption peak at 3422.62 cm-1 is due to stretch vibration absorption peak of N—H bond. It was shown that organic compound adsorbed on activated carbon was association compound [16]. After repeated use of the activated carbon many times, glycine adsorbed on activated carbon was changed from simple molecule to association compound with N—H bond. This association compound may be a non-catalytic activated compound which can not be degraded by electrochemical oxygen. It will occupy the catalytic point of activated carbon, thus causing a decrease in the catalytic activity of activated carbon.
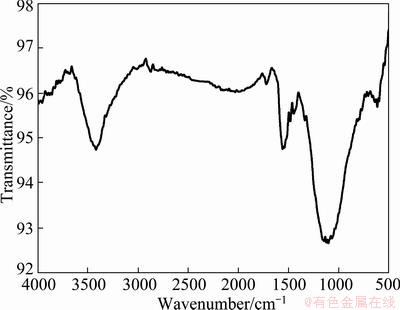
Fig. 4 Infrared spectrum of activated carbon after electrolysis
There were two reasons for the production of association compound. First, glycine can react with some adsorption site on activated carbon to generate strong chemical bond. Second, glycine can not be degraded quickly because of volume filling and capillary condensation of activated carbon. In order to accelerate the oxidation speed of the association compound, three-dimensional electrode was used to generate hydroxyl radicals. Due to the degradation of association compound by hydroxyl radicals, the catalytic activity of activated carbon was recovered. In electrolytic process, no organic compound was detected in solution, so all the hydroxyl radicals were assumed to degrade glycine. Thus, the catalytic activity of activated carbon could be effectively recovered by the electrochemical method.
3.2 Effect of current
Figure 5 shows the residual TOC in the three- dimensional electrode reactor at different currents. It was indicated that the removal efficiency of TOC dropped with current increasing. At the current of 100-300 mA, the residual TOC was below 10 mg/L. At the current of 400-700 mA, the residual TOC was 10-20 mg/L. However, when the current was enhanced to 1000 mA, the residual TOC increased to 42 mg/L. It was indicated that a greater current caused a weaker degradation of organic compounds. The highest TOC removal efficiency was obtained at the current of 100-300 mA.
The observed effect of current on TOC removal can be explained by the electrode reaction mechanism. Based on this mechanism, ·OH radical can easily turn to O2 at a high current.
3.3 Effect of conductivity
Conductivity can significantly affect the degradation of organic compounds. Figure 6 shows the change of residual TOC in solution with conductivity. With the increase of conductivity from 1.03 mS/cm to 1.39 mS/cm, the residual TOC dropped from 43 mg/L to 10 mg/L. But when the conductivity was higher than 1.39 mS/cm, the degradation efficiency tended to decrease and then became stable.
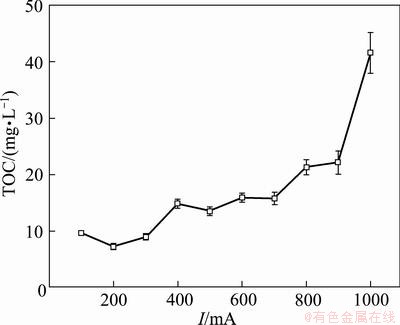
Fig. 5 Effect of current on residual TOC (σ=2.0 mS/cm, t=1 h, θ=25 °C, v=200 mL/min, pH=8.0)
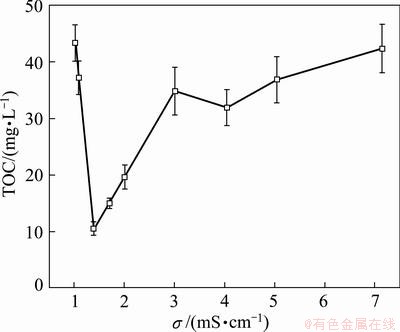
Fig. 6 Effect of electric conductivity on residual TOC (I=100 mA, t=1 h, θ=25 °C, v=200 mL/min, pH=8.0)
The greatest degradation efficiency appeared at 1.39 mS/cm. It was likely that in such condition the solution conductivity is close or equal to that for the activated carbon. Thus, the changes of electric potential in solution phase and solid phase in the three-dimensional electrode reactor are similar. The potential differences at solid-liquid interfaces of different sites on activated carbon are almost constant, resulting in the high TOC removal.
3.4 Effect of cycle electrolysis time
The cycle electrolysis time is the time of certain amount of wastewater flowing through the reactor in a circular reaction. Figure 7 shows the change of degradation rate with cycle electrolysis time. It is clearly illustrated that the residual TOC dropped quickly within 1.0 h, but after that it decreased slowly. The residual TOC was 65 mg/L at 0.25 h and 14 mg/L at 1.0 h. The residual TOC concentration met the limited value (10.58 mg/L) after 1.0 h. Thus, the optimum cycle electrolysis time was 1.0 h.
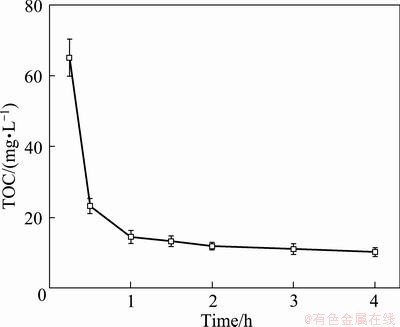
Fig. 7 Effect of cycle electrolysis time on residual TOC (I=100 mA, σ= 2.0 mS/cm, θ=25 °C, v=200 mL/min, pH=8.0)
3.5 Effect of pH
The effect of solution pH on the removal efficiency of TOC is shown in Fig. 8. The TOC removal efficiencies were low when pH value was lower than 3 and higher than 12. The residual TOC reached the lowest (8 mg/L) at pH value of 6-8. In the acidic or alkaline solution, ·OH radical generated from electrolysis can be quickly converted to O2 [17]. The low concentration of ·OH radical results in low degradation efficiency.
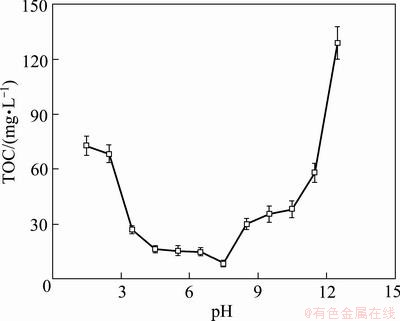
Fig. 8 Effect of solution pH on residual TOC (I=100 mA, σ=2.0 mS/cm, t=1 h, θ=25 °C, v=200 mL/min)
3.6 Recycle experiments with post-regeneration activated carbon
The optimized conditions for electrochemical regeneration of activated carbon were: 100-300 mA of current intensity, 1.39 mS/cm of electric conductivity, 60 min of electrolysis time and pH 6.0-8.0. The results further confirmed that the activated carbon regenerated by this electrochemical method could be used more than twenty times in electrolytic experiments. The regenerated activated carbon was used as particle electrode in the three-dimensional electrode to treat EDTA wastewater. As shown in Fig. 9, after the repeated use of the activated carbon 10 times, the residual TOC was lower than 10 mg/L. It was shown that the activity of activated carbon was effectively recovered by the electrochemical regeneration method.
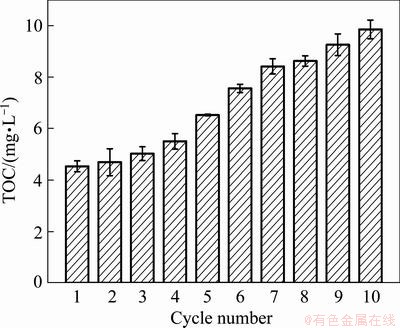
Fig. 9 Recycle experiments with post-regeneration activated carbon
Figure 10 shows the infrared spectra of fresh and regenerated activated carbon. The peaks at 3526.76 cm-1 and 3414.88 cm-1 are due to the asymmetric stretching vibration and symmetric stretch vibration of N—H respectively. Compared with the infrared spectrum of activated carbon after electrolysis (Fig. 4), it is obvious that the double peaks disappear; only asymmetric stretch vibration absorption peak of N—H bond appears in 3422.62 cm-1, indicating that the organic matter adsorbed on activated carbon is association compound and it can not be degraded. After regeneration with electrochemical method, the double peaks appeared again in 3400-3550 cm-1 as shown in Fig. 10, which was nearly the same with the fresh activated carbon [12]. The peak intensity and position were also almost the same in high frequency region. It was indicated that activated carbon can be effectively regenerated by this electrochemical regeneration method.
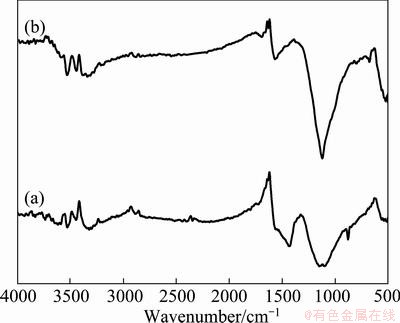
Fig. 10 Infrared spectra of fresh (a) and regenerated (b) activated carbon
4 Conclusions
1) The infrared spectra of activated carbon after adsorption and cyclic electrolysis showed that EDTA was degraded into glycine, and then non-catalytic activated association compound was formed by glycine with N—H bond, so catalytic activity of activated carbon was lost.
2) The optimum conditions for electrochemical regeneration of activated carbon were: 100-300 mA of current intensity, 1.39 mS/cm of electric conductivity, 60 min of electrolysis time and pH 6.0-8.0.
3) The infrared spectra of fresh and regenerated activated carbon showed that activated carbon can be regenerated under the optimum condition.
References
[1] GRATUITO M K B, PANYATHANMAPORN T, CHUMNANKLANG R A, SIRINUNTAWITTAYA N, DUTTA A. Production of activated carbon from coconut shell: Optimization using response surface methodology [J]. Bioresource Technology, 2008, 99(11): 4887-4895.
[2] ALTENOR S, CARENE B, EMMANUEL E, LAMBERT J, WHRHARDT J J, GASPARD S. Adsorption studies of methylene blue and phenol onto vetiver roots activated carbon prepared by chemical activation [J]. Journal of Hazardous Materials, 2009, 165(1-3): 1029-1039.
[3] KHAYOON M S, HAMEED B H. Acetylation of glycerol to biofuel additives over sulfated activated carbon catalyst [J]. Bioresource Technology, 2011, 102(19): 9229-9235.
[4] LOU J C, CHANG T W, HUANG C E. Effective removal of disinfection by-products and assimilable organic carbon: An advanced water treatment system [J]. Journal of Hazardous Materials, 2009, 172(2-3): 1365-1371.
[5] DELMAS H, CREANGA C, JULCOUR-LEBIGUE C, WIHELM A M. AD-OX: Asequential oxidative process for water treatment— Adsorption and bath CWAO regeneration of activated carbon [J]. Chemical Engineering Journal, 2009, 152(1): 189-194.
[6] CHAI Li-yuan, YOU Xiang-yu, SHU Yu-de, YANG Jie, WANG Yun-yan ZHAO Na. Electrochemical degradation of organic wastewater containing EDTA by three-dimensional electrode reactor [J]. Journal of Central South University: Science and Technology, 2010, 41(4): 1240-1245. (in Chinese)
[7] YUEN F K, HAMEED B H. Recent developments in the preparation and regeneration of activated carbons by microwaves [J]. Advances in Colloid and Interface Science, 2009, 149(1-2): 19-27.
[8] GUO D S, SHI Q T, HE B B, YUAN X Y. Different solvents for the regeneration of the exhausted activated carbon used in the treatment of coking wastewater [J]. Journal of Hazardous Materials, 2011, 186(2-3): 1788-1793.
[9] LU P J, LIN H C, YU W T, CHERN J M. Chemical regeneration of activated carbon used for dye adsorption [J]. Journal of the Taiwan Institute of Chemical Engineers, 2011, 42(2): 305-311.
[10] WENG C H, HSU M C. Regeneration of granular activated carbon by an electrochemical process [J]. Separation and Purification Technology, 2008, 64(2): 227-236.
[11] XU Shou-chang. Organic chemistry [M]. Beijing: Higher Education Press, 1999: 123-135. (in Chinese)
[12] CHANG Jian-hua, DONG Qi-gong. Principle and analytic of spectra [M]. Beijing: Science Press, 2005: 235-258. (in Chinese)
[13] ZOU Jian-ping, WANG Lu, ZENG Run-sheng. Structural analysis of organic compounds [M]. Beijing: Science Press, 2005: 46-52. (in Chinese)
[14] DENG Wan-yu. Treatment of the wastewater containing EDTA and heavy metals by ferrite process combined with Fenton’s method [D]. Kaohsiung: National Sun Yat-sen University, 2004: 28-29. (in Chinese)
[15] FANG Jing-li. Theory and application of coordination compounds in electroplating [M]. Beijing: Chemical Industry Press, 2008: 329-341. (in Chinese)
[16] PELLER J, WIEST O, KAMAT P V. Sonolysis of 2-dichloroph enoxyacetic acid in aqueous solutions. Evidence for ·OH-radical- mediated degradation [J]. Journal of Physical Chemistry A, 2001, 105(13): 3176-3181.
[17] LEITNER N K V, ROSHANI B. Kinetic of benzotriazole oxidation by ozone and hydroxyl radical [J]. Water Research, 2010, 44(6): 2058-2066.
吸附EDTA活性炭的电化学再生
尤翔宇1,2,3,柴立元1,2,王云燕1,2,苏艳蓉4,赵 娜1,5,舒余德1
1. 中南大学 冶金科学与工程学院,长沙 410083;
2. 国家重金属污染防治工程技术研究中心,长沙 410083;
3. 湖南省环境保护科学研究院,长沙 410004;
4. 中机国际工程设计研究院有限责任公司,长沙 410007;
5. 长沙有色冶金设计研究院,长沙 410011
摘 要:采用吸附饱和EDTA的活性炭作为三维电极反应器中的粒子电极,多次使用后采用电化学方法对其再生。通过对吸附饱和EDTA的活性炭和多次电解使用后的活性炭的红外光谱谱图的分析得出,EDTA被活性炭吸附后产生甘氨酸H2NCH2COOH,通过N—H键生成一种永久性占据活性炭活性点的非催化活性缔合物,导致其催化活性消失,降解效率下降。采用电解方法使活性炭再生,得出活性炭的最佳活化条件为:电流100~300 mA,溶液电导率1.39 mS/cm,pH值 6.0~8.0,电解1 h可以使活性炭恢复活性,电解后有机物的残余TOC浓度低于10 mg/L(初始浓度为200 mg/L)。
关键词:活性炭;电化学再生;三维电极;EDTA
(Edited by Hua YANG)
Foundation item: Project (2011467062) supported by National Scientific Research Project of Welfare (Environmental) Industry, China; Project (50925417) supported by China National Funds for Distinguished Young Scientists; Project (50830301) supported by the National Natural Science Foundation of China; Project (CX2010B121) supported by Hunan Provincial Innovation Foundation For Postgraduate, China
Corresponding author: Li-yuan CHAI; Tel: +86-731-88836921; E-mail: liyuan.chai@yahoo.com.cn
DOI: 10.1016/S1003-6326(13)62539-X
Abstract: Activated carbon after saturated adsorption of EDTA was used as particle electrode in a three-dimensional electrode reactor to treat EDTA-containing wastewater. Electrochemical method was used to regenerate activated carbon after many times of electrolysis. Based on the analysis of infrared spectra of activated carbon after adsorption and repeated electrolysis, EDTA was degraded into glycine, and then non-catalytic activated associated complex was formed with N—H bond on the activated carbon. The catalytic ability of the activated carbon vanished and the EDTA degradation efficiency was dropped. Activated carbon could be effectively regenerated by electrochemical method in the three-dimensional reactor. Effects of electric current, conductivity and pH on activated carbon regeneration were investigated, and the optimum conditions were concluded as follows: 100-300 mA of current intensity, 1.39 mS/cm of electric conductivity, 60 min of electrolysis time and pH 6.0-8.0. Under the optimized conditions, the activity of the activated carbon can be recovered and the residual total organic carbon (TOC) was below 10 mg/L (the initial TOC was 200 mg/L) in the three-dimensional electrode reactor.


