
Corrosion and mechanical properties of hot-extruded AZ31 magnesium alloys
ZHANG Zhi-min(张治民)1, XU Hong-yan(徐宏妍)1, 2, WANG qiang(王 强)1
1. School of Materials Science and Engineering, North University of China, Taiyuan 030051, China;
2. National Key Laboratory for Electronic Measurement Technology, North University of China,
Taiyuan 030051, China
Received 12 June 2008; accepted 5 September 2008
Abstract:
AZ31 magnesium alloys were hot-extruded at 573 K and 623 K with extrusion ratio (λ) of 20, 35 and 50. The corrosion and mechanical behavior of hot-extruded AZ31 were studied by galvanic tests and tensile tests. The microstructures of the studied AZ31 alloys were also investigated with optical microscope. The results show that, compared with the as-cast AZ31 alloy, the corrosion potentials of all hot-extruded AZ31 alloys are increased by 60 mV. Moreover, at the extrusion temperature of 623 K, the galvanic current of AZ31 alloy decreases with increasing extrusion and the galvanic corrosion resistance is increased by 10% with the extrusion ratio of 50. In addition, the tensile strength and elongation of the extruded alloys are significantly enhanced by about 20% and 140%, respectively. The improvement of corrosion resistance and obvious increasing of mechanical properties of AZ31 alloys by hot-extrusion are ascribed to grain refinement and microstructural modification together with the homogeneous distribution of intermetallic phases throughout the matrix.
Key words:
AZ31 magnesium alloy; hot-extrusion; corrosion properties; mechanical properties;
1 Introduction
Magnesium and its alloys have a wide prospect for application in the fields of automobiles, electronic products and aerospace industry, not only for their lightweight but also for their excellent electromagnetic ability[1]. However, due to the hexagonal close packed structure of magnesium, the ductility of magnesium and its alloys is rather poor at room temperature. Moreover, magnesium alloys have inferior corrosion resistance owing to their active electrochemical properties. These greatly resist their applications in structural fields, and it is necessary to produce magnesium alloys with not only good mechanical properties but also high corrosion resistance.
It is well known that thermomechanical processing can refine the microstructure of magnesium alloys, and has significant influence on their corrosion and mechanical properties[2-3]. Magnesium alloys produced by hot-extrusion have higher strength, better plasticity and various mechanical properties[4]. The processing parameters have great effect on the mechanical properties. LI et al[5] found that AZ91 bar extruded from ingot with short-time annealing has superior mechanical properties compared with that extruded from ingot with long-time annealing. MWEMBELA et al[6] observed that with the rolling temperature higher than 300 ℃, the grains of AZ31 magnesium alloy grew, and the strength of the alloy decreased. While there are little report on the corrosion properties of hot-extruded magnesium alloys.
In this work, the corrosion and mechanical properties of hot extruded AZ31 alloys were investigated by galvanic tests and tensile tests, respectively. For complementarity, the microstructures of the studied AZ31 alloys were also investigated with optical microscope.
2 Experimental
The chemical compositions of the AZ31 magnesium alloys are listed in Table 1. The as-cast AZ31 alloys were first homogenized at 673 K for 14 h, then they were hot-extruded at 573 K and 623 K with extrusion ratio (λ) of 20, 35 and 50, as listed in Table 2. The diameter of the extruded bars was 15 mm. For comparison, a reference specimen was processed from the as-cast AZ31 ingot to the same size as the hot-extruded one.
Table 1 Chemical compositions of AZ31 magnesium alloys (mass fraction, %)
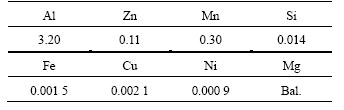
Table 2 Extrusion parameters for AZ31 alloy samples

Following standard metallographic procedures, the polished surfaces of the as-cast and hot-extruded AZ31 alloys were etched using a solution of 2% oxalic acid + 2% nitric acid, and their microstructures were examined by Image MAT A1 optical microscope.
Tensile specimens were machined to standard specimens from the extruded bar. Tensile tests were carried out at room temperature using a WDW-E100D electronic tensile testing machine with a tensile rate of 3.26 mm/min. The tensile direction was parallel to the extrusion direction.
In corrosion tests, AZ31 alloys with the size of d 15 mm×15 mm were sealed by epoxy resin with working surface of 1cm2 left. Galvanic corrosion properties of AZ31 alloys were studied by galvanic tests in 3% NaCl solution prepared by analytical grade reagent and distilled water. A ZF-3 potentiostat was used for the electrochemical measurements. A3 steel and saturated calomel electrode (SCE) were taken as the coupled cathode and reference electrode, respectively. The distance between anode and cathode is 5 cm, and surface area ratio of the anode to cathode is 1?1. Before tests, the working surface of AZ31 alloys and A3 steel were ground successively to 1000 grit SiC abrasive paper and degreased in ethanol.
3 Results and discussion
3.1 Microstructures of AZ31 alloys
The optical morphologies of the as-cast and extruded AZ31 alloys are shown in Fig.1 and Fig.2, respectively. It can be known from Fig.1 that the main phases in as-cast AZ31 alloy are α-Mg and the particle- like secondary phases, Mgl7All2 and Al-Mn distributing in the magnesium solid solution[7].
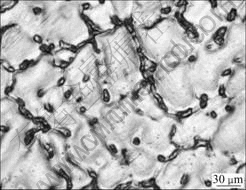
Fig.1 Optical morphology of as-cast AZ31 alloy
As shown in Fig.2, the extruded microstructures of AZ31 alloys are markedly refined. This indicates that the dynamic recrystallization occurs during hot extrusion of the AZ31 alloy[8]. At the extrusion temperature of 623 K, the average grain size of extruded alloys decreases with increasing extrusion ratio (Figs.2(a), (b) and (c)). This tendency was also found in AZ31B and AZ61A alloys[9]. Comparing Fig.2(b) with Fig.2(d), it can be seen that, with same extrusion ratio of 35, the grain is much refined at 623 K than at 573 K. This may be due to the fact that the dynamic recrystallization is complete at 623 K and incomplete at 573 K[7].
3.2 Tensile properties of AZ31 alloys
The tensile properties of extruded AZ31 alloys at room temperature are shown in Fig.3. The tensile strength and elongation of as-cast AZ31 alloy are measured as 250 MPa and 7%, respectively. It can be obviously observed that, compared with the as-cast AZ31 alloy, the tensile strength and elongation of the extruded alloys are significantly enhanced. The tensile strength and elongation of AZ31 alloy extruded at 623 K with extrusion ratio of 50 are 316 MPa and 19%, respectively. This is the result of grain refinement by hot-extrusion (Figs.1 and 2)[7]. With increasing extrusion ratio, tensile strength increases at both temperatures, while the elongation increases at 623 K and decreases at 573 K. This difference may be also caused by the incomplete dynamic recrystallization and a certain of work hardening at 573 K.
3.3 Corrosion properties of AZ31 alloys
The galvanic corrosion measurement of AZ31 alloys was divided into three phases: 1) measurement of the free corrosion potential at test beginning (15 min), 2) galvanic corrosion measurement (1.5 h), and 3) measurement of the free corrosion potential at test end (15 min).
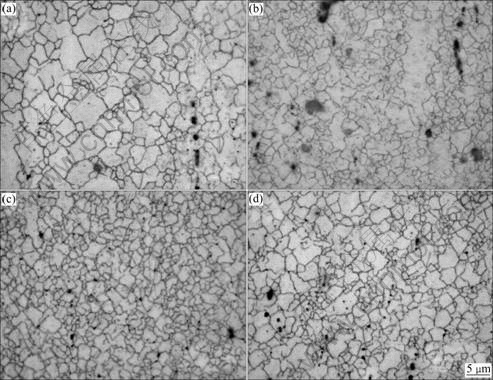
Fig.2 Microstructures of extruded AZ31 alloy at: (a) 623 K, λ=20; (b) 623 K, λ=35; (c) 623 K, λ=50; (d) 573 K, λ=35
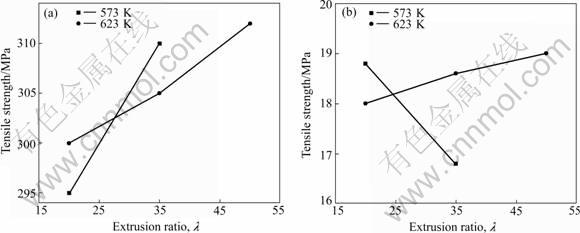
Fig.3 Tensile properties of extruded AZ31 alloys at room temperature
As seen from the recorded free corrosion potential curves of AZ31 alloys (Fig.4), the free corrosion potentials of the as-cast sample increases from -1 843 mV (vs SCE) in the initial test to -1 642 mV (vs SCE) after 15 min. While the free corrosion potentials of the hot-extruded AZ31 alloys are nearly constant of -1 580 mV (vs SCE) after 2 min immersion in NaCl solution. After the measurement, the free corrosion potentials of all AZ31 alloys reach to a value of -1 550 mV (vs SCE).
A general rule in the constructional sector for avoiding galvanic corrosion is to avoid large differences in electrochemical potentials between the contact partners[10]. Corrosion potentials of all hot-extruded AZ31 alloys are 60 mV higher than that of the as-cast one, therefore, theoretically, the hot-extruded AZ31 alloys have better galvanic corrosion resistance than the as-cast ones.
The development of galvanic corrosion can be shown by the change in average galvanic current over the whole AZ31 anode surface with time. The galvanic currents for extruded and as-cast AZ31 magnesium alloys are plotted in Fig.5. The most important feature is that the galvanic current increases steadily with time in the first few minutes and reaches the maximum. After
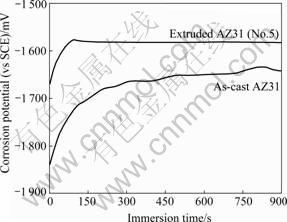
Fig.4 Dependence of free corrosion potential on immersion time of different AZ31 alloys immersed in 3.5%NaCl solution
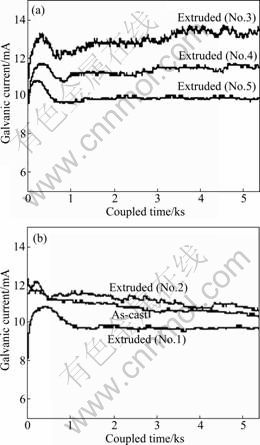
Fig.5 Typical change in average galvanic current density with coupled time
that, most of the galvanic currents fluctuate around a value within a certain range. This could be related to the roughening (increase in area) of tested surface caused by corrosion. The fluctuation of the galvanic currents can be interpreted as competition between the initiation and ceasing of corrosion in various areas. It is possible that corrosion stops in a corroded area, but initiates in a new area. The initial value of galvanic current of all the extruded AZ31 alloy is lower than that of the as-cast one (Table 3), and this corresponds to the free corrosion potentials (Fig.4). It can also be clearly observed from Table 3 that, for AZ31 alloys extruded at 623 K, the galvanic corrosion resistance increases with increasing of extrusion ratio, while it is contrary to AZ31 alloys extruded at 573 K.
Table 3 Galvanic current measured in different AZ31-A3 steel couples

The corrosion resistance of the extruded AZ31 alloys may be significantly influenced by their microstructures and distribution of β-Mg17Al12 phase. The refinement of the microstructure is beneficial to the corrosion properties. It can change the mechanism of corrosion by turning pitting corrosion of Mg-Al magnesium alloys into overall corrosion[11]. The distribution of β-Mg17Al12 phase determines the corrosion resistance of the Mg-Al alloys[12]. The β-Mg17Al12 phase mainly serves as a galvanic cathode and accelerates the corrosion process of the α-matrix if the volume fraction of β-Mg17Al12 phase is small; however, for high volume fraction, the β-Mg17Al12 phase might act as an anodic barrier to inhibit the overall corrosion of the alloy[13]. The better corrosion resistance of die casting AZ91D alloy is due to the very fine grains, high β volume fraction and continuous distribution of β phase along the grain boundaries[14]. Sometimes the β-Mg17Al12 phase can enrich the corrosion product, thereby possibly inhibiting the corrosion rate[15].
4 Conclusions
1) The corrosion and mechanical properties of the extruded AZ31 alloys are significantly enhanced, which are attributed to the refinement of microstructures and distribution of β-Mg17Al12 phase in matrix.
2) The optimized extrusion parameters for AZ31 alloys are 623 K and extrusion ratio of 50. AZ31 alloys extruded with above parameters have good combination of corrosion and mechanical properties: 10% increase in galvanic corrosion resistance compared with the as-cast AZ31 alloys, together with high tensile strength (316 MPa) and high elongation (19%).
References
[1] MORDIKE B L, EBERT T. Magnesium properties—applications—potential [J]. Materials Science and Engineering A, 2001, A302: 37-45.
[2] PARDO A, MERINO M C, COY A E, ARRABAL R, VIEJO F, MATYKINA E. Corrosion behaviour of magnesium/aluminium alloys in 3.5% NaCl [J]. Corrosion Science,2008, 50(3): 823-834.
[3] ZHANG Kai-feng, YIN De-liang, WANG Guo-feng, HAN Wen-bo. Superplastic deformation behavior of hot-rolled AZ31 magnesium alloy sheet at elevated temperatures [J]. Journal of Wuhan University of Technology—Materials Science, 2006, 21(3): 1-6.
[4] GUAN Shao-kang, ZHU Shi-jie, WANG Li-guo, YANG Qing, CAO Wen-bo. Microstructures and mechanical properties of double hot-extruded AZ80+xSr wrought alloys [J]. Trans Nonferrous Met Soc China, 2007,17(6): 1143-1151.
[5] LI Zhi-feng, DONG Jie, ZENG Xiao-qing, LU Chen, DING Weng-jiang. Influence of Mg17Al12 intermetallic compounds on the hot extruded microstructures and mechanical properties of Mg-9Al-1Zn alloy [J]. Materials Science and Engineering A, 2007, 466: 134-139.
[6] MWEMBELA A, KONOPLEVA E B, MCQUEEN H J. Microstructural development in Mg alloy AZ31 during hot working [J]. Scripta Materialia, 1997, 37(11): 1789-1795.
[7] ZHANG Hui, YAN Yun-qi, WENG Wen-ping, ZHONG Hao, CHEN Qi. Effects of hot extrusion and annealing treatment on microstructures, properties and texture of AZ31 Mg alloy [J]. Trans Nonferrous Met Soc China, 2006, 16(s3): s1732-s1735.
[8] WANG Ling, TIAN Su-gui, MENG Fan-lai, DU Hong-qiang. Influence of hot extrusion on microstructure and mechanical properties of AZ31 magnesium alloy [J]. Trans Nonferrous Met Soc China, 2006, 16(s3): s1770-s1773.
[9] UEMATSU Y, TOKAJI K, KAMAKURA M, UCHIDA K, SHIBATA H, BEKKU N. Effect of extrusion conditions on grain refinement and fatigue behaviour in magnesium alloys [J]. Materials Science and Engineering A,2006, 434(1/2): 131-140.
[10] HPCHE H, BLAWERT C, BROSZEIT E, BERGER C. Galvanic corrosion properties of differently PVD-treated magnesium die cast alloy AZ91D [J]. Surface & Coating Technology, 2005, 193: 223-229.
[11] DALOZ D, STEINMETZ P, MICHOT G. Corrosion behavior of rapidly solidified magnesium-aluminum-zinc alloys [J]. Corrosion, 1997, 53(12): 944-954.
[12] ZENG R C, ZHANG J, HUANG W J, DIETZEL W, KAINER K U, BLAWERT C, KE W. Review of studies on corrosion of magnesium alloys [J]. Trans Nonferrous Met Soc China,2006, 16: s763-s771.
[13] SONG G L, ATRENS A. Corrosion mechanisms of magnesium alloys [J]. Advanced Engineering Materials, 1999, 1(1): 11-33.
[14] SONG G L, ATRENS A, DARGUSCH M. Influence of microstructure on the corrosion of die cast AZ91D [J]. Corrosion Science, 1999, 41: 249-273.
[15] CAARLSON B E, JONES J W. The metallurgical aspects of the corrosion behavior of cast Mg-Al alloys [C]// Light Metals Processing and Applications. METSOC Conference. Quebec, 1993.
(Edited by YANG Bing)
Corresponding author: XU Hong-yan; Tel: +86-351-3557485; E-mail: xuhongyan@nuc.edu.cn


