
Preparation of pure SbCl3 from lead anode slime bearing high antimony and low silver
CAO Hua-zhen(曹华珍) 1, CHEN Jin-zhong (陈进中)2, YUAN Hai-jun(袁海军)1, ZHENG Guo-qu(郑国渠)1
1. College of Chemical Engineering and Materials, Zhejiang University of Technology, Hangzhou 310014, China;
2. Department of Science and Technology, Guangxi China Tin Group Co., Ltd., Liuzhou 545006, China
Received 3 December 2009; accepted 23 April 2010
Abstract:
A novel treatment process of lead anode slime bearing high antimony and low silver was developed by a potential-controlled chloridization leaching and continuous distillation. The experimental results show a high Sb3+concentration, 489.2 g/L, in the leaching solution for two-stage countercurrent leaching process, and the leaching rates of Sb, Cu, Bi more than 99% when the potential is controlled at 450 mV. When the leaching solution is distillated and concentrated at 120 °C, almost all the silicon compound is evaporated into the concentration distillate and exists as amorphous hydrated silica. By the continuous distillation, high pure SbCl3 could be prepared, and AsCl3 is enriched in the distillate while metals Bi, Cu are enriched in the continuous distillation residue. As a result, the recovery rate of Sb is more than 95%.
Key words:
lead anode slime; potential-controlled chloridization leaching; continuous distillation; antimony trichloride;
1 Introduction
Lead anode slime bearing high antimony and low silver is usually produced during the electrolysis of lead from jamesonite concentrate[1]. However, the components in this slime are complicated. Traditional treatment process for this slime, which is called pyrometallurgy, has the problems of low sliver recovery, high energy-consumption and serious environment pollution.
QIU et al[2-3] reported a treatment process of lead anode slime bearing high antimony using a vacuum volatilization technique. The removal of antimony reached 96%, and the antimony trioxide obtained was white powder or crystal solid. Noble lead remained in the residue of volatilization. However, once the lead anode slime contains a high concentration of arsenic, it is difficult to separate arsenic from antimony by this technique.
Hydrometallurgical process is a novel technique for treating lead anode[4-7], which was developed in recent years and has been applied in industrial community. But it is rarely reported to treat lead anode slime bearing high antimony and low silver by hydrometallurgical process.
NIE et al[8] treated the lead anode slime bearing high antimony and low silver by a chloridization leaching using sodium chlorate as oxidant. The noble metal could be effectively separated from the base metals. The cubic antimony white was achieved by hydrolyzing the leaching solution, and the valuable metals were recovered. However, a large quantity of waste acid was produced during the hydrolysis process and hard to be recycled.
TANG et al[9] used an AC process to treat the lead slime bearing high antimony and lower sliver in order to increase silver recovery rate and avoid the environmental problem. The noble metal could be effectively separated by the chloridization leaching process. Pure SbCl3 solution containing antimony with the concentration of 650 g/L was obtained by the subsequent dry-distillation. The difficulty for the continuous operation of dry-distillation limits the application of this technique.
XIONG et al[10] leached the lead slime bearing high antimony by a potential-controlled chloridization in HCl-NaCl aqueous solution. The leaching rate of base metals was more than 98%, and the mass fraction of gold in the leaching residue increased from 0.81% to 63.54%. 0.1%-0.3% of gold in the lead anode slime was transferred into the leaching solution. The noble metal could be effectively separated by this method. However, the recovery of antimony needs to be further studied.
In our previous studies[11-12], a combination technique containing potential-controlled chloridization leaching, distillation at low pressure and oxidization- crystalline was proposed to prepare SbCl5 from lead anode slime bearing high antimony and low silver. A high antimony recovery rate could be achieved by this method. Additionally, it could be operated in a continuous mode. Other valuable metals could also be recovered at the same time. In the process, the solution was closely recycled and no waste was discharged. In this work, a two-stage countercurrent and potential- controlled chloridization leaching process was used to treat the lead anode slime bearing high antimony and low silver. Silicon compound was separated from the leaching solution by a concentration-distillation process. High pure SbCl3 was achieved by the continuous distillation technique.
2 Experimental
2.1 Raw material
Lead anode slime was taken from Hechi smelter of Huaxi Group Corp. in Guangxi, China. The raw material contained high antimony (63.6%) and low silver(Table 1). Compared with the slime used in our previous studies, the content of antimony in this slime is lower, while the content of arsenic is much higher, which will lead to a great increase of AsCl3 concentration in the potential-controlled chloridization leaching solution. When the antimony concentration in the leaching solution reaches 500 g/L, the arsenic concentration can be more than 30 g/L, which causes the hydrolysis of leaching solution. At the same time, the distilled liquid with high arsenic content cannot be recycled to the leaching process and has to be individually treated.
Table1 Chemical composition of lead slime taken from Hechi smelter

2.2 Experimental process
Fig.1 shows the principle flowsheet for preparation of antimony trichloride from the lead anode slime bearing high antimony. This process has some advantages as follows: 1) The process is a closed cycle and no waste is discharged, which is benign to the environment; 2) The valuable metal co-existed in the lead anode slime can also be recovered; 3) High pure SbCl3 can be obtained directly, and a high antimony recovery rate can be achieved in a cost-effective way.

Fig.1 Principle flowsheet for preparation of antimony trichloride from lead anode slime bearing high antimony and low silver
Potential-controlled chloridization leaching process consisted of two steps. Firstly, the lead anodic slime was leached in an HCl aqueous solution for 1 h under a pre-specified temperature. Then chlorine was added into the leached solution at a certain velocity and a potentiometer was used to measure the solution potential. The chloridization process was finished when no Sb5+ ion is detected in the solution. At the same time, the solution potential reached 260 mV. After that, the solution was stirred for half an hour and then filtered. This step was named as one-stage chloridization leaching process. The filtered liquor, i. e. one-stage chloridization leaching solution, was applied in the following concentration- distillation process. The filtered residue was leached again with a 3.5 mol/L HCl aqueous solution. Chlorine was added into the leached solution at a certain velocity, which was called two-stage chloridization leaching process. The two-stage chloridization process was finished when the solution potential reached 450 mV. Then the solution was stirred for half an hour and filtered. Two-stage chloridization leaching solution was sent back to one-stage chloridization leaching process. The filtered residue was rinsed with 2 mol/L HCl aqueous solution and dried for analysis.
One-stage chloridization leaching solution was heated to 120 °C in the concentration-distillation process. During this process, part of low boiling point species such as HCl, H2O, fluosilicic acid were distilled and cooled in the condenser. The removal of some low boiling point species is beneficial to the subsequent continuous distillation, which can solve the problem of pipe block caused by their deposition during the evaporation and cooling process. The solution in the condenser was filtered and the resultant residue was dried at vacuum condition for analysis. The concentration liquor was sent to the next process for continuous distillation.
Fig.2 shows the schematic diagram for continuous distillation of antimony trichloride. The concentration liquor flows to the top of the distillation tower. The low boiling point species such as HCl, H2O and AsCl3 are evaporated and cooled, and then flow to the storage tank. The flow velocity of concentrated liquor is adjustable by a valve. The molten salt of SbCl3 as well as other high boiling point species such as PbCl2, BiCl3, CuCl2 and FeCl2 exists in the distillation still 1. When the temperature in the distillation still 1 reaches the design value, the molten salt is discharged into the distillation still 2. Then SbCl3 is distillated and condensed into the storage tank. Other high boiling point species remain in the distillation still 2. Finally, the residue is removed by dilute hydrochloric acid when it is accumulated to a certain amount. Therefore, the whole distillation process achieves continuity.
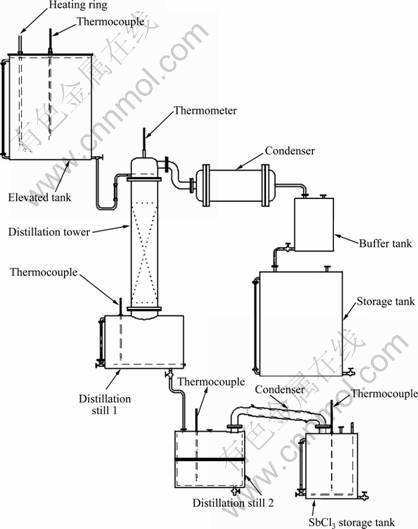
Fig.2 Schematic diagram for continuous distillation of antimony trichloride
The contents of Sb, As, Bi, Cu, Ag and Pb in the experimental samples were analyzed by means of oxidation-reduction titration using cerium sulfate, potassium bromate, EDTA, iodimetry, atomic absorption spectrometry and EDS(Thermo Noran Vantage-EST), respectively. The XRD patterns were obtained with an X-ray diffractometer(Thermo ARL X'TRA) using Cu Kα radiation.
3 Results and discussion
3.1 Potential-controlled chloridization leaching process
Table 2 lists the composition of one-stage potential-controlled chloridization leaching solution. The content of trivalent antimony ion in the leaching solution is as high as 489.2 g/L. With the increased antimony concentration, not only could leaching equipment investment be reduced, but also could the distillation efficiency be improved in the follow-up process, which results in a lower industrial cost and higher productivity. At the same time, the solubility of PbCl2, AgCl can also be reduced because of high concentration of trivalent antimony ion according to the thermodynamic analysis results in Ref. [13]. The concentrations of lead and silver in the leaching solution are only 4.27 g/L and 0.71 g/L respectively. But the concentrations of arsenic and fluoride in the leaching solution are as high as 30.5 g/L and 16.8 g/L, respectively. So, leaching solution can be recycled.
Table 2 Composition of one-stage chloridization leaching solution (g/L)

Fig.3 shows XRD pattern for the leached residue of two-stage chloridization leaching process at the controlled potential of 450 mV. The main species in the leached residue are PbCl2 and AgCl while no metallic Sb, Bi, Cu is observed, indicating a high leaching rate under such leaching potential. When the addition of chlorine is stopped prematurely, a dramatic decrease in solution potential is observed. The reason can be attributed to the fact that some metals are remained in the leaching residue. These metals reduce the pentavalent antimony ion into trivalent antimony ion, which causes a decrease in the solution potential.

Fig.3 XRD pattern for leached residue of two-stage chloridization leaching at controlled potential of 450 mV
The leaching rates of Sb, Cu and Bi are more than 99% when the solution potential of two-stage chloridization leaching is controlled at 450 mV. 91.54% of silver and 95.65% of lead in the lead anode slime are shifted into the leached residue. Therefore, the metals are highly enriched and the contents of Ag and Pb in the leached residue are more than 3% and 63%,respectively. The high concentration of metals in leached residue can benefit the following recovery process.
3.2 Concentration-distillation of chloridization leaching solution
Table 3 lists the composition of concentration liquor and concentration distillate. The distillation rate of arsenic is only 5.4% at the distillation temperature of 120 °C, which differs from the results reported by DUAN et al[14] in which arsenic trichloride can be entirely separated from antimony trichloride at about 110 °C. The concentrations of fluorine in the concentration distillate and concentration liquor are 31.72 g/L and 9.65 g/L, respectively. The distillation rate of fluoride is around 62.1%.
Fig.4 shows the XRD pattern of precipitate from the concentration distillate. It is found that the precipitate has an amorphous structure suggested by a broad scattering peak in the XRD pattern. The bulge peak denotes glass sample holder.
Table 3 Composition of concentration liquor and concentration distillate

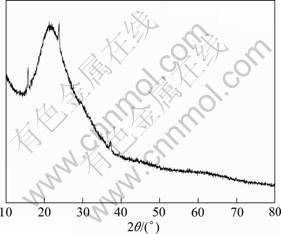
Fig.4 XRD pattern of precipitate from concentration distillate
Fig.5 shows the IR spectrum of precipitate from the concentration distillate. The peaks at 1104.2, 804.3 and 465.5 cm-1 are the characteristic absorption peaks of SiO2[15]. The absorption peaks at 1 104.2 and 804.3 cm-1 correspond to asymmetric and symmetric stretching vibration of Si—O bond, and the absorption peak of 465.5 cm-1 corresponds to bending vibration of Si—O—Si bond. The absorption peaks of water molecule (capillary water, the surface absorbed water, structural water) emerge at 1 634.6 and 3 426.5 cm-1. The former is resulted from the bending vibration of H—O—H band, which is related to free water (capillary water, the surface absorbed water). The latter is the stretching vibration of antisymmetric O—H band, which is relevant to structure water. The IR spectrum indicates that the precipitate from the concentration distillate is amorphous hydrated silica.
Table 4 lists the composition of precipitate from the concentration distillate. It is found that the main component of precipitate is silicon with the content of 43.71%. The small amount of antimony and arsenic is caused by mechanical entrainment during the concentration-distillation process.
Table 4 Composition of precipitate from concentration distillate (mass fraction, %)

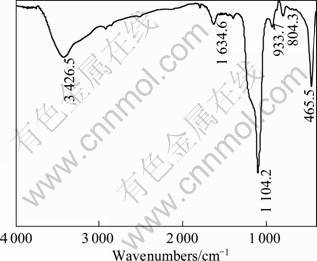
Fig.5 IR spectrum of precipitate from concentration distillate
3.3 Condensed liquid under continuous distillation
Fig.6 shows the relationship between boiling point and SbCl3 content in SbCl3-HCl-H2O system. It is found that the boiling point changes with the composition of liquid phase. The content of SbCl3 in the distillation mother liquor is more than 97% at 120 °C and reaches 99% at 140 °C. With increasing the temperature, the content of SbCl3 in the distillation mother liquor approaches 100% gradually. As already mentioned above, the purpose of continuous distillation is to evaporate the species with lower boiling point, such as HCl, H2O and AsCl3, and obtain the anhydrous antimony trichloride molten salt. High pure antimony trichloride can be achieved by evaporating the anhydrous antimony trichloride. By considering the fluidity of the molten salt and the loss of antimony trichloride, the ultimate temperature should be controlled between 180 and 190 °C during the distillation process of low boiling point species.
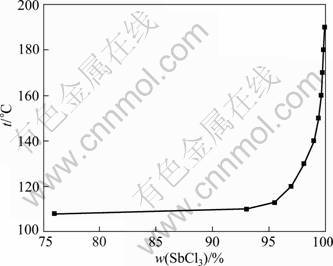
Fig.6 Temperature—content curve of antimony trichloride solution
Fig.7 shows the relationship between arsenic distillation rate and temperature. It is found that the distillation rate of As is only 5.4% at 120 °C, while it increases rapidly when the temperature exceeds 120 °C. The distillation rate of As is 36.3% at 130 °C and reaches 90% at 150 °C. The increasing of arsenic distillation rate with increased temperature becomes slow when the temperature exceeds 150 °C. The distillation rate of As is less than 100% at 180 °C. It is different from the results by DUAN et al[14] in which arsenic trichloride can be entirely evaporated at 110 °C. The difference might be caused by high concentration of SbCl3 in leaching solution, which impedes the evaporation of AsCl3.
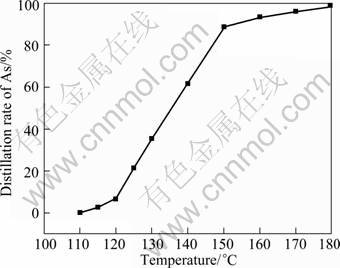
Fig.7 Relationship between arsenic distillation rate and temperature ([Sb3+]: 500 g/L)
Fig.8 shows the influence of temperature on fluoride distillation rate in the continuous distillation process. The distillation rate of fluoride is 65% at 120 °C and it increases to 80.6% at 130 °C. The trend becomes gentle when the distillation temperature is higher than 150 °C. The distillation ratio of F is less than 90% at 180 °C. The existence of F in the leaching solution is mainly caused by hydrofluosilic acid and lead fluosilicate in this lead anode slime. Fluoride distillation rate depends directly on the behavior of hydrofluosilic acid in continuous distillation process. According to the above analysis, hydrofluosilic acid can be evaporated or decomposed into amorphous hydrous silicon dioxide and HF at 120 °C. HF can be chelated with antimony ion, which makes the fluoride distillation more difficult. The fluoride distillation rate is less than 90%, even at 180 °C.
Table 5 lists the composition of antimony chloride prepared by continuous distillation. It is found that the product is high-purity SbCl3, and the contents of Fe, Bi, Pb, Ag, Cu are very low. However, the content of As is relatively high due to the low distillation separation efficiency for As in the leaching solution with a high concentration of antimony trichloride.
Table 6 lists the composition of continuous distillate. There are 5.48 g/L Sb and trace amount of Cu, Bi, Fe introduced into the continuous distillate by the mechanical entrainment. The main composition of distillate includes hydrochloric acid, arsenic trichloride and fluoride. The concentration of arsenic is up to 71.65 g/L, which is helpful for As recovery.
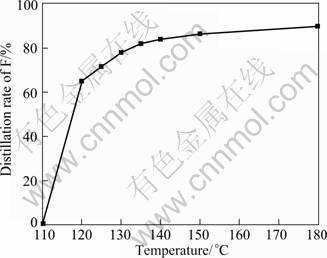
Fig.8 Relationship between fluoride distillation rate and temperature during continuous distillation process
Table 5 Composition of antimony chloride product (mass fraction, %)

Table 6 Composition of continuous distillate (g/L)

Table 7 lists the composition of continuous distillation residue. It is clear that Bi, Pb, Cu, Ag in the chloridization leaching solution are remained in continuous distillation residue, and more than 99% of bismuth chloride and copper chloride are enriched in the continuous distillation residue. Due to the high concentration, it becomes easy to recycle these valuable metals from lead anode slime. Antimony trichloride, bismuth trichloride, copper chloride in residue can be dissolved in 2 mol/L HCl solution, but lead chloride and silver chloride are almost insoluble.
Table 7 Composition of continuous distillation residue (mass fraction, %)

4 Conclusions
1) High-purity SbCl3 can be prepared from lead anode slime bearing high antimony and low silver by a potential-controlled chloridization leaching and continuous distillation. The recovery rate of Sb is high by this cost-effective and short process. It is a closed circulation and no waste is produced in the whole process, which is benign to the environmental protection.
2) The leaching rates of Sb, Cu, Bi are more than 99% when the solution potential of two-stage chloridization leaching is controlled at 450 mV. The content of trivalent antimony ion in the leaching solution is as high as 489.2 g/L. 91.54% of silver and 95.65% of lead in the lead anode slime are shifted into the leached residue.
3) When the leaching solution is distilled and concentrated at 120 °C, almost all the silicon compounds are evaporated into the concentration distillate and exist as the form of amorphous hydrated silica.
4) AsCl3 is enriched in the distillates while Bi and Cu are enriched in the continuous distillation residue. The high degree of enrichment is helpful for the following recovery process.
References
[1] LAGER T, FORSSBERG K S E. Beneficiation characteristics of antimony minerals a review—Part 1[J]. Minerals Engineering, 1989, 2(3): 321-336.
[2] QIU Ke-qiang, YANG Xue-lin, ZHANG Lu-lu, CHEN Qi-yuan. The new treatment technology of antimony-rich lead anode slime [J].Gold, 2003, 24(11): 37-39. (in Chinese)
[3] ZHANG Lu-lu, YANG Xue-lin, QIU Ke-qiang, CHEN Qi-yuan. Research on antimony removal from antimony-rich lead anode slime by vacuum reduction [J]. Chemical Industry and Engineering Progress, 2004, 23(8): 869-873. (in Chinese)
[4] HAVUZ T, DONMEZ B, CELIK C. Optimization of removal of lead from bearing-lead anode slime [J]. Journal of Industrial and Engineering Chemistry, 2010, 16(2): 355-358.
[5] AMER A M. Processing of copper anodic-slimes for extraction of valuable metals [J]. Waste Management, 2003, 23(8): 763-770.
[6] FERN?NDEZ M A, SEGARRA M, ESPIELL F. Selective leaching of arsenic and antimony contained in the anode slimes from copper refining [J]. Hydrometallurgy, 1996, 41(3): 255-267.
[7] DONMEZ B, EKINCI Z, ?ELIK C, ?OLAK S. Optimisation of the chlorination of gold in decopperized anode slime in aqueous medium [J]. Hydrometallurgy, 1999, 52(1): 81-90.
[8] NIE Xiao-jun, CHEN Qing-bang, LIU Ru-yi. Studies on hydrometallurgiced recovery of silver and other metals from high-antinomy and low-silver lead anode slimes [J]. Journal of Guangdong University of Technology, 1996, 13(4): 51-57. (in Chinese)
[9] TANG Mo-tang, TANG Zhao-bo, YANG Sheng-hai, WEI Yuan-ji. AC process for treating lead electrolysis refining anodicslime bearing high antimony and lower sliver: Enlarge experiments of chlorination-leaching and dry-distillation [J].Journal of Central South University of Technology: Natural Science, 2002, 33(4): 360-363. (in Chinese)
[10] XIONG Zhong-guo. Studies on new hydrometallurgiced method in dealing with lead anode slimes [J]. Non-ferrous Smelting, 1994(5): 26-30. (in Chinese)
[11] ZHI Bo. The preparation of antimony pentachloride from antimony-rich lead anode slime and its process of hydrolysis [D]. Hangzhou: Zhejiang University of Technology, 2006. (in Chinese)
[12] CHEN Jin-zhong, CAO Hua-zhen, ZHENG Guo-qu, ZHI Bo, YANG Tian-zu. Novel technology for preparation of SbCl5 from lead anode slime with high antimony and low silver content [J]. The Chinese Journal of Nonferrous Metals, 2008, 18(11): 2094-2099. (in Chinese)
[13] CHEN Jin-zhong, CAO Hua-zhen, LI Bo, YUAN Hai-jun, ZHENG Guo-qu, YANG Tian-zu. Thermodynamic analysis of separating lead and antimony in chloride system [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(3): 730-734.
[14] DUAN Xue-chen, YANG Xiang-ping, HUANG Wei-chuang. Preparation of high purity antimony chloride [J]. Journal of Central South University of Technology: Natural Science, 2001, 32(2): 169-172. (in Chinese)
[15] RYU S R, TOMOZAWA M. Fictive temperature measurement of amorphous SiO2 films by IR method [J]. Journal of Non-Crystalline Solids, 2006, 352(36): 3929-3935.
(Edited by LI Xiang-qun)
Foundation item: Project(2006BAB02B04-4-1) supported by the National Science and Technology Pillar Program during the 11th Five-Year Plan Period, China
Corresponding author: ZHENG Guo-qu; Tel: +86-571-88320429; E-mail: zhenggq@zjut.edu.cn
DOI: 10.1016/S1003-6326(10)60661-9


