
J. Cent. South Univ. (2019) 26: 806-812
DOI: https://doi.org/10.1007/s11771-019-4050-6
Flotation behavior and adsorption mechanism of salicylhydroxamic acid in artificial mineral anosovite
WANG Xue-feng(王雪峰)1, 2, WANG Yi-jie(王伊杰)2, WEN Shu-ming(文书明)3, YIN Peng-gang(殷鹏刚)1
1. School of Chemistry and Environment, Beijing University of Aeronautics and Astronautics,Beijing 100191, China;
2. Chinese Academy of Natural Resources Economics, Beijing 101149, China;
3. State Key Laboratory of Complex Nonferrous Metal Resources Clean Utilization, Kunming University of Science and Technology, Kunming 650093, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract:
Flotation is often employed to separate valuable natural minerals and gangue minerals. However, few studies have been conducted on artificial mineral flotation. Anosovite, the primary mineral in titanium slag, is a typical artificial mineral that can be enriched by flotation. In the present work, flotation behavior and adsorption mechanism of anosovite in salicylhydroxamic acid (SHA) solution were studied. The influence of pH and SHA dosage on anosovite flotability was investigated. Micro-flotation test results show that a pH range of 7–8.5 is available for SHA to collect anosovite. A maximum recovery of 93.26% can be obtained with SHA dosage of only 4×10-5 mol/L. In addition, TOC, zeta potential, FTIR, SEM-EDS, and XPS analyses were used to study the adsorption mechanism. Results demonstrated that SHA adsorption is governed by chemisorption. XPS studies further suggested that chemical adsorption occurred at the Ti sites on the anosovite surface.
Key words:
anosovite; salicylhydroxamic acid; flotation; adsorption;
Cite this article as:
WANG Xue-feng, WANG Yi-jie, WEN Shu-ming, YIN Peng-gang. Flotation behavior and adsorption mechanism of salicylhydroxamic acid in artificial mineral anosovite [J]. Journal of Central South University, 2019, 26(4): 806–812.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-019-4050-61 Introduction
Titanium slag is an important intermediate product in titanium industry, and its quality is directly related to the production process and product quality of titanium dioxide and titanium sponge [1, 2]. Therefore, the upgrading of titanium slag is a vital topic [3]. The melting slag produced by direct reduction and smelting process contains 40%–50% of TiO2, and the enrichment of titanium minerals and their separation from gangue minerals are also key issues in the process. At present, most studies of titanium purification focus on hydrometallurgy or pyrometallurgy [4–6]. CHO et al [7] studied the leaching characteristics of iron removal from the reduced upgraded titania slag with mild hydrochloric and boric acids under ambient and elevated pressures. ZHENG et al [8] investigated the hydrochloric acid leaching of titanium from titanium-bearing electric furnace slag under different particle sizes, hydrochloric acid concentration, and reaction temperature. Results showed that iron and calcium were almost dissolved, but other species were still in the leaching residue. LASHEEN [9] presented a soda ash roasting process for upgrading titania slag product of Rosetta ilmenite to high-grade TiO2.
Hydrometallurgy and pyrometallurgy are complicated and expensive. Thus, flotation, as a mature beneficiation technology, can be applied to the purification process of titanium and melting slags. However, few reports are involved in this subject. The minerals in titanium and melting slags are artificial minerals, whose surface properties largely differ with natural minerals because of their different minerogenetic environments [10–14]. Hence, flotation performance of natural minerals greatly differs from that of artificial minerals. The primary titanium mineral in titanium and melting slags is anosovite solid solution, which can be expressed as m(AO·TiO2)·n(B2O3·TiO2), where A is Ti2+, Fe2+, Mg2+, Mn2+, etc.; B is Ti3+, Fe3+, Al3+, Cr3+, etc.; and m and n are constant coefficients [15–17]. Rare study on flotation behavior of anosovite has been reported, and its flotation characteristics have seldomly been studied; thus, such study is a meaningful work.
Salicylhydroxamic acid (SHA) has a good selectivity as collector because of its chelating properties. Therefore, it is widely used in the flotation process of ilmenite, cassiterite, rare earth, bauxite, and other ores [18, 19]. SHA was chosen as the collector for this study to explore the flotation behavior of anosovite pure mineral, and its adsorption mechanism was also studied by analytical and testing methods. This study provides theoretical support for titanium and melting slag purification by flotation and also enriches the basic theory of artificial mineral flotation.
2 Experimental
2.1 Materials
The anosovite samples used in the study were obtained from Wuding County, China. They were produced by the electric furnace smelting process with the raw materials of ilmenite concentrates. The chemical composition of the anosovite samples is shown in Table 1. As can be seen from the table, the TiO2 has a high grade of 93.45%. The X-ray diffraction (XRD) results of the anosovite samples are shown in Figure 1. The spectra indicate that the anosovite crystal is well developed, and the main characteristic peaks are obvious. Furthermore, the anosovite crystal is doped with magnesium, and its chemical composition is Mg0.09Ti2.91O5.
Table 1 Chemical composition of anosovite ore (mass fraction, %)

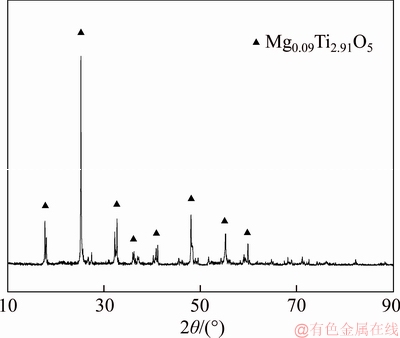
Figure 1 XRD pattern of anosovite samples
2.2 Experimental method
2.2.1 Flotation test
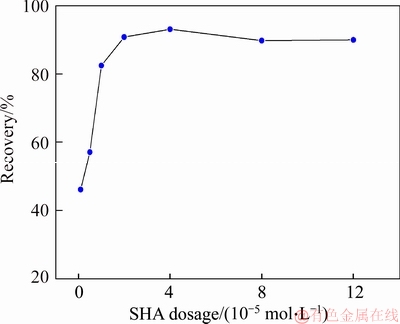
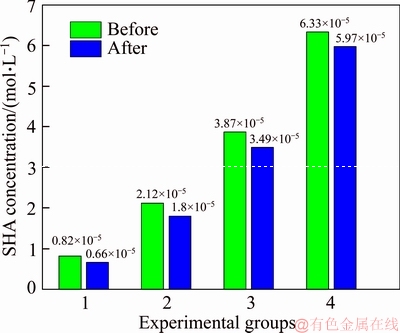
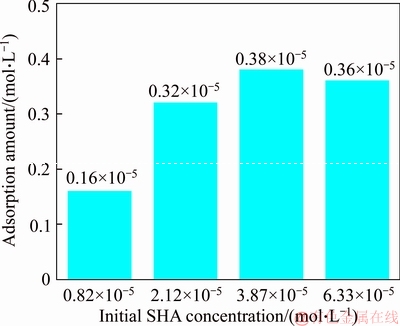
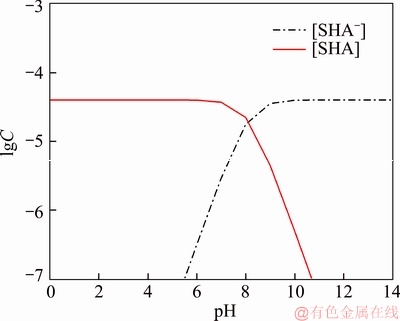
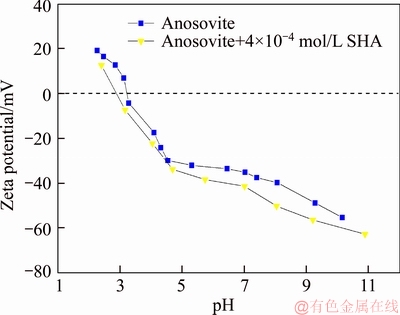
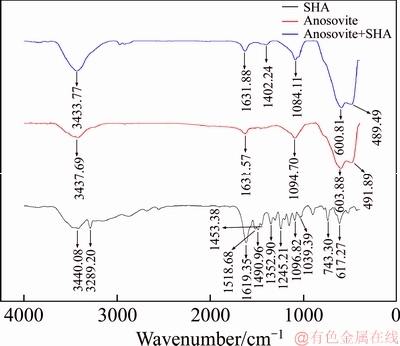
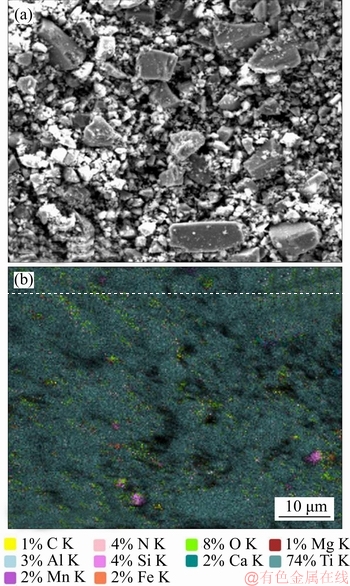
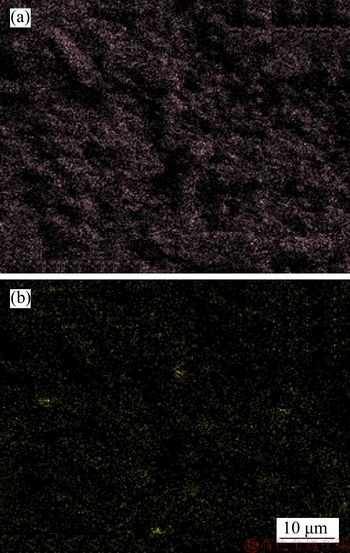
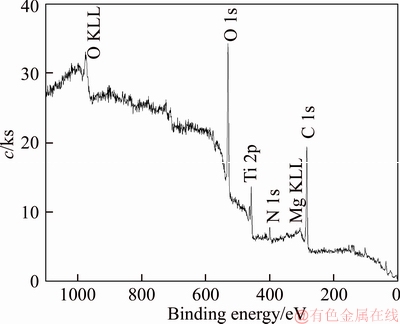
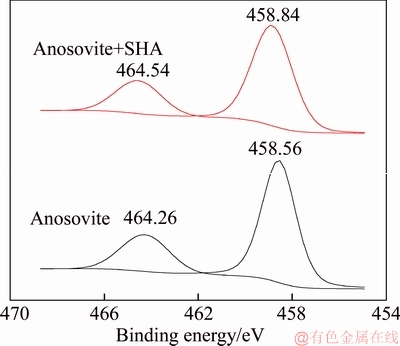
A 40 mL hanging laboratory flotation machine at a revolving speed of 1900 r/min was used to test the flotation behavior of anosovite. Deionized water with a resistivity of <18 MΩ·cm was used throughout the flotation tests, and all tests were performed at room temperature. Up to 3 g of anosovite (74 μm 2.2.2 XRD analysis The mineral composition of the anosovite ore was characterized using XRD (D/Max 2200, Rigaku, Japan) with Cu-Kα radiation (λ=0.154056 nm, 40 kV, 200 mA) over the 2θ range of 10°–90° at a scanning rate of 3 (°)/min. 2.2.3 Total organic carbon (TOC) measurement A total of 3 g of anosovite sample (74 μm 2.2.4 Zeta potential measurement The zeta potential was measured with Zeta Plus from Brookhaven Instruments Corporation. Up to 0.1 g of anosovite (d<5 μm) was placed in a solution with a certain pH, and SHA was added to the solution. The solution was stirred for 10 min using a magnetic stirrer and then allowed to rest for 10 min. The pH of up to 1.5 mL of supernatant, pipetted by a sampler, was measured in a measurement cell; the ζ- potential was measured 10 times to calculate the average. 2.2.5 FTIR analysis The potassium bromide squash method was used in the FTIR study. One gram of anosovite (d<5 μm) was added to the 40 mL SHA solution and stirred for 5 min. Subsequently, the suspensions were filtered and rinsed three times with deionized water. Finally, the samples were air-dried. The FTIR spectra (Tensor 27, Bruker, Germany) were recorded in the range of 400 to 4000 cm-1 with a spectral resolution of 4 cm-1 and a scan number of 10. 2.2.6 SEM-EDS analysis Surface micromorphological characteristics were examined with a Quantan 200 scanning electron microscope (SEM; FEI, the Netherlands). Element contents were determined by Genesis 2000 energy dispersive spectroscopy (EDS; EDAX, USA). The preparation methods of the test samples used in the SEM-EDS analysis were consistent with the methods used in the FTIR analysis. 2.2.7 XPS analysis The preparation methods of the test samples used in the XPS study were consistent with the methods used in the FTIR analysis. XPS analysis was conducted with a PHI 5000 Versa Probe II spectrometer (ULVAC-PHI, Japan) using an Al Kα irradiation X-ray source; the pressure in the analyser chamber was 1×10-8 Pa. MultiPak Spectrum software was used to analyse the spectra. C1s binding energy (284.8 eV) was used to calibrate the Ti2p3/2 and Ti2p1/2 peaks. 3 Results and discussion 3.1 Effect of pH and SHA dosage on anosovite flotability The pulp pH and collector dosage are two major factors affecting mineral recovery in flotation, thus this study focuses on these two factors. The effect of pH on anosovite flotation recovery at a SHA dosage of 1×10-5 mol/L is shown in Figure 2. Results show that the recovery of anosovite in a neutral and weak alkaline environment is higher than that in acid and alkaline environment, especially in alkaline environment. The collecting ability of SHA for anosovite reduces in acid and alkaline environment; thus, the neutral and weak alkaline pH ranges are available for SHA to collect anosovite. The effect of SHA dosage on the flotation recovery of anosovite at pulp pH=7 was studied, and the results are shown in Figure 3. As can be seen from Figure 3, with the increase of SHA dosage, the anosovite recovery gradually increases and then levels off. The recovery reaches a maximum of 93.26% when SHA dosage is 4×10-5 mol/L. Figure 2 Effect of pH on flotation recovery of anosovite at SHA concentration of 1×10-5 mol/L Figure 3 Effect of SHA dosage on flotation recovery of anosovite at pH=7 3.2 Adsorption amount of SHA on anosovite surface The collector concentration before and after mineral adsorption is often measured to indirectly calculate the amounts of the collector adsorption on mineral surface. TOC method was employed in this study to calculate the concentration of SHA. At a pH of 7, the adsorption amount of SHA on anosovite surface at different initial SHA concentrations were evaluated. Figures 4 and 5 show that the adsorption amount of SHA on anosovite surface increases when the initial concentration increases and then remains stable. The adsorption amount of anosovite to SHA was 0.32×10-5 mol/L to 0.38×10-5 mol/L, when the SHA initial concentration ranged from 2.12×10-5 mol/L to 6.33×10-5 mol/L.The adsorption amount of SHA becomes saturated when the initial concentration exceeds 2.12×10-5 mol/L, and the recovery of anosovite also leveled off at that point (Figure 3), thus proving that they are positively correlated. Figure 4 SHA concentration in solution before and after anosovite adsorption Figure 5 Adsorption amount of SHA at different initial concentrations 3.3 Solution chemistry and zeta potential SHA dissociates in water as it is a weak acid. The ratio of undissociated SHA molecules to dissociated SHA ions depends on the pH of aqueous solution. The lgC– pH diagram of SHA solution is shown in Figure 6. Figure 6 Relationship between lgC and pH of SHA solution components at CT of 4×10-5 mol/L Figure 7 shows the zeta potential of anosovite in deionized water and in SHA solution at a concentration of 4×10-4 mol/L. The point of zero charge (PZC) is visible near pH 3.2. The zeta potential of the anosovite surface shifted toward negative direction in the presence of SHA, indicating that SHA adsorbed on the surface of the anosovite. At pH<3.2, the adsorption is expected as electrostatic adsorption or chemisorption, whereas at pH>3.2, the adsorption is expected as chemisorption. As can be seen from Figure 6, the concentrations of [SHA–] increase rapidly in a range of 7<pH<8.5. Therefore, the increasing level of anion adsorption on the anosovite surface leads to a decrease in zeta potential. The above analysis of the zeta potential results is further supported by flotation experiments (as shown in Figure 2). In a weak alkaline solution (7<pH<8.5), the higher anosovite recovery indicates that more collectors are adsorbed onto the anosovite surface. Figure 7 Effects of SHA and pH on zeta potential of anosovite 3.4 FTIR spectra analysis The FTIR was conducted at a SHA concentration of 1×10-2 mol/L and pH=8. Figure 8 shows the FTIR spectra of SHA, and the FTIR spectra of the anosovite before and after reaction with SHA. SHA has many characteristic absorption peaks, which have been reported in the literature. The peak at 3289.20 cm-1 is due to the N–H stretching vibration peak. The peak at 1619.35 cm-1 is the C=O vibration peak. Benzene skeleton vibration is shown at 1518.68 and 1490.96 cm-1, and the peak at 1453.38 cm-1 is the C=C stretching vibration peak in benzene. The peaks at 1096.82 and 1039.39 cm-1 are the split peaks of N—O vibration. Figure 8 FTIR spectra of anosovite before and after reaction with SHA As shown in Figure 8, a new peak at 1402.24 cm-1 was detected after anosovite reacted with SHA, and this peak may be the characteristic peak of the benzene ring. In addition, a characteristic peak of anosovite at 1094.70 cm-1 moved to 1084.11 cm-1 after SHA adsorption due to N—O vibration, indicating that a chemical reaction occurred between the N—O bond and the atoms on the mineral surface. 3.5 SEM-EDS and XPS analysis The experimental conditions used in SEM-EDS and XPS analysis were consistent with FTIR analysis. Figures 9 and 10 show the element distributions of anosovite surface after SHA adsorption. N element and C element were evenly distributed on the anosovite surface after reaction with SHA, indicating adsorbance. A peak of N element was detected in XPS broad scan spectra (Figure 11), further supporting SEM-EDS analysis. The binding energies of Ti2p before and after SHA adsorption are shown in Figure 12. The binding energies of Ti2p3/2 and Ti2p1/2 on the anosovite mineral surface were measured as 458.56 and 464.26 eV [20–22]. After conditioning anosovite with SHA, the binding energies of Ti2p changed from 458.56 and 464.26 eV to 458.84 and 464.54 eV, respectively, with energy differences of 0.28 eV. The change in Ti2p binding energy demonstrates that chemical adsorption occurred at the Ti sites on the anosovite surface. XPS analysis results are consistent with the FTIR spectra. Figure 9 SEM images (a) and EDS spectrum (b) of anosovite surface after SHA adsorption Figure 10 Distribution of N (a) and C (b) elements on mineral surface after SHA adsorption Figure 11 XPS broad scan spectrum of anosovite after SHA adsorption Figure 12 Ti2p XPS spectra of anosovite before and after SHA adsorption 4 Conclusions This work demonstrates the flotation behavior and adsorption mechanism of SHA to the artificial mineral anosovite. Analyses of the anosovite properties show that the TiO2 grade of the anosovite ore sample is 93.45%, and its chemical composition is Mg0.09Ti2.91O5. A neutral and weak alkaline environment (7<pH<8.5) is reasonable for SHA to collect anosovite. Furthermore, a maximum recovery of 93.26% can be acquired at an SHA dosage of 4×10-5 mol/L. TOC results indicate that the adsorption amount of SHA are positively correlated with flotation recovery. The zeta potential decreased to a negative direction largely at 7<pH<8.5 when the concentrations of [SHA–] increased rapidly. Moreover, FTIR and SEM-EDS analyses showed that SHA adsorption depends on chemisorptions. XPS studies further suggested that the chemical adsorption occurred at the Ti sites as a result of the change in the binding energy for Ti2p. References [1] SAMAL S. Synthesis and characterization of titanium slag from ilmenite by thermal plasma processing [J]. JOM, 2016, 68(9): 1–10. [2] XUE Ya-zhou, WANG Xue-feng, WANG Hai-jun, LI Wen-chao. On comprehensive utilization of vanadium- titanium magnetite resources in Panzhihua-Xichang region of Sichuan province [J]. Chinese Land Resources Economy, 2017, 30(4): 9–13. (in Chinese) [3] ZHANG Ying, FANG Zhi-gang Zak, XIA Yang, HUANG Zhe, LEFLER H, ZHAGN Tuo-yang, SUN Pei, FREE M L, GUO Jun. A novel chemical pathway for energy efficient production of Ti metal from upgraded titanium slag [J]. Chemical Engineering Journal, 2016, 286: 517–527. [4] CHEN Xu-ling, HUANG Yun-song, FAN Xiao-hui, GAN Min. Oxidation roasting behavior and concretion properties of vanadium-titanium magnetite pellet [J]. Journal of Central South University: Science and Technology, 2016, 47(2): 359–366. (in Chinese) [5] SUI Li-li, ZHAI Yu-chun, MIAO Li-hua. Recovery of titania from high titanium slag by roasting method using concentrated sulfuric acid [J]. Rare Metals, 2015, 34(12): 895–900. [6] WAN He-li, XU Bao-qiang, DAI Yong-nian, YANG Bin, LIU Da-chun, SEN Wei. Preparation of titanium powders by calciothermic reduction of titanium dioxide [J]. Journal of Central South University, 2012, 19(9): 2434–2439. [7] CHO J, ROY S, SATHYAPALAN A, ZENG W. Purification of reduced upgraded titania slag by iron removal using mild acids [J]. Hydrometallurgy, 2016, 161: 7–13. [8] ZHENG Fu-qiang, CHEN Feng, GUO Yu-feng, JIANG Tao, TRAVYANOV A Y, QIU Guan-zhou. Kinetics of hydrochloric acid leaching of titanium from titanium- bearing electric furnace slag [J]. JOM, 2016, 68(5): 1476–1484. [9] LASHEEN T A. Soda ash roasting of titania slag product from Rosetta ilmenite [J]. Hydrometallurgy, 2008, 93(3, 4): 124–128. [10] SZCZE [11] MA Jun-wei, SUI Zhi-tong, CHEN Bing-chen, NIE Yong-feng. Flotation behavior and mechanism on perovskite in Ti-bearing blast furnace slag [J]. Chinese Journal of Nonferrous Metals, 2002, 12(1): 171–177. (in Chinese) [12] WANG Wei-qing, ZHU Yang-ge, ZHANG Shi-qiu, DENG Jie, HUANG Yang, YAN Wu. Flotation behaviors of perovskite, titanaugite, and magnesium aluminate spinel using octyl hydroxamic acid as the collector [J]. Minerals, 2017, 7(8): 134–152. [13] MARABINI A M, BARBARO M. A study of the surface electrical properties of titanium oxide [J]. International Journal of Mineral Processing, 1980, 7(2): 159–166. [14] MEHDILO A, IRANNAJAD M, REZAI B. Effect of crystal chemistry and surface properties on ilmenite flotation behavior [J]. International Journal of Mineral Processing, 2015, 137: 71–81. [15] GRASS V, ISTOMIN P, NAZAROVA L. X-ray diffraction refinement of the crystal structure of anosovite prepared from leucoxene [J]. Crystal Research and Technology, 2009, 44(1): 117–122. [16] GREY I E, CRANSWICK L M D, LI C, WHITE T J, BURSILL L A. New M3O5-anatase intergrowth structures formed during low-temperature oxidation of anosovite [J]. Journal of Solid State Chemistry, 2000, 150(1): 128–138. [17] XIROUCHAKIS D, SMIRNOV A, WOODY K, LINDSLEY D H, ANDERSEN F J. Thermodynamics and stability of pseudobrookite-type MgTi2O5 (karrooite) [J]. American Mineralogist, 2002, 87(5, 6): 658–667. [18] QIN Wen-qing, REN Liu-yi, XU Yang-bao, WANG Pei-pei, MA Xi-hong. Adsorption mechanism of mixed salicylhydroxamic acid and tributyl phosphate collectors in fine cassiterite electro-flotation system [J]. Journal of Central South University, 2012, 19(6): 1711–1717. [19] FENG Qi-cheng, ZHAO Wen-juan, WEN Shu-ming, CAO Qin-bo. Activation mechanism of lead ions in cassiterite flotation with salicylhydroxamic acid as collector [J]. Separation and Purification Technology, 2017, 178: 193–199. [20] ESKANDARLOO H, HASHEMPOUR M, VICENZO A, FRANZ S, BADIEI A, BEHNAJADY M A, BESTETTI M. High-temperature stable anatase-type TiO2 nanotube arrays: A study of the structure-activity relationship [J]. Applied Catalysis B: Environmental, 2016, 185: 119–132. [21] HOANG S, BERGLUND S P, HAHN N T, BARD A J, MULLINS C B. Enhancing visible light photo-oxidation of water with TiO2 nanowire arrays via cotreatment with H2 and NH3: Synergistic effects between Ti3+ and N [J]. Journal of the American Chemical Society, 2012, 134: 3659–3662. [22] LIU Jian, DUAN Xiu-lan, ZHANG Yang, LI Zi-qing, YU Fa-peng, JIANG Huai-dong. Growth, electronic structure and properties of Rb2Ti1.95Yb0.05(PO4)3 crystals [J]. Journal of Alloys & Compounds, 2016, 660: 356–360. (Edited by HE Yun-bin) 中文导读 人造矿物黑钛石的浮选行为及水杨羟肟酸的吸附机理 摘要:浮选经常被应用于分离天然的有用矿物和脉石矿物,但是很少有研究聚焦于人造矿物的浮选。黑钛石作为钛渣中的主要矿物,是一种典型的人造矿物,可以通过浮选来富集。本文研究了黑钛石在水杨羟肟酸溶液中的浮选行为及吸附机理,重点研究了pH和水杨羟肟酸用量对黑钛石浮选性能的影响。纯矿物浮选试验结果表明,以水杨羟肟酸为捕收剂,黑钛石最佳的浮选区间是7<pH<8.5,当水杨羟肟酸的用量只有4×10-5mol/L时,黑钛石的回收率可以达到93.26%。另外,采用总有机碳分析、zeta电位、红外光谱分析、扫描电镜能谱分析、X射线光电子能谱分析等测试手段研究吸附机理。研究结果表明水杨羟肟酸在黑钛石表面是化学吸附,X射线光电子能谱分析显示化学吸附发生在Ti位点上。 关键词:黑钛石;水杨羟肟酸;浮选;吸附 Foundation item: Project(51090385) supported by the Major Program of the National Natural Science Foundation of China; Projects(121102000000160001, 121102000000170013) supported by the Ministry of Land and Resources Department Budget, China; Project(DD20179133) supported by the Geological Survey and Evaluation Project of China; Project(2018M641439) supported by China Postdoctoral Science Foundation Received date: 2017-11-23; Accepted date: 2018-07-23 Corresponding author: WANG Yi-jie, PhD; Tel: +86-10-61592381; E-mail: yjwang2012@126.com; ORCID: 0000-0001-7918-8779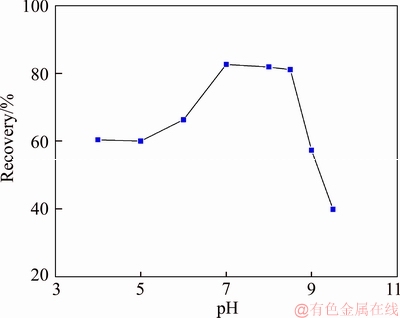
 A, YAN Ying-di, CHIBOWSKI E,
A, YAN Ying-di, CHIBOWSKI E,  L, BANACH M. Properties of natural and synthetic hydroxyapatite and their surface free energy determined by the thin-layer wicking method [J]. Applied Surface Science, 2017, 434: 1232–1238.
L, BANACH M. Properties of natural and synthetic hydroxyapatite and their surface free energy determined by the thin-layer wicking method [J]. Applied Surface Science, 2017, 434: 1232–1238.
Abstract: Flotation is often employed to separate valuable natural minerals and gangue minerals. However, few studies have been conducted on artificial mineral flotation. Anosovite, the primary mineral in titanium slag, is a typical artificial mineral that can be enriched by flotation. In the present work, flotation behavior and adsorption mechanism of anosovite in salicylhydroxamic acid (SHA) solution were studied. The influence of pH and SHA dosage on anosovite flotability was investigated. Micro-flotation test results show that a pH range of 7–8.5 is available for SHA to collect anosovite. A maximum recovery of 93.26% can be obtained with SHA dosage of only 4×10-5 mol/L. In addition, TOC, zeta potential, FTIR, SEM-EDS, and XPS analyses were used to study the adsorption mechanism. Results demonstrated that SHA adsorption is governed by chemisorption. XPS studies further suggested that chemical adsorption occurred at the Ti sites on the anosovite surface.


