
Preparation of supported nano-copper oxide and its sulfation kinetics
YU Qing-chun(郁青春)1, ZHANG Shi-chao(张世超)2, YANG Bin(杨 斌)1,
LIU Da-chun(刘大春)1, XU Bao-qiang(徐宝强)1, MA Wen-hui(马文会)1
1. National Engineering Laboratory of Vacuum Metallurgy, Kunming University of Science and Technology,
Kunming 650093, China;
2. School of Materials Science and Engineering, Beijing University of Aeronautics and Astronautics,
Beijing 100083, China
Received 10 August 2009; accepted 15 September 2009
Abstract:
CuO/γ-Al2O3 sorbents were prepared by means of impregnation. Thermogravimetric technique was used to study the sulfation of CuO/γ-Al2O3 sorbents. The sulfation tests were performed using gas containing 0.1%-0.9% SO2, 5% O2, 3% H2O steam, and N2 as the balance. Experimental conditions including temperature, SO2 concentration and pore structure were studied. The sulfation experiment results show that the sulfation reaction rate increases with increasing temperature and SO2 concentration, and the surface and pore volume decrease after sulfation. Sulfation kinetics analysis shows that the reaction between CuO/γ-Al2O3 and SO2 obeys pore-blocking model well. Proportionality (pore-blocking constant) 1/λ decreases with increasing temperature. The activation energy and reaction order with respect to SO2 obtained are 37.9 kJ/mol and the first order, respectively. The existing state of CuO exerts an influence on activation energy.
Key words:
CuO; SO2; sulfation; pore-blocking model; flue gas desulfurization;
1 Introduction
Air pollution arising from the emission of SOx from combustion of fossil fuels has increasingly been recognized as a problem[1-3]. Currently, flue gas desulfurization (FGD) is being carried out mainly using two basic FGD processes: regenerable and throwaway. Throwaway process uses inexpensive scrubbing media that are cheaper to replace than to regenerate, but a lot of waste disposals are needed. With increasing sever environmental regulations, there is a strong incentive worldwide to the application of efficient processes for dry and recycling solid sorbents. The removal of SO2 by CuO/γ-Al2O3 sorbent, a dry and regenerable means to remove SO2, has received much attention recently[4-8].
To use gas-solid reaction models in modeling of SO2 retension in boilers, it is necessary to know the sulfation kinetics. CENTI et al[9] proposed that SO2 interacted CuO sites to give rise to the formation of SO3 that in turn reacted with a further copper site to form a bidentate sulfate, and an integral kinetic model based on two parallel pathways of formation of copper- or alumina-sulfate was obtained. In the case of industrial copper-on-alumina pellets for industrial applications[10], a grain model modified was used for the simulation of sulfation behavior in fixed-bed flow reactor tests. Most grain models are based on the assumption that the initial structure is maintained throughout and is not affected by the progress of the reaction[11-12]. These models generally require a numerical solution. In recent years, the regeneration of sulfated CuO/γ-Al2O3 sorbents indicated that the surface area and pore volume of sorbent decreased after sulfation due to the pore plugging caused by the formation of sulfates[13]. Furthermore, few literatures reported the sulfation of nano-CuO. Based on considerations above, a more general and easily resolved model, i.e. the “pore-blocking” model was believed to be a logical choice to give satisfactory predictions in sulfation reactions.
The aim of the present work is to prepare nano- scale CuO supported on γ-Al2O3. The effects of SO2 concentration and temperature were investigated by thermogravimetric technique. The sulfation kinetics of CuO/γ-Al2O3 sorbent was studied using pore-blocking model, and the change of kinetic parameters was discussed.
2 Experimental
2.1 Instruments and materials
Instruments included a self-made thermogravimetric setup[14], an muffle furnace (SX2-12-10), a surface adsorption and desorption instrument (AUTOSORB-1C), an X-ray diffractometer (D/max-RBX), and a scanning electron microscopy (JSM-5800).
The gas composition was 2.86-25.74 g/m3 SO2, 5% O2, 3% H2O steam, and N2 as the balance. Cu(NO3)2?3H2O (A.R.) and γ-Al2O3(BET surface area 277.8 m2/g) were used.
2.2 Experimental procedure
CuO/γ-Al2O3 sorbent was prepared by wet impregnation with a known mass of γ-Al2O3 and a solution containing a calculated amount of Cu(NO3)2?3H2O. Being kept in static conditions at room temperature for 1 h, followed by evaporating at 90 ℃ with stirring, the samples were dried in oven at 120 ℃ for 24 h, and subsequently calcined at 450 ℃ in stagnant air for 5 h in muffle furnace to convert the impregnated Cu(NO3)2 to CuO. In this way, sorbents containing 0.30 g CuO, 0.40 g CuO and 0.5 g CuO per gram γ-Al2O3 were obtained, respectively.
A sample pan loaded with 50 mg of sorbent was placed into the reaction zone of the thermogravimetric setup and then heated to the desired temperature. Humidified mixture of H2O steam, N2 and O2 with a rate of 300 mL/min flew into the reactor. When the mass change of the sample reached a steady state, SO2 was introduced into the reactor. The mass gain was recorded automatically.
2.3 Data evaluation
The chemical reaction taking place during the sulfation of sorbents is as follows:
CuO+SO2+1/2O2=CuSO4 (1)
Data of mass gain vs time during sulfation are obtained from the TG results. ?m=mt-m0 is calculated at each time, where m0 is the mass of the sorbent at the start of sulfation, and mt is the mass of the sorbent at time t. The conversion of CuO to CuSO4, X, defined as ?m/?mmax is calculated for each time and plotted as a function of time. ?mmax calculated according to Eq.(1) is the maximal mass gain.
2.4 Kinetic model
Pore-blocking model was derived by EVANS[15] based upon the reaction rate influenced by the products in a given pore set up compressional stresses in the solid, causing blockage of neighboring pores. The conversion of copper oxide (X) is expressed by
X=λln(1+Kd t/λ) (2)
where t is time; 1/λ is proportionality constant (pore- blocking constant) that depends only on the basic pore structure and should be independent of gas concentration; Kd is constant. Eq.(2) can be transformed to the following equation:
exp(X/λ)=1+Kd t/λ (3)
The pore-blocking model given by Eq.(3) can be tested by the range of λ, which gives a straight line at each temperature on plotting exp(X/λ) against t. Kd influenced by temperature and gas concentration can be expressed as
Kd =a?k?f (CSO2) (4)
where a is the stoichiometry coefficient (a=1 in this system); k is the rate constant; CSO2 is the SO2 concentration in the bulk; and f designates the concentration dependence. According to Arrhenius equation:
ln k =-E/(RT)+B (5)
where B is a constant, and R is the gas constant.
The slope is then used to calculate activation energy.
3 Results and discussion
3.1 XRD characterization
XRD patterns were used for nano-CuO identification. Fig.1 shows XRD patterns of the samples, indicating the peaks of CuO at 35? and 38?.

Fig.1 XRD patterns of CuO supported on γ-Al2O3: (a) 0.3 g CuO/g (γ-Al2O3); (b) 0.4 g CuO/g (γ-Al2O3); (c) 0.5 g CuO/g (γ-Al2O3)
The intensities of peaks increase with increasing the amount of CuO loading. γ-Al2O3 is noncrystallite and cannot be detected by XRD. Scherer’s formula (Eq.(6)) was used for the calculation of CuO crystallite sizes.
![]() (6)
(6)
where D is the crystallites size; λ is the X-ray wavelength used; β is the broadening of diffraction line measured as half of its maximum intensity; and θ is the corresponding angle. Based on Eq.(6), an estimated crystallite size of 0.3 g CuO/g(γ-Al2O3) found to be 85 nm was used in the following sulfation experiments.
3.2 Sulfation experiments
3.2.1 Effect of SO2 concentration
SO2 concentrations were chosen as follows: 2.86, 5.72, 14.30, 25.74 g/m3. The effect of the SO2 concentration on the conversion at 400 ℃ is shown in Fig.2.
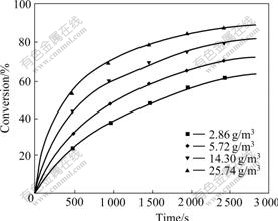
Fig.2 Curves of conversion vs time at different SO2 concentrations
The reaction rate increases with an increase in the SO2 concentration. The curves exhibit two regions: a rapid reaction region, and a slow reaction region in which the conversion either levels off or continues at a slow rate. The decreasing slope may be attributed to the decreasing pores and the increasing diffusional resistance through pores.
3.2.2 Effect of temperature
The experiments were carried out at SO2 concentration of 14.30 g/m3 in the temperature range of 250-450 ℃. The conversion vs time at different temperatures is shown in Fig.3.
The sulfation curves were characterized by an initial increase in conversion with increasing temperature. Due to size expansion from CuO to CuSO4 and possibly pore plugging by the formed CuSO4, a slow rate is found in the final stage of sulfation, which is similar to the curves of conversion vs time at different SO2 concentrations shown in Fig.2.

Fig.3 Curves of conversion vs time at different temperatures
3.2.3 Effect of pore structure
In order to study the effect of sulfation on pores, pore structure parameters of CuO/γ-Al2O3 sorbent before and after the sulfation were tested using N2 isothermal adsorption method. The pore size distribution is shown in Fig.4.
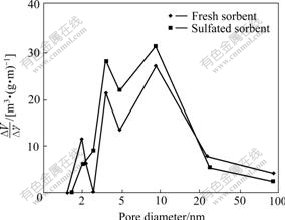
Fig.4 Pore-size distribution of 0.3 g (CuO)/g(γ-Al2O3) sorbent before and after sulfation
The pore size distribution shifts to smaller pores with a decrease of surface area during sulfation reaction.
The total average pore diameter is smaller than 20 nm. Most pores of the sorbent are mesopores and micropores. Pore volumes of sulfated samples are observed to decrease, indicating that the reaction takes place inside the pores and the reaction products (CuSO4) formed on the pore walls have larger molar volumes than the solid reactants. The surface area decreases after sulfation of CuO/γ-Al2O3 sorbent because sulfates may plug the pores of the alumina support, as confirmed by Table 1.
Table 1 Pore structure parameters of 0.3 g (CuO)/g(γ-Al2O3) sorbent before and after sulfation

3.3 Kinetic analysis
In order to check the validity of pore-blocking model, experimental data at different temperatures were analyzed according to Eq.(3). λ and Kd determined by nonlinear regression analysis[16] are shown in Table 2. A straight line at each temperature on plotting exp(X/λ) vs t is obtained, as shown in Fig.5, which means that the pore-blocking model fits the sulfation of nano-CuO well.
Table 2 λ and Kd at different temperatures

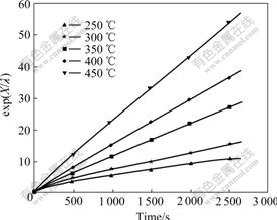
Fig.5 Plots of exp(X/λ) vs time at different temperatures
The chemical reaction was confined to the external crystallites of the individual particle at the initial stage. However, at higher temperatures, the penetration of SO2 increases, and the reaction takes place within the interior structure of the particle. These phenomena are supported indirectly by the higher pore-blocking rate constants. 1/λ is obtained at lower temperatures and shown in Table 2. As supported by the data for CuSO4 crystallite size listed in Table 1, larger 1/λ is undoubtedly associated with a larger size of CuSO4 crystallites locally formed in the external layer of CuO particles.
According to Eq.(4), a plot of Kd vs CSO2 yields a straight line passing through the origin, as shown in Fig.6, indicating that the reaction is of the first order with respect to SO2 concentration in the gas phase. It should be pointed out that O2 concentration is far more than SO2 concentration, and the complete reaction of SO2 only consumes less than 3% of the total oxygen. Therefore, it is reasonable to assume that O2 concentration is unchangeable.

Fig.6 Plot of Kd vs SO2 concentration
In order to obtain the activation energy, rate constants (k) at different temperatures were calculated according to Eq.(4) and Kd in Table 2. Then, substituting k into Eq.(5), the plot of ln k vs 1/T is a straight line relationship, as shown in Fig.7. The slope of the straight line through the experimental points corresponds to an activation energy of 37.9 kJ/mol and the frequency factor is 13 360 s-1.
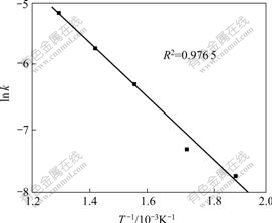
Fig.7 Arrhenius plot of ln k vs 1/T
Due to the influence of heat transfer and mass transfer on the phase surface, kinetic parameters of gas-solid reaction vary with many factors, such as the change of reaction condition and crystal form. DEBERRY and SLADEK[17], and YU et al[18] reported that the reaction activation energies of pure CuO and highly dispersed CuO with SO2 were 112.44 and 19.98 kJ/mol, respectively. There exists a dramatical decrease of activation energy in the case of nono-scale reductants compared with the normal sized CuO. This is normally attributed to the excessive energy stored in the phase boundaries of nano-CuO. The reaction activation energy of highly dispersed CuO is even lower than that of nano-CuO, since the highly dispersed CuO has higher surface energy than nano-CuO. The existing state of CuO exerts an influence on its reaction activity.
4 Conclusions
1) Under the studied conditions, the sulfation reaction rate increases with increasing temperature and SO2 concentration, and the surface area and pore volume decrease after sulfation.
2) The sulfation reaction between CuO/γ-Al2O3 and SO2 obeys pore-blocking model well. Proportionality (pore-blocking constant) 1/λ decreases with increasing temperature. Activation energy and reaction order with respect to SO2 obtained are 37.9 kJ/mol and the first order, respectively.
3) The activation energy of nano-CuO supported on γ-Al2O3 decreases much compared with that of pure CuO, and a little higher than that of highly dispersed CuO supported on γ-Al2O3. The existing state of CuO exerts an influence on its reaction activity.
References
[1] GULDUR C, DOGU G, DOGU T. Kinetics of trona sulfur dioxide reaction [J]. Chemical Engineering and Processing, 2001, 40: 13-18.
[2] WEI Zai-shan, NIUHE Jing-ying, JI Yong-feng. Simultaneous removal of SO2 and NOx by microwave with potassium permanganate over zeolite [J]. Fuel Processing Technology, 2009, 90: 324-329.
[3] LIN Cui, LI Xiao-gang. Initial corrosion of AZ91D magnesium alloy in atmosphere containing SO2 [J]. Trans Nonferrous Met Soc China, 2004, 14: 1658-1665.
[4] SUYADAL Y, OGUZ H. Dry desulfurization of simulated flue gas in a fluidized-bed reactor for a broad range of SO2 concentration and temperature: A comparison of models [J]. Industrial and Engineering Chemistry Research, 1999, 38: 2932-2939.
[5] KARVANA O, ATAKULB H. Investigation of CuO/mesoporous SBA-15 sorbents for hot gas desulfurization [J]. Fuel Processing Technology, 2008, 89: 908-915.
[6] AKYURTLU J F, AKYURTLU A. Behavior of ceria-copper oxide sorbents under sulfation conditions [J]. Chemistry Engineering Science, 1999, 54: 2991-2997.
[7] STROHMEIER B R, LEYDEN D E, FIELD R S, HERCULES D. Surface spectroscopic characterization of Cu/Al2O3 catalysts [J]. Journal of Catalysis, 1985, 94: 514-530.
[8] LIN Y S, DEN S G. Removal of trace sulfur dioxide from gas stream by regenerative sorption processes [J]. Separation and Purification Technology, 1998, 13: 65-77.
[9] CENTI G, RIVA A, PASSARINI N, BRAMBILLA G, HODNETT B K, DELMON B, RUWET M. Simultaneous removal of SO2/NOx from flue gases, sorbent/catalyst design and performances [J]. Chemistry Engineering Science, 1990, 45: 2679-2686.
[10] CENTI G, PERATHONER S. Role of the size and texture properties of copper-on-alumina pellets during the simultaneous removal of SO2 and NOx from flue gas [J]. Industrial and Engineering Chemistry Research, 1997, 36: 2945-2953.
[11] SZEKELY J, EVANS J W. A structural model for gas-solid reactions with a moving boundary (Ⅵ): The effect of grain size distribution on the conversion of porous solids [J]. Chemical Engineering Science, 1975, 25: 1091-1096.
[12] HARTMAN M, GOUGHLIN R W. Reaction of sulfur dioxide with limestone and the grain model [J]. American Institute of Chemical Engineers Journal, 1976, 24: 490-498.
[13] XIE Guo-yong, LIU Zhen-yu, ZHU Zhen-ping, LIU Qing-ya, MA Jian-rong, Reductive regeneration of sulfated CuO/Al2O3 catalyst-sorbent in ammonia [J]. Applied Catalysis B: Environmental, 2003, 45: 213-221.
[14] YU Qing-chun, ZHANG Shi-chao, WANG Xin-dong. Thermo- gravimetric study of CuO/γ-Al2O3 sorbents for SO2 in simulated flue gas [J]. Industrial and Engineering Chemistry Research, 2007, 46: 1975-1980.
[15] EVANS U R. The corrosion and oxidation of metals: Scientific principles and practical applications [M]. London: Edward Arnold Ltd, 1960: 20-25.
[16] WANG Qi. Introduction of chemical kinetics [M]. Changchun: Jilin People Press, 1982: 100-102. (in Chinese)
[17] DEBERRY D W, SLADEK K J. Rates of reaction of SO2 with metal oxides [J]. Canadian Journal of Chemistry Engineering, 1971, 49: 781-785.
[18] YU Qing-chun, ZHANG Shi-chao, WANG Xin-dong, ZHANG Jie, LU Zhen-ming. Sulfation behavior of CuO/γ-Al2O3 sorbent for the removal of SO2 from flue gas [J]. Journal of University of Science and Technology Beijing, 2008, 15: 500-504.
(Edited by CHEN Wei-ping)
Foundation item: Project(u0837604) supported by United Foundation of Natural Science Foundation of China and Yunnan Province; Project(08Z0017) supported by the Provincial Education Department of Yunnan Province, China; Project(2008ZC011M) supported by the Applied Fundamental Research of Yunnan Province, China; Project(2008-06) supported by the Scientific Research Foundation of Kunming University of Science and Technology
Corresponding author: YU Qing-chun; Tel: +86-871-5114017; E-mail: yqcy@163.com


