文章编号:1004-0609(2008)02-0307-05
高温质子导体BaZr0.45Ce0.45Gd0.1O3-δ的制备及性能
吕敬德,王 岭,樊丽华,李跃华,戴 磊,郭红霞
(河北理工大学 化工与生物技术学院,唐山 063009)
摘 要:
利用高温固相反应法制备高温质子导体BaZr0.45Ce0.45Gd0.1O3-δ。采用X射线衍射仪、扫描电子显微镜和交流阻抗谱法对不同烧结温度试样的相组成、微观形貌和导电性能进行表征,并测试试样在沸水和CO2气氛中的化学稳定性。结果表明:1 600 ℃烧结的试样为纯相且致密;此温度下制备的试样具有最高的电导率,800 ℃时其电导率达到0.82×10-2 S/cm,电导活化能为0.74 eV;样品对CO2表现出良好的化学稳定性,而在沸水中其稳定性稍差。
关键词:
BaZr0.45Ce0.45Gd0.1O3-δ;高温质子导体;烧结;化学稳定性;电导率;
中图分类号:O 614.33 文献标识码:A
Preparation and characteristic of proton conductor BaZr0.45Ce0.45Gd0.1O3-δ
L? Jing-de, WANG Ling, FAN Li-hua, LI Yue-hua, DAI Lei, GUO Hong-xia
(College of Chemical Engineering and Biotechnology, Hebei Polytechnic University, Tangshan 063009, China)
Abstract: The high temperature proton conductor BaZr0.45Ce0.45Gd0.1O3-δ was synthesized by solid-state reaction. The phase composition, microstructures and electrical conducting property of samples sintered at different temperatures were investigated by XRD, SEM and AC impedance spectroscopy. The chemical stability of specimen in boiling water and CO2 atmosphere was tested. The results indicate that the sample sintered at 1 600 ℃ is single phase, with high density and electrical conductivity. Its conductivity at 800 ℃ reaches 0.82×10-2 S/cm. The conducting activity energy is 0.74 eV. BaZr0.45Ce0.45Gd0.1O3-δ shows better chemical stability against carbon dioxide than boiling water.
Key words: BaZr0.45Ce0.45Gd0.1O3-δ; high temperature proton conductor; sintering; chemical stability; conductivity
高温质子导体(HTPC)是指在高温含氢气氛中具有质子传导能力的一类物质。由于掺杂三价金属阳离子的钙钛矿型氧化物在300~1 000 ℃之间具有优良的质子传导能力[1],因此,该领域的研究主要集中在钙钛矿型氧化物上。其化学式为ABO3,属于立方晶系,较大的阳离子A和O-按立方紧密堆积构成骨架,而较小的B离子填隙在6个O-所组成的八面体间隙中。A位通常由碱土金属以及其它一些离子半径较大的离子占据,如Ca2+、Sr2+、Ba2+等。B位则常由元素周期表中第三、四、五周期的过渡金属离子占据,如Ce4+、Ti4+和Zr4+等。这类高温质子导体具有许多用途,如用于中高温燃料电池、气体传感器、水电解制氢、氢气的分离和提纯、有机物的催化加氢/脱氢、常压氨的合成及各种电化学反应器[2-6]。与稳定的氧化锆固体电解质一样,钙钛矿型质子导体也是由于晶体中产生氧缺陷而传导质子[7-8]。
尽管相同条件下,BaCeO3基电解质比其它钙钛矿型氧化物具有更高的电导率[9],但其碱性较强,暴露于CO2/H2O气氛中容易分解为BaCO3/Ba(OH)2和CeO2[10-12],因而其应用受到限制。为提高高温质子导体的化学稳定性,各国学者进行大量研究[13-16]。一般认为,BaZrO3具有较好的化学稳定性和机械强度,但电导率较低。另外,由于BaCeO3 和BaZrO3能形成固溶体,且Zr4+能任意比例取代Ce4+[4]。因此,本文作者采用高温固相反应制备新型高温质子导体BaZr0.45Ce0.45Gd0.1O3-δ(以下简写为BZCG10),探讨不同烧结温度对其物相结构、微观形貌、电导率的影响,并对其在CO2和沸水中的稳定性进行研究。
1 实验
按化学计量比1?0.45?0.45?0.05称取BaCO3 (99.0%)、ZrO2(99.0%)、CeO2(99.99%)和Gd2O3(99.0%)。将混合料、ZrO2球、无水乙醇按1?2?0.5的配比放入聚四氟乙烯球磨罐中,球磨10 h。磨好的浆料自然晾干后,放入电阻炉中,在1 250 ℃预烧10 h。然后在粉末中加入1%PVB,再次在无水乙醇介质中球磨10 h。30 MPa的压力下将干燥后的粉体压成d 10.27 mm×1.2 mm的圆片,将其分别于1 400、1 500、1 600和1 650 ℃空气气氛中烧结10 h,升、降温速度均控制在2 ℃/min。
用X’pert Pro型X射线衍射仪测定样品的XRD图谱,X射线源为Cu Kα(λ=0.154 056 nm),管电压45 kV,管电流为40 mA,扫描范围2θ=10?~90?。根据所测得的XRD谱,计算样品的晶胞参数和理论密度,采用阿基米德原理测定样品的体积密度。
运用KYKY2800型扫描电镜对试样的微观结构进行表征。试样测试前对其表面进行喷金处理,喷金设备为中国科学院仪器厂生产的SBC-2表面处理机。
CO2中的稳定性实验在管式炉中进行,将试样置于100%流动CO2气氛中,在900 ℃处理2 h,观察试样物相的变化。为了测试试样在水中的稳定性,将其放入沸腾的蒸馏水中,煮沸6 h,然后观察试样物相结构的变化。
将样品的两面磨平后,超声波清洗15 min,两面均匀涂上一薄层Pt浆,待自然干燥后950 ℃焙烧1 h。通过机械挤压将铂丝与铂电极紧密接触,并与IM6e型电化学工作站相连,组成的电池为:Air,Pt|质子导体|Pt,Air。
测试温度范围为25~900 ℃,测量间隔100 ℃。测量时微扰电压5 mV,频率10 mHz~1 MHz,测量装置示意图如图1所示。根据阻抗谱图计算得到质子导体的电导率和相应的电导活化能。
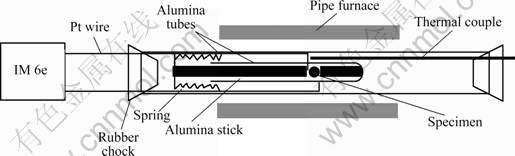
图1 电导率测量装置示意图
Fig.1 Schematic setup for conductivity measurement
2 结果与讨论
图2所示为不同烧结温度下试样的X射线衍射谱。可以看出,不同烧结温度烧结片的X射线衍射谱与检索到的JCPDS 82-2373的BaCe0.9-Gd0.1O3-δ标准卡片基本一致,说明已形成斜方晶钙钛矿结构。1 400 ℃烧结试样的峰型较宽且比较模糊,表明晶格不完整。随着烧结温度的升高,衍射峰型越来越尖锐,说明晶格更加饱满,晶粒进一步长大,当烧结温度达到1 600 ℃时,峰型非常清晰尖锐,形成完整的晶型。温度再进一步升高达到1 650 ℃时,发现有少量的新相单斜晶ZrO2生成。根据1 600 ℃烧结试样的X射线衍射谱,可以计算出其晶胞参数为:a=0.867 2 nm,b=0.611 7 nm,c=0.610 0 nm,V=0.323 6 nm3。这组数据与JCPDS 82-2373给出的BaCe0.9Gd0.1O3-δ晶格常数小,其原因是由于离子半径较小的Zr4+部分取代了离子半径大的Ce4+所致。
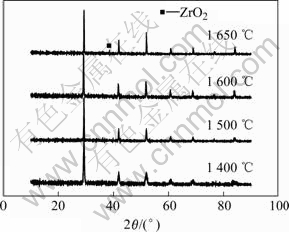
图2 不同温度烧结BZCG10试样的XRD谱
Fig.2 XRD patterns of BZCG10 sintered at different temperatures
烧结试样的理论密度(ρd)可用如下公式计算 [17]:

图3所示为不同烧结温度的试样表面和断面扫描电镜照片。由图3(a)~(d)可知,随着烧结温度的提高,晶粒不断增大(与XRD谱信息一致),平均粒径由1 400 ℃时的0.5~1.0 μm增长到1 650 ℃时的5~8 μm,单位面积内的晶界数量减少。试样表面晶粒大小不等,较大晶粒周围环绕着一些较小的晶粒,主要由于掺杂的离子填入晶系,增加体系的混乱度。烧结温度较低的样品(图3(a)和3(b)),试样表面孔隙较多,不够致密,且晶型较模糊,而温度达到1 600 ℃以上时,试样微观组织结构已相当致密,晶粒饱满,晶界清晰(图3(c)和3(d))。从断面的SEM图(图3(e)~(f)) 也进一步看出1 600 ℃烧结的试样非常致密。
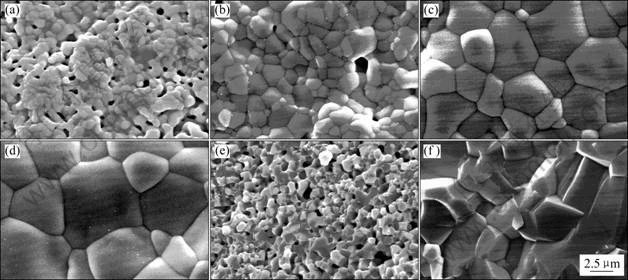
图3 不同烧结温度BZCG10试样的SEM照片
Fig.3 SEM images of BZCG10 sintered at different temperatures (Surface: (a)-(d); Cross-section: (e)-(f)): (a) 1 400 ℃; (b) 1 500 ℃; (c) 1 600 ℃; (d) 1 650 ℃; (e) 1 400 ℃; (f) 1 600 ℃
为了测试在1 600 ℃下制备的高温质子导体BZCG10在CO2气氛中的化学稳定性,将其置于100%流动CO2中900 ℃处理2 h。对经过CO2稳定性实验后试样观察发现,样品表面陶瓷光泽和颜色未发生任何变化。化学稳定实验前后试样的XRD谱(图4)也表明,经CO2处理后的试样组成基本不变。
为了测试高温质子导体BZCG10在水中的化学稳定性,将其放入蒸馏水中煮沸6 h。经处理后的试样表面明显变得粗糙,颜色变浅,并且水中有沉淀物出现,说明样品发生了部分分解,一般认为是样品在水中分解成Ba(OH)2造成的。样品在沸水中处理前后的XRD谱(图4)也可以看出,处理后试样的XRD谱基线明显变得粗糙不平,毛刺较多,表明有一些少量的新相生成,但晶相结构仍以斜方晶钙钛矿为主。
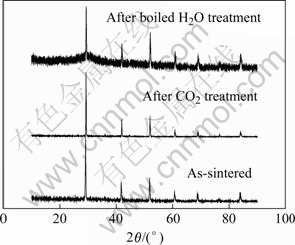
图4 1 600 ℃下制备的BZCG10化学稳定性实验前后的XRD谱
Fig.4 XRD patterns of BZCG10 sintered at 1 600 ℃ before and after chemical stability test
2.4.1 交流阻抗谱
固体电解质的交流阻抗谱从高频至低频一般由以下4部分组成[18]:1) 导线及接触电阻R0;2) 晶粒电阻Rg及其电容Cg;3) 晶界电阻Rgb及其电容Cgb;4) 电极反应电阻Rct及电极-电解质界面双电层电容Cdl,总电阻Rtot=R0+Rg+Rgb+Rct。在给定的测量温度范围内,各部分对总电阻的贡献有所不同。低温时主要表现晶粒和晶界阻抗半圆,当温度升高时,晶粒半圆逐渐减小直至消失,晶界半圆也逐渐减小,界面阻抗半圆出现。图5所示为1 600 ℃烧结试样在400 ℃的交流阻抗谱图。图中第一个小半圆为试样晶粒阻抗形成的半圆,与实轴的交点为晶粒电阻,第二个半圆对应于晶界阻抗。700 ℃时,晶粒半圆与晶界半圆已经融合在一起(图6)。图6所示为不同烧结温度试样700 ℃时的交流阻抗谱图。
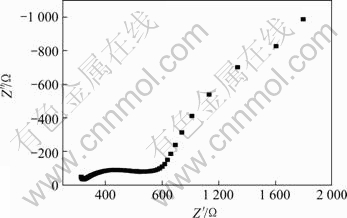
图5 1 600 ℃时制备的BZCG10试样在400 ℃下的交流阻抗谱
Fig.5 AC impedance spectroscopy at 400 ℃ for BZCG10 sintered at 1 600 ℃
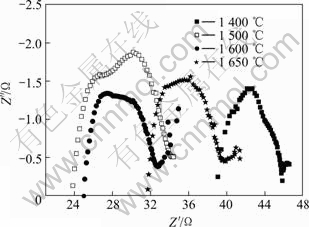
图6 不同温度下制备的BZCG10试样在700 ℃下的交流阻抗谱
Fig.6 AC impedance spectroscopy at 700 ℃ for BZCG10 sintered at different temperatures
2.4.2 电导率和活化能的测定
首先测量BZCG10圆片的厚度L,截面积S,由σ = L/RS可求出相应烧结试样的电导率。图7所示为试样在800 ℃时的电导率与烧结温度的关系。由图可以看出,1 600 ℃烧结的试样电阻最小,电导率最高,800 ℃时其电导率达到0.82×10-2 S/cm。低于1 600 ℃时,试样的孔隙率较大,晶体结构不够致密,因此电阻较大;高于1 600 ℃时,试样过烧产生新相ZrO2,因此阻抗也较大。
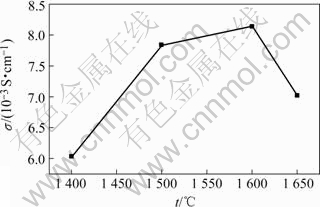
图7 800 ℃时BZCG10试样的电导率与烧结温度的关系
Fig.7 Relationship between BZCG10 electrical conductivity at 800 ℃ and sintering temperature
假设温度与电导率符合Arrhenius 公式:

图8所示为不同温度烧结试样的Arrhenius曲线,其中 1 600 ℃烧结试样空气气氛下的电导活化能为 0.74 eV。
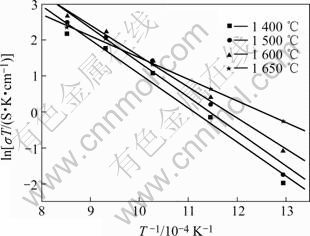
图8 不同温度烧结的BZCG10试样的Arrhenius曲线
Fig.8 Arrhenius plots of BZCG10 sintered at different temperatures
REFERENCES
[1] IWAHARA H, UCHIDA H, ONO K, OGAKI K. Proton conduction in sintered oxides based on BaCeO3[J]. Journal of the Electrochemical Society, 1988, 135(2): 529-533.
[2] PENG R R, WU Y, YANG L Z, MAO Z Q. Electrochemical properties of intermediate temperature SOFCs based on proton conducting Sm-doped BaCeO3 electrolyte thin film[J]. Solid State Ionics, 2006, 177(3/4): 389-393.
[3] SCHOBER T. Applications of oxidic high-temperature proton conductors[J]. Solid State Ionics, 2003, 162/163: 277-281.
[4] ZUO C D, LEE T H, DORRIS S E, BALACHANDRAN U, LIU M L. Composite Ni-Ba(Zr0.1Ce0.7Y0.2)O3 membrane for hydrogen separation[J]. Journal of Power Sources, 2006, 159(2): 1291-1295.
[5] LEE T H, DORRIS S E, BALACHANDRAN U. Thin film preparation and hydrogen pumping characteristics of BaCe0.8Y0.2O3-δ[J]. Solid State Ionics, 2005, 176(15/16): 1479-1484.
[6] KOKKOFITIS C, OUZOUNIDOU M, SKODRA A, STOUKIDES M. High temperature proton conductors: Applications in catalytic processes[J]. Solid State Ionics, 2007, 178(7/10): 507-513.
[7] MATSUMOTO H, SHIMURA T, HIGUCHI T, TANAKA H, KATAHIRA K, OTAKE T, KUDO T, YASHIRO K, KAIMAI A, KAWADA T, MIZUSAKI J. Protonic-electronic mixed conduction and hydrogen permeation in BaCe0.9-xY0.1RuxO3-α [J]. Journal of the Electrochemical Society, 2005, 152(3): A488-A492.
[8] HILLS M P, SCHWANDT C, KUMAR R V. Oxide Ion conduction in indium-oxide-substituted calcium zirconate[J]. Journal of the Electrochemical Society, 2006, 153(10): H189-H194.
[9] HIGUCHI H, TSUKAMOTO T, MATSUMOTO H, SHIMURA T, YASHIRO K, KAWADA T, MIZUSAKI J, SHIN S, HATTORI T. Electronic structure of proton conducting BaCe0.90Y0.10O3-δ[J]. Solid State Ionics, 2005, 176(39/40): 2967-2970.
[10] Snijkers F M M, Buekenhoudt A, Cooymans J, Luyten J J. Proton conductivity and phase composition in BaZr0.9Y0.1O3-δ[J]. Scripta Materialia, 2004, 50(5): 655-659.
[11] SHIMADA T, WEN C, TANIGUCHI N, OTOMO J, TAKAHASHI H. The high temperature proton conductor BaZr0.4Ce0.4In0.2O3-α [J]. Journal of Power Sources, 2004, 131(1/2): 289-292.
[12] 庞兆宝, 孟 波, 谭小耀. 高温质子导体Ba(Ce0.8Zr0.2)0.9- Y0.1O3-δ的合成与性能[J]. 中国有色金属学报, 2006, 16(5): 858-861.
PANG Zhao-bao, MENG Bo, TAN Xiao-yao. Synthesis and characteristics of Ba(Ce0.8Zr0.2)0.9Y0.1O3-δ high temperature proton conductor [J]. The Chinese Journal of Nonferrous Metals, 2006, 16(5): 858-861.
[13] SCHOLTEN M J, SCHOONMAN J, MILTENBURG J C V, OONK H A J. Synthesis of strontium and barium cerate and their reaction with carbon dioxide[J]. Solid State Ionics, 1993, 61(1/3): 83-91.
[14] RYU K H, HAILE S M. Chemical stability and proton conductivity of doped BaCeO3-BaZrO3 solid solutions [J]. Solid State Ionics, 1999, 125(1/4): 355-367.
[15] KATAHIRA K, KOHCHI Y, SHIMURA T, IWAHARA H. Protonic conduction in Zr-substituted BaCeO3[J]. Solid State Ionics, 2000, 138(1/2): 91-98.
[16] ZHONG Z M. Stability and conductivity study of the BaCe0.9-xZrxY0.1O2.95 systems[J]. Solid State Ionics, 2007, 178(3/4): 213-220.
[17] SAMMES N, PHILLIPS R, SMIRNOVA A. Proton conductivity in stoichiometric and sub-stoichiometric yittrium doped SrCeO3 ceramic electrolytes[J]. Journal of Power Sources, 2004, 134(2): 153-159.
[18] JIANG Kai, HE Zhi-qi, MENG Jian, REN Yu-fang, SU Qiang. Low temperature preparation and fuel cell properties of rare earth doped barium cerate solid electrolytes[J]. Science in China B, 1999, 42(3): 298-304.
基金项目:国家自然科学基金资助项目(50772030, 50572024);教育部归国人员启动基金资助项目;唐山市科技局资助项目(04364001B-10)
收稿日期:2007-04-16;修订日期:2007-10-22
通讯作者:王 岭,教授,博士;电话:0315-2597148;E-mail: tswling@126.com; lvjingde82@tom.com
(编辑 龙怀中)


