Trans. Nonferrous Met. Soc. China 23(2013) 841-848
Production of nanocrystalline silver particles by hydrogen reduction of silver nitrate aerosol droplets
 EBIN, Elif YAZICI, Sebahattin
EBIN, Elif YAZICI, Sebahattin 
Department of Metallurgical & Materials Engineering, Istanbul Technical University, 34469, Istanbul, Turkey
Received 24 February 2012; accepted 25 January 2013
Abstract:
Nanocrystalline silver particles were produced by hydrogen reduction of silver nitrate aerosol droplets formed by high frequency ultrasonic generator. The dependences of the particle size, morphology and crystallite size on the precursor concentration and the reaction temperature were investigated. Ultrasonic spray pyrolysis process was combined with hydrogen reduction to research the effects on the silver particle production. Nanocrystalline silver particles including slight oxide structure were prepared at temperature as low as 200 °C from silver nitrate under hydrogen atmosphere. X-ray diffraction (XRD) studies showed that pure silver particles were obtained above 200 °C reaction temperature. The crystallite sizes of the samples ranged from 29 to 47 nm. The results indicate that the crystallite sizes hardly ever depended on the reaction temperature. Crystallites slightly enlarged by increasing precursor concentration. SEM observations showed that particles were obtained in spherical morphology with particle sizes between 210 and 525 nm. Reaction temperature and precursor concentration strongly influenced the particle size.
Key words:
silver particles; nanocrystalline materials; aerosol process; hydrogen reduction;
1 Introduction
Nanocrystalline silver particles have received an increasing amount of attention in the last decade due to their novel electrical, thermal, optical, catalytic and antibacterial properties [1-4]. Silver particles, which have fine size and uniform particle size distribution, not only are preferential in many fields of commercial application, but also have various potential usages in electronic, medical and chemistry industries. Silver particles can be incorporated in several kinds of materials and these commercial products are sterile and can be useful as an antibacterial to prevent or to minimize infection with pathogenic bacteria [3-7]. Also, silver particles are the major constituent of conductive inks, pastes and adhesives for various electronic components due to their high oxidation resistance, high electrical and thermal conductivity [5,8]. They are useful for chemical and biological sensing based on surface-enhanced Raman scattering (SERS), localized surface plasmaresonance (LSPR) and metal-enhanced fluorescence (MEF). A large surface to volume ratio of fine silver particles and combination of their surface and optoelectronic features make them perfect catalyst and photocatalyst for many organic reactions [4,9].
The properties of nanostructured metal particles are directly related with shape, size, composition, crystallinity and structure (dense, solid) of the powders. Thus, there are many attempts to produce silver particles in different morphologies such as nano or microspheres, cubes, tetrahedrons, rods, tubes, fibers, plates, disks, prisms and dendrites [10-13]. In the last decade, many methods have been developed to prepare silver nano/microparticles, such as irradiation method [3], sol-gel technique [7], chemical reducing processes [8,14], photoreduction in microemulsions [10], polyol process [12], supercritical carbon dioxide assisted polyol process [15], colloidal aggregation mechanism [16] and spray pyrolysis based methods [17]. Among them, ultrasonic spray pyrolysis (USP) method is a suitable process to fabricate spherical non-agglomerated particles in wide size range and different compositions. USP method is based on thermal decomposition or reduction of constant sized aerosol droplets of the precursor solution obtained by powerful ultrasound source [18-21].
ZHANG et al [22] produced nanophase silver particles by an evaporation/condensation aerosol process in a jet flow reactor. They studied a high temperature aerosol process consisting of two heating zones, where the droplets decomposed to silver at 1000 °C in the first stage of the furnace, followed by vaporization of silver particles in superheater section at 1350 °C. PINGALI et al [23] synthesized silver nanoparticles by flash pyrolysis of a liquid feed solution of silver nitrate nebulized by an ultrasonic atomizer, and found out that solution concentration has important effect on the result particle size. SHI et al [24] fabricated nano silver powder in two steps; primarily, Ag/MgO composite powder was produced by spray pyrolysis at 790 °C, and then MgO, which was used as a template, was dissolved to obtain silver powders. LEE et al [25] synthesized silver nanoparticles in polymeric matrix by spray pyrolysis using aqueous solution of silver nitrate and polyvinlypyrollidone (PVP). Also, they showed the aggregation of primary silver nanoparticles and particle growth by sintering due to elevating reaction temperature from 100 °C to 1000 °C. YANG and KIM [17] produced silver particles by spray pyrolysis method under nitrogen and air atmospheres, and reported that silver particle size was almost independent of temperature in the range of 600-1000 °C and residence time in the range of 12.7-25.4 s. PARK et al [26] showed that silver particles prepared by spray pyrolysis method under nitrogen and air atmospheres exhibited spherical morphology with smooth surface. These particles were in the mixed phases of silver nitrate, silver oxide and silver until 400 °C and became phase-pure silver at a temperature between 400 °C and 500 °C. These results were different from those of PLUYM et al [27] who reported that the minimum temperatures for complete reduction were 600 and 900 °C with nitrogen and air, respectively. Although, various studies were carried out about production of silver particle by spray pyrolysis, there is still lack of systematic studies on spray pyrolysis method for Ag particles mass production, such as the role of the hydrogen gas in the process.
In this work, silver particles were produced by hydrogen reduction of ultrasonically atomized aerosol droplets of silver nitrate aqueous solution which was prepared without any additive. The production possibility of silver particles at lower temperature than that given in literature was studied. The effects of the reaction set temperature and the concentration of the corresponding solution on the particle size, morphology, chemical composition, crystal structure and crystallite size were investigated.
2 Experimental
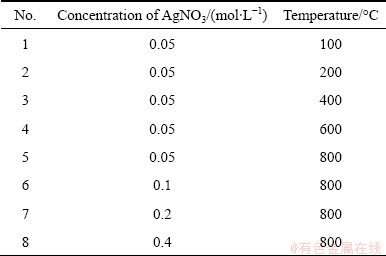
The precursor solution was prepared by dissolving the appropriate amount of AgNO3 in distilled water. The concentrations of the corresponding solutions were between 0.05 and 0.4 mol/L. Table 1 lists the chemical composition of the applied solutions and conditions of the production process. A schematic diagram of the USP setup was provided in our previous paper [19]. Nitrogen with a flow rate of 1.0 L/min was used during heating of the furnace to remove oxygen in the experimental set-up, and create an inert atmosphere before reduction process to avoid H2(g) and O2(g) (from air) reaction. The precursor solution, maintained at 30 °C, was atomized using ultrasonic nebulizer at a frequency of 1.3 MHz. The aerosol droplets were carried to the heated furnace by H2 gas. The flow rate of H2 gas was fixed to 1.0 L/min and the furnace temperature was changed from 100 °C to 800 °C, which are below the melting temperature of the silver (tm,Ag=962 °C). Continuous reduction process of the aerosol droplets under hydrogen atmosphere took place in the heated zone (250 mm). The residence time of the droplets was calculated as 4.7 s by assuming that the rates of droplets and the carrier gas are equal.
Table 1 Composition of precursor solutions, and conditions of process (tprecursor=30 °C, volume of used solution 250 mL)
The thermodynamic analysis of the hydrogen reduction of the silver nitrate was investigated by HSC Software. The crystal structures of the samples were identified by X-ray diffraction (XRD, Philips 1700 diffractometer) using Cu Ka radiation (l=1.54187 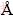 , 2θ range 30°-80°). The crystallite sizes of the particles were determined by Scherrer equation using XRD patterns. Energy dispersive spectroscopy (EDS) analyses were performed to investigate the chemical compositions of the particles. The particle size and morphology were examined by field emission scanning electron microscopy (FE-SEM, Jeol JSM 700F) and transmission electron microscopy (TEM, JEOL 2000 EX). The geometric mean diameter and standard deviation were determined from SEM images using Leica image manager. All the clearly observed particles on the SEM images were taken into account in these analyses.
, 2θ range 30°-80°). The crystallite sizes of the particles were determined by Scherrer equation using XRD patterns. Energy dispersive spectroscopy (EDS) analyses were performed to investigate the chemical compositions of the particles. The particle size and morphology were examined by field emission scanning electron microscopy (FE-SEM, Jeol JSM 700F) and transmission electron microscopy (TEM, JEOL 2000 EX). The geometric mean diameter and standard deviation were determined from SEM images using Leica image manager. All the clearly observed particles on the SEM images were taken into account in these analyses.
3 Results and discussion
3.1 Thermodynamic analysis of hydrogen reduction
The thermodynamic analysis was done using HSC Software in temperature ranging between 0 °C and 900 °C (Fig. 1). The hydrogen reduction of silver nitrate for the formation of Ag metal can be described as
AgNO3+H2(g)→Ag+H2O+NO2(g) (1)
The values of Gibbs free energy (△GΘ) for reaction (1) in the temperature up to 900 °C confirm the probability for the formation of Ag by hydrogen reduction of silver nitrate. The Gibbs free energy is always negative between 0 °C and 900 °C and it decreases through the negative value at elevated temperature. Silver formation by hydrogen reduction of silver nitrate is thermodynamically possible at desired temperatures of 100-800 °C.
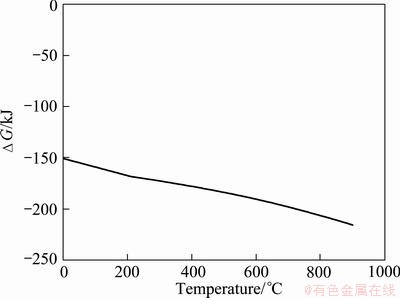
Fig. 1 Change of Gibbs free energy for hydrogen reduction of silver nitrate
3.2 Structural analysis of Ag particles
The XRD patterns of the particles obtained at various temperatures are given in Fig. 2. The reflections of Ag, AgO and AgNO3 were recorded on the XRD pattern of the sample obtained at 100 °C and AgNO3 is the dominant phase in the structure. This result indicated that silver nitrate did not sufficiently decompose to silver in the short residence time (4.7 s) at 100 °C. However, silver became main phase in the structure by increasing the reaction temperature to 200 °C, but silver oxide peak still slightly exists in the pattern. The particles, produced at temperature above 200 °C under reducing hydrogen atmosphere, have pure silver phase. Figure 3 shows the XRD patterns of the particles which were obtained by increasing silver nitrate concentration at 800 °C furnace temperature. The observed silver diffraction peaks in the samples were indexed with face centered cubic structure (Space Group: 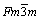 ), which is consistent with the reported data of silver (JCPDS Card No: 01-089-3722).
), which is consistent with the reported data of silver (JCPDS Card No: 01-089-3722).
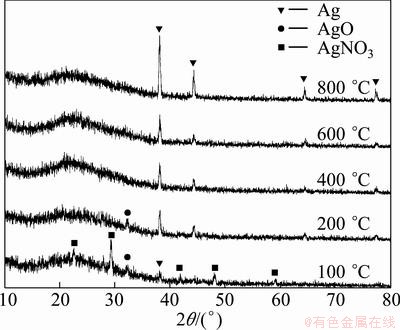
Fig. 2 XRD patterns of particles prepared at different furnace temperatures
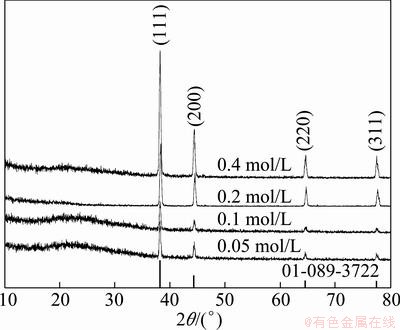
Fig. 3 XRD patterns of particles prepared from various solution concentrations
The crystallite sizes of the silver particles were calculated by Scherrer formula [19-21] using XRD patterns. The (111) peaks in Fig. 2 and Fig. 3 were used for the crystallite size determination and instrumental broadening was taken into account for all calculations to determine accurate crystallite sizes. Figure 4 exhibits the variation in the crystallite size of the silver particles, obtained using 0.05 mol/L AgNO3 solution at different reaction temperatures. The crystallite sizes of the particles were between 29 and 39 nm, and changed slightly by elevating temperature. According to Fig. 4, the crystallite sizes of the particles produced by hydrogen reduction of aerosol droplets were not distinguishably affected by reaction temperature. The results corresponded to those of PARK et al [26] who reported that the crystallite sizes of the silver particles prepared using air and nitrogen flows were around 50 nm.
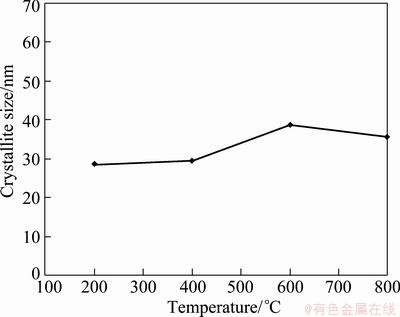
Fig. 4 Variation of crystalline size of silver particles prepared from 0.05 mol/L AgNO3 solution by elevating reaction temperature
Figure 5 shows the effect of the precursor concentration on the crystallite size of the silver particles produced at 800 °C under hydrogen atmosphere. The crystallite sizes of the silver particles were between 36 nm and 47 nm, and slightly enlarged by increasing silver nitrate concentration.
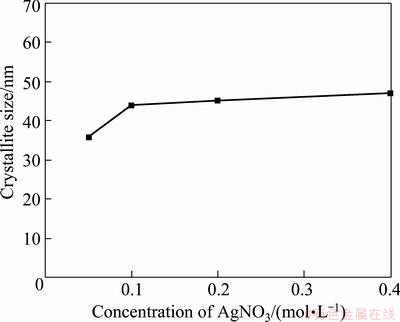
Fig. 5 Variation of crystalline size of silver particles prepared from different solution concentrations at 800 °C reaction temperature
The compositions of the products prepared by hydrogen reduction of the silver nitrate precursor were further studied by EDS. Figure 6 displays the EDS analyses of the particles produced under the endpoint conditions. Only Ag was detected for particles obtained at 800 °C, and any impurity due to the non-reacted precursor was not observed. However, a small oxygen peak was observed at 0.5 keV for particles prepared at 200 °C in Fig. 6(c). This EDS results confirmed the silver oxide peak shown in the XRD pattern.
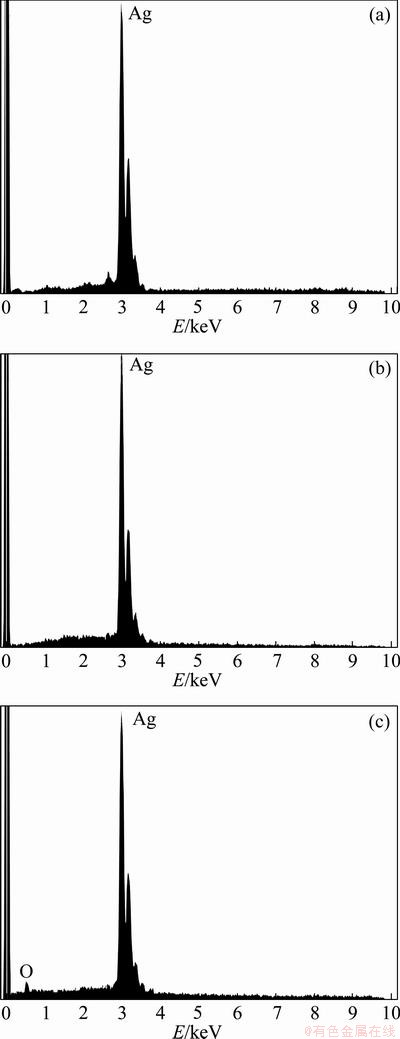
Fig. 6 EDS analyses of nanocrystalline silver particles produced from 0.4 mol/L AgNO3 precursor at 800 °C (a), 0.05 mol/L AgNO3 precursor at 800 °C (b), and 0.05 mol/L AgNO3 precursor at 200 °C (c)
3.3 Morphological characterization
SEM micrographs of the nanocrsytalline silver particles produced from 0.05 mol/L AgNO3 precursor concentrations at various temperatures between 200 and 800°C are given in Fig. 7. Silver particles have spherical morphology with nearly smooth surface. Also, initial crystallites as nanoparticles were partly observed on the submicron particle surfaces in high magnified SEM micrographs. This result indicates that initial crystallites (nanoparticles) aggregate and submicron (secondary) particles form as reported by LEE et al [25] and in our previous studies [19,21,28,29]. Nanosize particles appearing on secondary particles surfaces confirm the crystallite sizes calculated using XRD data. Geometric mean particle size and standard deviation values are shown in Fig. 8. As a result of elevating reaction temperature, the particle sizes decreased from about 490 to 214 nm, and at the same time, particles had a narrower size distribution as indicated by the bar shown in Fig. 8. The cause of the particle size reduction by elevating temperature was the effective sintering of the primary particles and densification. TEM micrograph of silver particles produced using 0.05 mol/L AgNO3 solution at 800 °C is given in Fig. 9. This micrograph shows that totally dense and spherical nanocrystalline silver particles were obtained at 800 °C.

Fig. 7 SEM micrographs of nanocrystalline silver particles produced using 0.05 mol/L AgNO3 solution at 200 °C (a, b), 400 °C (c, d), 600 °C (e, f), and 800 °C (g, h)
Figure 10 exhibits the SEM micrographs of the nanocrsytalline silver particles produced using 0.1-0.4 mol/L AgNO3 precursor at 800 °C. As shown in this figure, spherical silver particles had smooth surface.
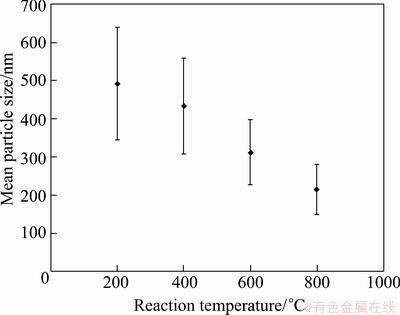
Fig. 8 Effect of reaction temperature on mean particle size
It was clear that increasing the precursor concentration did not have any effect on the Ag particle morphology. On the other hand, the dependence of the particle size to the solution concentration was investigated by measuring the particle sizes in SEM micrographs. Figure 11 shows the geometric mean particle size and standard deviation for the particles prepared using 0.05-0.4 mol/L AgNO3 precursor at 800 °C. The average particle sizes were 214, 280, 345 and 525 nm for 0.05, 0.1, 0.2 and 0.4 mol/L AgNO3 solution, respectively. Ag particle sizes became coarse by increasing solution concentration, which was typically observed for particles produced by aerosol based methods, such as nickel [18,30], iron [29], FeNi alloy [19] and FeCo alloy [20] particles. Also, wider particle size distributions were obtained by increasing solution concentration, as seen in Fig. 11. According to particle formation principle for USP process, submicron Ag particles are formed by aggregation of initial crystallites. Although the crystallite sizes of particles changed slightly by increasing solution concentration, the particle size increased regularly. The results show that coarse particles should contain more crystallites than the fine.

Fig. 9 TEM micrograph of silver particles obtained at 800 °C from 0.05 mol/L AgNO3 solution

Fig. 10 SEM micrographs of nanocrystalline silver particles produced at 800 °C using 0.1 mol/L (a, b), 0.2 mol/L (c, d), and 0.4 mol/L (e, f) AgNO3 solution
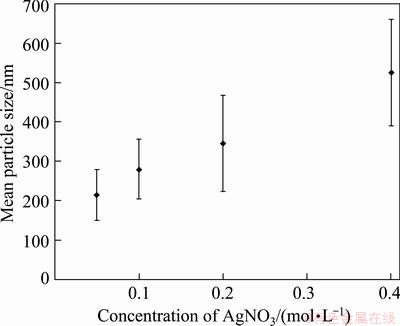
Fig. 11 Effect of solution concentration on mean particle size
4 Conclusions
Nanocrystalline silver particles in spherical morphology were produced by hydrogen reduction of silver nitrate aerosol droplets. Effects of reaction temperature in the range from 100 °C to 800 °C and solution concentration in the range from 0.05 to 0.4 mol/L on the silver particles were investigated. Although the formation of silver by hydrogen reduction of the silver nitrate was thermodynamically possible at 100 °C, the short residence time prevents the completion of the reduction process at the given temperature. Silver particles with trace of silver oxide were obtained at such a low temperature as 200 °C under the reducing hydrogen atmosphere. The crystallite sizes of the silver particles prepared using 0.05 mol/L precursor at the temperature between 200 and 800 °C were around 29 to 39 nm. Also, the crystallite size of the particles slightly became larger from 36 nm to 47 nm by increasing the silver nitrate concentration. The elevating reaction temperature from 200 °C to 800 °C caused the decrease of the particle size from about 490 nm to 210 nm for samples obtained by 0.05 mol/L solution due to the effective densification of the particles. Moreover, the precursor concentration has a direct effect on the silver particle sizes which grew from nearly 210 nm to 525 nm by increasing concentration from 0.05 mol/L to 0.4 mol/L, respectively. Finally, it could be possible for the production of silver nanoparticles by reducing the precursor concentration.
Acknowledgement
This research was supported by The Scientific and Technological Research Council of Turkey with Grant No: 107M505. Authors would like to thank to Prof. Dr. Gultekin GOLLER, Prof. Dr. Mustafa URGEN and Technician Huseyin SEZER for XRD and SEM studies.
References
[1] SUBER L, SONDI I, MATIJEVIC E,GOIA D V. Preparation and the mechanisms of formation of silver particles of different morphologies in homogeneous solutions [J]. Journal of Colloid and Interface Science, 2005, 288: 489-495.
[2] SHAHVERDI A R, FAKHIMI A, SHAHVERDI H R, MINAIAN S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli [J]. Nanomedicine: Nanotechnology, Biology, and Medicine, 2007, 3: 168-171.
[3] SHARMA VIRENDER K, YNGARD R A, LIN Y. Silver nanoparticles: Green synthesis and their antimicrobial activities [J]. Advances in Colloid and Interface Science, 2009, 145: 83-96.
[4] LU W W, LIU G, GAO S, XING S, WANG J. Tyrosine-assisted preparation of Ag/ZnO nanocomposites with enhanced photocatalytic performance and synergistic antibacterial activities [J]. Nanotechnology, 2008, 19: 445711-445721.
[5] CHOU K S, REN C Y. Synthesis of nanosized silver particles by chemical reduction method [J]. Materials Chemistry and Physics, 2000, 64: 241-246.
[6] DURAN N, MARCATO P D, DE SOUZA G I H, ALVES O L, ESPOSITO E. Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment [J]. Journal of Biomedical Nanotechnology, 2007, 3: 203-208.
[7] KAWASHITA M, TSUNEYAMA S, MIYAJI F, KOKUBA T, KOZUKA H, YAMAMOTO K. Antibacterial silver-containing silica glass prepared by sol-gel method [J]. Biomaterials, 2000, 21: 393-398.
[8] JANARDHANAN R, KARUPPAIAH M, HEBALKAR N, RAO T N. Synthesis and surface chemistry of nano silver particles [J]. Polyhedron, 2009, 28: 2522-2530.
[9] WIDONIAK J, EIDEN-ASSMAN S, MARET G. Silver particles tailoring of shapes and sizes [J]. Colloids and Surfaces A, 2005, 270–271: 340-344.
[10] HARADA M, KIMURA Y, SAIJO K, OGAWA T, ISODA S. Photochemical synthesis of silver particles in Tween 20/water/ionic liquid microemulsions [J]. Journal of Colloid and Interface Science, 2009, 339: 373-381.
[11] SUN Y G, XIA Y. Shape-controlled synthesis of gold and silver nanoparticles [J]. Science, 2002, 298: 2176-2179.
[12] WILEY B, HERRICKS T, SUN Y, XIA Y. Polyol synthesis of silver nanaoparticles: Use of chloride and oxygen to promote the formation of single-crystal, truncated cubes and tetrahedrons [J]. Nano Letters, 2004, 4(9): 1733-1739.
[13] SONG W, JIA H, CONG Q, ZHAO B. Silver microflowers and large spherical particles: Controlled preparation and their wetting properties [J]. Journal of Colloid and Interface Science, 2007, 311: 456-460.
[14] MARTINEZ-CASTANON G A, NINO-MARTINEZ N, LOYOLA- RODRIGUEZ J P, PATINO-MARIN N, MARTINEZ-MENDOZA J R, RUIZ F. Synthesis of silver particles with different sizes and morphologies [J]. Materials Letters, 2009, 63: 1266-1268.
[15] CHIH Y W, CHENG W T. Supercritical carbon dioxide-assisted synthesis of silver nano-particles in polyol process [J]. Materials Science and Engineering B, 2007, 145: 67-75.
[16] YAKUTIK I M, SHEVCHENKO G P, RAKHMANOV S K. The formation of monodisperse spherical silver particles [J]. Colloids and Surfaces A, 2004, 242: 175-179.
[17] YANG S Y, KIM S G. Characterization of silver and silver/nickel composite particles prepared by spray pyrolysis [J]. Powder Technology, 2004, 146: 185-192.
[18] EBIN B,  S. Sythesis and characterization of nickel particles by hydrogen reduction assisted ultrasonic spray pyrolysis (USP-HR) method [J]. KONA Powder and Particle Journal, 2011, 29: 134-140.
S. Sythesis and characterization of nickel particles by hydrogen reduction assisted ultrasonic spray pyrolysis (USP-HR) method [J]. KONA Powder and Particle Journal, 2011, 29: 134-140.
[19]  S, EBIN B, STOPIC S, FRIEDRICH B. Nanocrystalline spherical iron–nickel (Fe–Ni) alloy particles prepared by ultrasonic spray pyrolysis and hydrogen reduction (USP-HR) [J]. Journal of Alloys and Compounds, 2009, 480: 529-533.
S, EBIN B, STOPIC S, FRIEDRICH B. Nanocrystalline spherical iron–nickel (Fe–Ni) alloy particles prepared by ultrasonic spray pyrolysis and hydrogen reduction (USP-HR) [J]. Journal of Alloys and Compounds, 2009, 480: 529-533.
[20]  S, GUVEN A, EBIN B, STOPIC S, FRIEDRICH B. Synthesis of nano-crystalline spherical cobalt–iron (Co–Fe) alloy particles by ultrasonic spray pyrolysis and hydrogen reduction [J]. Journal of Alloys and Compounds, 2009, 481: 600-604.
S, GUVEN A, EBIN B, STOPIC S, FRIEDRICH B. Synthesis of nano-crystalline spherical cobalt–iron (Co–Fe) alloy particles by ultrasonic spray pyrolysis and hydrogen reduction [J]. Journal of Alloys and Compounds, 2009, 481: 600-604.
[21]  S, EBIN B. Production and characterization of the nanostructured hollow iron oxide spheres and nanoparticles by aerosol route [J]. Journal of Alloys and Compounds, 2010, 492: 585-589.
S, EBIN B. Production and characterization of the nanostructured hollow iron oxide spheres and nanoparticles by aerosol route [J]. Journal of Alloys and Compounds, 2010, 492: 585-589.
[22] ZHANG L, RANADE M B, GENTRY J W. Synthesis of nanophase silver particles using an aerosol reactor [J]. Journal of Aerosol Science, 2002, 33: 1559-1575.
[23] PINGALI K C, ROCKSTRAW D A, DENG S. Silver nanoparticles from ultrasonic spray pyrolysis of aqueous silver nitrate [J]. Aerosol Science and Techology, 2005, 39: 1010-1014.
[24] SHI X L, WANG S, DUAN X, ZHANG Q. Synthesis of nano Ag powder by template and spray pyrolysis technology [J]. Materials Chemistry and Physics, 2008, 112: 1110-1113.
[25] LEE K H, RAH S C, KIM S G. Formation of monodisperse silver nanoparticles in poly(vinylpyrrollidone) matrix using spray pyrolysis [J]. Journal of Sol-Gel Science and Technology, 2008, 48: 187-193.
[26] PARK E K, RAH S, KIM S G. Effect of gas environment on properties of particles prepared by spray pyrolysis of metal nitrates [J]. Korean Journal of Chemistry and Engineering, 2008, 25(4): 885-891.
[27] PLUYM T C, POWELL Q H, GURAV A S, WARD T L, KODAS T T, WANG L M, GLICKSMAN H D. Solid silver particles production by spray pyrolysis [J]. Journal of Aerosol Science, 1993, 24: 383-392.
[28] EBIN B, ARIG E, OZKAL B,  S. Production and characterization of ZnO nanoparticles and porous particles by ultrasonic spray pyrolysis using a zinc nitrate precursor [J]. International Journal of Minerals, Metallurgy and Materials, 2012, 19(7): 651-656.
S. Production and characterization of ZnO nanoparticles and porous particles by ultrasonic spray pyrolysis using a zinc nitrate precursor [J]. International Journal of Minerals, Metallurgy and Materials, 2012, 19(7): 651-656.
[29] EBIN B,  S. Aerosol synthesis of nano-crystalline iron particles from iron(II) chloride solution[J]. Metall, 2011, 65(4): 151-154.
S. Aerosol synthesis of nano-crystalline iron particles from iron(II) chloride solution[J]. Metall, 2011, 65(4): 151-154.
[30] JUNG K Y, LEE J H, KOO H Y, KANG Y C, PARK S B. Preparation of solid nickel nanoparticles by large-scale spray pyrolysis of Ni(NO3)2·6H2O precursor: Effect of temperature and nickel acetate on the particle morphology [J]. Materials Science and Engineering B, 2007, 137: 10-19.
氢还原硝酸银气溶胶制备纳米银粉
 EBIN, Elif YAZICI, Sebahattin
EBIN, Elif YAZICI, Sebahattin 
Department of Metallurgical & Materials Engineering, Istanbul Technical University, 34469, Istanbul, Turkey
摘 要:采用高频超声波发生器生成硝酸银气雾滴,然后用氢气还原硝酸银制备纳米银粉。研究前驱体浓度和反应温度对产物粒径、形貌和晶粒尺寸的影响。在200 °C的氢气气氛中,以硝酸银为原料制得的纳米银粉中含有氧化物。当反应温度超过200 °C时可以制得纯银粉;X射线衍射分析表明,所制得样品的晶粒尺寸为29~47 nm。研究表明反应温度对产物的粒径有明显影响。随着前驱体浓度的增加,所得纳米粉末的晶粒尺寸增加。SEM观察表明,产物银粉呈球形,粒径为210~525 nm。反应温度和前驱体浓度对产物粒径有明显影响。
关键词:银粉;纳米晶材料;气溶胶工艺;氢还原
(Edited by Hua YANG)
Corresponding author: Sebahattin  ; Tel: +90-212-285-68-62; Fax: +90-212-285-34-27; E-mail: gurmen@itu.edu.tr
; Tel: +90-212-285-68-62; Fax: +90-212-285-34-27; E-mail: gurmen@itu.edu.tr
DOI: 10.1016/S1003-6326(13)62537-6
Abstract: Nanocrystalline silver particles were produced by hydrogen reduction of silver nitrate aerosol droplets formed by high frequency ultrasonic generator. The dependences of the particle size, morphology and crystallite size on the precursor concentration and the reaction temperature were investigated. Ultrasonic spray pyrolysis process was combined with hydrogen reduction to research the effects on the silver particle production. Nanocrystalline silver particles including slight oxide structure were prepared at temperature as low as 200 °C from silver nitrate under hydrogen atmosphere. X-ray diffraction (XRD) studies showed that pure silver particles were obtained above 200 °C reaction temperature. The crystallite sizes of the samples ranged from 29 to 47 nm. The results indicate that the crystallite sizes hardly ever depended on the reaction temperature. Crystallites slightly enlarged by increasing precursor concentration. SEM observations showed that particles were obtained in spherical morphology with particle sizes between 210 and 525 nm. Reaction temperature and precursor concentration strongly influenced the particle size.


