
Simulated small-scale pilot heap leaching of low-grade copper sulfide ore with selective extraction of copper
QIN Wen-qing(覃文庆), ZHANG Yan-sheng(张雁生), LI Wei-zhong(黎维中), WANG Jun(王 军)
School of Resource Processing and Bioengineering, Central South University, Changsha 410083, China
Received 20 September 2008; accepted 5 November 2008
Abstract:
The bioleaching of low-grade copper sulfide ore and the selective extraction of copper were investigated. Lix984 dissolved in kerosene was used as extractant. The results show that it is possible to selectively leach copper from the ores by heap leaching. The copper concentration of leaching liquor after 250 d is 2.17 g/L, and the copper concentration is 0.27 g/L after solvent extraction. The leach liquor was subjected to solvent extraction, scrubbing and selective stripping for the enrichment of copper and the removal of impurities. The pregnant copper sulfate solution produced from the stripping cycle is suitable for copper electro-winning.
Key words:
bioleaching; chalcopyrite; extraction; Lix98; copper;
1 Introduction
Bioleaching has been widely used in the commercial extraction of copper, uranium and gold from ores, and it is being exploited in the extraction of other base metals and rare noble metals such as zinc, cobalt, nickel, molybdenum, gallium, and germanium[1-4]. The metal sulfides are oxidized by some special bacteria, such as Thiobacillus ferrooxidans, Thiobacillus thiooxidanin and Leptospirillum ferrooxidans, into soluble metal sulfates, elemental sulfur and sulfuric acid[5-8]. In order to enhance the leaching rate, many studies have been done in the aspects of microbiology, electrochemistry, metallurgy, etc. One of the solutions proposed is to cultivate effective microorganism used in bioleaching[9].
Chalcopyrite is the most abundant and refractory copper sulfide. And the bioleaching of chalcopyrite is the main industry target. The slow dissolution rate of chalcopyrite is the main factor hindering the commercial application of bioleaching because the polysulphides, elemental sulfur and iron-hydroxy precipitate layer form on the surface of chalcopyrite, which restrict the flow of bacteria, nutrients, oxidants, and reaction products to and from the chalcopyrite surface[10-13].
Solvent extraction is regarded as a highly effective technique of separation and purification, which has been used in the extraction of gold, copper, cobalt, nickel, etc [14-15]. Copper extraction has been successfully commercialized for decades due to the availability of copper selective extractants[16-17].
In the present work, the bioleaching process of low-grade copper sulfide ore and the solvent extraction of copper from bioleaching liquor using Lix984 in treated kerosene as diluent are investigated.
2 Experimental
2.1 Low grade copper sulfide ore
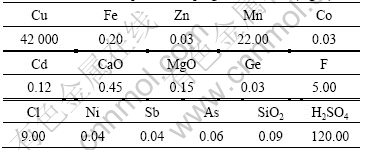
The low-grade copper sulfide ore was obtained from Jiangtian Mine in Yunnan Province, China, whose chemical composition is given in Table 1. Microscopic examinations in thin slides of the groundmass showed that the copper minerals are mainly chalcopyrite(CuFeS2) and bornite (CuS), and gangue minerals are quartz, gypsum, hematite, and sericite (fine grained mica) in both crystalline and microcrystalline forms. Table 2 shows the mineral composition.
Table 1 Chemical composition of copper sulfide ore (mass fraction, %)
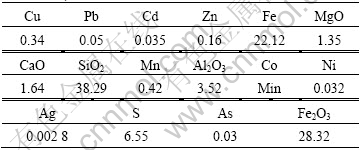
Table 2 Minerals composition of copper sulfide ore (mass fraction, %)

2.2 Elective culture of bacteria on pyrrhotite
The original microorganisms were collected from Jiangtian Mine. They were grown in the modified 9K nutrient medium, which was added with 6%(w/v)pyrrhotite sample to replace 10% iron (Fe2+) as the only energy source. The flasks were placed on an orbital shaker (170 r/min) and incubated at 30 ℃. The pH value was measured periodically, and when it dropped below 1.80, it was adjusted to 1.80 with 5 mol/L sulfuric acid. The number of bacterial cells in solution was determined by counting with a hemacytometer under a biomicroscope. Repeatedly, when the bacteria reached an exponential growth phase, one-fourth of the culture volume was transferred to the next incubation. The concentration of bacterial cells was diluted with iron-free 9K nutrient solution to the density of 107 cell/mL, which was used as the inoculum of the next experiments.
2.3 Column leaching
The experiments were carried out in a column reactor that was fabricated with 5 mm-thick 304L stainless steel. The height of column was 2.5 m with an internal diameter of 0.2 m, and it stood in a shallow tank with a capacity of 0.1 m3, which collected the PLS solution draining from the column. The solution was applied to the surface of column charge with a simple garden sprinkler head used in drip irrigation systems. A granite rock was sourced from a local supplier. Mineralogical analysis showed that the major minerals were quartz. The ore size was 99% passing 25-30 mm with only 0.6% passing 10 mm.
The original concentration of H2SO4 was 0.306 mol/L, and the initial density of bacterial cells in the liquor phase was about 107 cell/mL. The leach liquor was recycled without the addition of acid after solvent extraction of copper. A tank with a capacity of 0.1 m3 was used to collect the PLS solution draining from the column. Leach liquor was sampled from this tank to determine the solution concentrations, metal dissolution and acid balance by an atomic absorption spectrophotometer.
Ore was coated onto the support rock by tumbling weighed batches. The leach liquor passed through the ore sample by gravity and re-circulated through a side loop with a peristaltic pump. In the leaching experiments, the column system was comprised of 100 kg ore. For all of the tests the initial temperature and pH value of the feed were 25 ℃ and 1.8, respectively.
When the concentration of copper(Ⅱ) from the PLS was 2 g/L, it was recovered by solvent extraction with Lix984. The evaporation loss and sample removal were made up with water (pH=2.0). After leaching, liquor in the column was allowed to drain, and the column contents were then rinsed to remove residual copper and other soluble species. The column charge was rinsed first with dilute sulphuric acid solution (pH=1.2) followed by water rinse.
2.4 Extraction and stripping processes
Lix984 with purity of 95% was obtained from Henkel Corporation. Kerosene (260#) was obtained from Sinopec Shanghai Gpc Oil Refinery in China and distilled to collect the fraction by distilling over 260 ℃. It was mostly aliphatic in nature. Fig.1 shows the schematic diagram for the copper solvent extraction process.
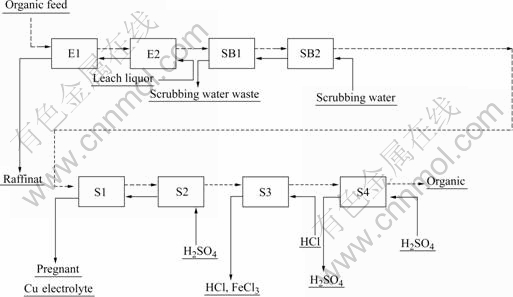
Fig.1 Schematic diagram for copper solvent extraction process
The mixer-settler cascade used in this work was composed of box-type mixer-settlers which were made of Teflon with similar internal arrangement and dimension (width 400 mm, depth 150 mm and height 200 mm). The active volume of one mixer-settler or stage was 2.0 L, while the ratio of the mixer to settler volumes was 1?4. The stirring speed was 1 200 r/min. Each mixer unit was provided with a pump-mixer impeller made of Teflon. The residence time of each phase in the mixer was 2 min, so the flow rate for the feed and solvent streams was 120 mL/min in the extraction section, and 30 mL/min for the stripping solution stream in the stripping section.
When the copper-loaded organic was stripped by sulfuric acid, copper transferred from organic phase to aqueous phase and little iron (Ⅲ) remained in the organic phase, which was directly stripped by concentrated hydrochloric acid in a stripper. After the iron (Ⅲ) was removed, the organic phase was again stripped by sulfuric acid. It was composed of box-type mixer-settlers that were connected with one section of each stage, one for hydrochloric acid stripping and others for sulfuric acid stripping.
3 Results and discussion
3.1 Column bioleaching of copper sulfide ore
In the experiments, the column was set up initially with pH 1.8 media. The initial density of bacterial cells in the liquor phase was about 107 cell/mL. No pH control was exercised during the experiment. The leach liquor was recycled without adding compensating acid after solvent extraction process. After about 250 d, the solution pH level in the experiment was typically about 2.2.
Copper extraction from the column was determined from solution assays, mass of concentrate filled and the initial head grade, as well as on the basis of assays of the residual solids. The extractions of copper, iron and silica as a function of time are shown in Figs.2(a) and (b), respectively. After the 250 d leaching the recovery of copper was above 55% (Fig.2(a)).
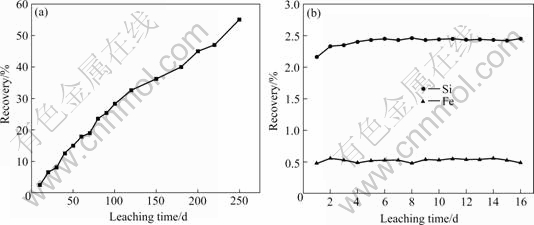
Fig.2 Column leaching of zinc oxide ores: (a) Recovery of copper; (b) Recovery of iron and silica
In the closed circuit leaching, the pH value of solution was changed from pH 1.8 to pH 2.2. The recovery rates of iron and silica were 0.6% and 2.4%, respectively.
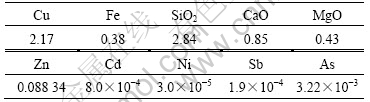
The components of leaching liquor after leaching for 200 d are listed in Table 3. The content of copper was 2.71 g/L, and the contents of iron, calcium and silica were lower than 0.40, 1.20 and 3.00 g/L, respectively. The other impurities such as zinc, cadmium, nickel, antimony and arsenic, which are deleterious to copper electrowinning, were at very low level. Hence, the leaching liquor obtained from column leaching was suitable to the next process.
Table 3 Chemical components of leach liquor (leaching time 200 d) (g?L-1)
The optimum recovery of copper was obtained after 200 d leaching. The reason is that, the particle size of ores is relatively larger and the acidity of lixiviant is moderate, which decreases the speed of leaching.
However, heap leaching of copper sulfide ores has some advantages, such as low acid consumption and low dissolved impurities. In the leaching circuit, the accumulation of iron, silica and other impurities was not observed. This is probably due to the absorption of the accumulated ores. In fact, the pH value of the solution soaked into the ore particles increases when the leaching reaction occurs, which results in iron and silica precipitating in the residues.
3.2 Extraction and stripping
The pH—extraction isotherms of copper, calcium, cadmium, cobalt and nickel were determined by shake-out test at different equilibrium pH values. The metal concentration in aqueous solution was 2 g/L. The extraction experiments were carried out with mechanically agitated beakers. The solution was agitated by a mechanical stirrer with a constant rate. After shaking beakers for 3 min, the organic phase was separated from the aqueous phase. The pH value of the aqueous solution was adjusted to the desired value by adding a small amount of HNO3 or NaOH. After phase disengagement, the aqueous phase was separated and its equilibrium pH was measured with a pH meter. The Lix984 content was 10%, the phase ratio (VO/VA) was 1.5?1, and the contact time was 3 min. The results are shown in Fig.3.
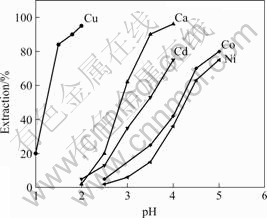
Fig.3 pH—extraction isotherms of Lix984 for six elements at 25 ℃
At the equilibrium pH values of about 2.0, the copper extraction was 95%, while both of the Fe and Ca extraction was less than 5%, and the cobalt and nickel extraction was negligible. Therefore, copper was easily separated from ferric, cadmium, cobalt and nickel at equilibrium pH value of 2.0.
The solvent extraction process is shown in Fig.1. When the concentration of copper(Ⅱ) from the PLS was higher than 2 g/L after 200 d leaching, copper was recovered by solvent extraction with Lix984. One extraction process was carried out, and about 30 L leaching liquor was used in the extraction process. The results of leaching-extraction circuit are shown in Table 4. The pH value of the extraction solution was less than 1.8. After the extraction of copper, the aqueous pH value increases to about 2.2.
Table 4 Results of leaching-extraction circuit
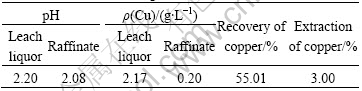
The copper concentration of the leaching liquor in the first leaching cycle was 2.17 g/L. Some leaching liquor was recovered by solvent extraction, and the raffinate was recycled to leaching process. After the copper extraction, about 0.20 g/L of copper was not extracted and remained in the raffinate when the VO/VA ratio was close to 1.5. So the copper concentration of leaching liquor was decreased to 0.27 g/L.
In solvent extraction, the co-extraction of impurities was inevitable using Lix984 as extractant. Therefore, to obtain the pregnant metal solution with low impurities, the loaded organic must be scrubbed to remove the co-extracted impurities.
The scrubbing solution was copper sulfate solution, and the pH value was 2.2. To keep water phase balance, the high phase ratio (VO/VA) was likely used. The co-extracted calcium and silica in loaded organic can be removed by scrubbing, and the phase ratio (VO/VA) of 10?1 was acceptable.
Hence, the aqueous phase in stripping must be of high acidity to keep the equilibrium moving rightwards. The copper-loaded organic phase contained little iron(Ⅲ), and minim of calcium and silica. The rate of stripping increased with the increase of acidity. It was found that the iron in the loaded-organic phase was hardly stripped by sulfuric acid in the high acidity of 1.53 mol/L. When the copper-loaded organic was stripped by sulfuric acid, copper transferred from organic phase to aqueous phase while iron(Ⅲ) remained in the organic phase.
Though the iron concentration in the leaching liquor was low, it accumulated in the recycling organic, which decreased the loading capacity of the extractant. Hence, the recycle organic must be treated periodically to remove the iron(Ⅲ). The conventional method is that Fe3+ is directly stripped by concentrated hydrochloric acid.
The test indicated that the performance of organic phase is stable in recycling process, and the emulsion or the third phase was not found in solvent extraction.
The chemical analysis of the pregnant copper sulfate produced from solvent extraction is shown in Table 5. Copper electrowinning is dependent on high hydrogen overpotential on the metal. A small amount of impurities can result in the decrease of hydrogen overpotential, which decreases the current efficiency and the quality of cathode copper. The impurities in the electrolyte were at levels as follows (mg/L): Zn<0.10, Ni<0.30, Co<0.30, Fe<10.00, Cd<0.30, Sb<0.03, As<1.00, Ge<0.03, and Sn<0.10. The electrolyte is suitable to the broad current density.
Table 5 Chemical components of pregnant solution (mg/L)
4 Conclusions
1) It is possible to selectively leach copper from the ores by heap leaching. The copper concentration of the leach liquor after 200 d is 2.17 g/L, and the recovery of copper is 55.01%.
2) The leaching liquor is subjected to solvent extraction, scrubbing and selective stripping for the enrichment of copper and the removal of impurities. The pregnant copper sulfate solution produced from the stripping cycle is suitable for copper electrowinning.
References
[1] MILLER P C, RHODES M K, WINBY R. Commercialization of bioleaching for base-metal extraction [J]. Minerals & Metallurgical Processing, 1999, 16(4): 42-50.
[2] EHRLICH H L. Past, present and future of bioleaching [J]. Hydrometallurgy, 2001, 59: 127-134.
[3] KEELING S E, PALMER M L, CARACATSANIS F C, JOHNSON J A, WATLING H R. Leaching of chalcopyrite and sphalerite using bacteria enriched from a spent chalcocite heap [J]. Minerals Engineering,2005, 18(13/14): 1289-1296.
[4] WATLING H R. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review [J]. Hydrometallurgy, 2006, 84: 81-108.
[5] BOON M, BRASSER H J, HANSFORD G S. Comparison of the oxidation kinetics of different pyrite in the presence of Thiobacillus ferrooxidants or Leptospirillum ferrooxidants [J]. Hydrometallurgy, 1999, 53: 57-72.
[6] SAND W, RHODE K, SOBOTKE B. Evaluation of Leptospirillum ferrooxidants for leaching [J]. Appl Environ Microbiol, 1992, 58(1): 85-92.
[7] BRIERLEY J A. A perspective on developments in bio- hydrometallurgy [J]. Hydrometallurgy, 2008, 94(1/4): 2-7.
[8] CANCHO L, BL?ZQUEZ M L, BALLESTER A, GONZ?LEZ F, ANDMU?OZ J A. Bioleaching of a chalcopyrite concentrate with moderate thermophilic microorganisms in a continuous reactor system [J]. Hydrometallurgy, 2007, 87(3/4): 100-111.
[9] GARCIA J O, BIGHAM J M, TUOVINEN O H. Sphalerite oxidation by Thiobacillus ferrooxidants and thiobacillus thiooxidants [J]. Can J Microbiol, 1995, 41: 578-584.
[10] JOHNSON D B, OKIBE NAOKO, WAKEMAN K, LIU Y J. Effect of temperature on the bioleaching of chalcopyrite concentrates containing different concentrations of silver [J]. Hydrometallurgy, 2008, 94(1/4): 42-47.
[11] CANCHO L, BL?ZQUEZ M L, BALLESTER A, GONZ?LEZ F, MU?OZ J A. Bioleaching of a chalcopyrite concentrate with moderate thermophilic microorganisms in a continuous reactor system [J]. Hydrometallurgy, 2007, 87(3/4): 100-111.
[12] CLARK M E, BATTY J D, BUUREN C B, DEW D W, EAMON M A. Biotechnology in minerals processing: Technological breakthroughs creating value [J]. Hydrometallurgy, 2006, 83(1/4): 3-9.
[13] PRADHAN N, NATHSARMA K C, RAO K S, SUKLA L B, MISHRA B K. Heap bioleaching of chalcopyrite: A review [J]. Minerals Engineering,2008, 21(5): 355-365.
[14] QIU Guan-zhou, WU Bo-zeng, QIN Wen-qing, LAN Zhuo-yue. Bioleaching of marmatite in high concentration of iron [J]. Trans Nonferrous Met Soc China, 2002, 12(6): 1435-1439.
[15] HSU C H, HARRISON R G. Bacterial leaching of zinc and copper from mining wastes source [J]. Hydrometallurgy, 1995, 37(2): 169-179.
[16] ARBITER N, FLETCHER A W. Copper hydrometallurgy evolution and milestones [J]. Mining Engineering, 1994, 46(2): 118-123.
[17] AMINIAN H, BAZIN C. Solvent extraction equilibria in copper(Ⅱ)-iron(Ⅲ)-LIX984 system [J]. Minerals Engineering,2000, 13(6): 667-672.
Foundation item: Project(2004CB619205) supported by the National Basic Research Program of China; Project(50621063) supported by the National Natural Science Foundation of China; Project(2007AA060902) supported by High-tech Research and Development Program of China
Corresponding author: QIN Wen-qing; Tel: +86-731-8830545; E-mail: qinwenqinq1@126.com
(Edited by YUAN Sai-qian)


