文章编号:1004-0609(2015)12-3370-11
纳米结构铁素体合金中氦捕获的第一性原理研究
江 勇1, 2, 3,杨力通1, 3,金亚楠4,周张健5,吕 铮6
(1. 中南大学 材料科学与工程学院,长沙 410083;
2. 中南大学 粉末冶金国家重点实验室,长沙 410083;
3. 中南大学 有色金属材料科学与工程教育部重点实验室,长沙 410083;
4. 清华大学 材料科学与工程学院,北京100084;
5. 北京科技大学 材料科学与工程学院,北京100083;
6. 东北大学 材料与冶金学院,沈阳110819)
摘 要:
基于第一性原理计算研究纳米结构铁素体合金(NFAs)对中子辐照引发的氦的选择性捕获能力。结果表明:与铁基体中的空位相比,氦更容易被NFAs中Y2Ti2O7的八面体间隙和Y2TiO5沿b轴方向的中空通道捕获;被氧化物捕获的氦相互间存在排斥作用,说明氦在NFAs中的分散度将在很大程度上取决于氧化物的析出分数和弥散度;通过计算界面相图推断,铁素体/Y2Ti2O7界面应为富Y/Ti型,与实验观测到的Y2Ti2O7析出相的非化学计量比特性吻合;氦对Fe/Y2Ti2O7界面有较强的偏聚倾向,且可能对氧化物颗粒尺寸敏感。较大尺度的Y2Ti2O7能够将氦捕获在界面和体内,而低纳米尺度的Y2Ti2O7更倾向于将氦捕获在界面。
关键词:
纳米结构铁素体合金;氦;Y2Ti2O7;Y2TiO5;第一性原理;
中图分类号:TG142 文献标志码:A
First principles study of helium trapping in nano-structured ferritic alloys
JIANG Yong1, 2, 3, YANG Li-tong1, 3, JIN Ya-nan4, ZHOU Zhang-jian5, LU Zheng6
(1. School of Materials Science and Engineering, Central South University, Changsha 410083, China;
2. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
3. Key Laboratory of Nonferrous Materials, Ministry of Education, Central South University, Changsha 410083, China;.
4. School of Materials Science and Engineering, Tsinghua University, Beijing 100084, China;
5. School of Materials Science and Engineering, University of Science and Technology, Beijing 100083, China;
6. School of Materials and Metallurgy, Northeastern University, Shenyang 110819, China)
Abstract: First principles was studied for assessing the relative stabilities of transmutant helium trapping in Y2Ti2O7, Y2TiO5 and the ferrite matrix in nano-structured ferritic alloys (NFAs). The results suggest that, helium in NFAs prefers to occupy individual octa-interstitial sites of Y2Ti2O7 and the open channels along the b axis of Y2TiO5. The helium self-interaction in Y2Ti2O7 is essentially repulsive, suggesting that the dispersion of helium in NFAs might, to a large extent, depend on the number and dispersion of Y2Ti2O7 in the iron matrix. The calculated interface phase diagram predicts the ferritic/Y2Ti2O7 interfaces in NFAs as Y/Ti-rich, in agreement with the experimentally observed non-stoichiometric nature of Y2Ti2O7 precipitates in NFAs. Segregation tendency of helium to the ferritic/Y2Ti2O7 interface can be sensitive to the size of Y2Ti2O7.
Key words: nano-structured ferritic alloy; helium; Y2Ti2O7; Y2TiO5; first principle
纳米结构铁素体合金(Nano-structured ferritic alloys,NFAs),也称纳米特征增强超级钢,是新一代核反应堆用理想堆体结构材料,也是近年来被美国能源部、欧洲原子能共同体、日本原子能机构、ITER国际聚变能组织等重点研究的新一类合金材料。该型合金通过特殊粉末冶金方式制备,在铁铬合金基体中原位生成大量、弥散、具有纳米尺度特征(1~10 nm)的微结构。这些纳米结构或以共格富(Y-Ti-O)原子簇团(类似GP区),或以近化学计量比的复杂氧化物(Y2TiO5或Yi2Ti2O7)形式存在,连同所获得的细晶组织、超高位错密度(约1×1024 m-3),可使该合金获得远超过常规铁基合金的高温力学性能(蠕变强度、断裂韧性)以及高抗辐照损和高吸收氦等特殊性能[1-3]。
NFA合金中纳米析出结构具有极高的热力学稳定性,经长时间时效(800 ℃,约4.4年)不发生明显粗化,晶粒和位错变化也很小[1, 4]。如何充分认识和理解这些纳米特征析出物(原子簇团或氧化物相)的化学组成、微观结构、析出形貌和变形协调性,在基体中的形核与长大以及相关界面的化学组成和微观结构、化学键合、及与材料宏观性能的相关性等,成为目前国内外核材料领域的研究热点。针对这些问题的研究,已采用了一切可能的现代实验表征技术[1-13],包括小角度中子散射(SANS)、高分辨透射电镜(HRTEM)、扫描隧道电镜(HAADF-STEM)、小角度X光散射(SAXS)、原子探针(APT)、X射线衍射(XRD)、X射线吸收谱(XRAS)、电子能量损失谱分析(EELS)等。然而,可能是由于合金制备工艺或各种表征技术原理之间的差异性,不同表征技术的分析研究结果往往相互矛盾,这对该型合金的研究造成了极大困扰。目前倾向于接受的一般性结论如下:1) 不同类型的纳米特征结构可能共存,其结构细节与其尺度强烈相关;2) 较大纳米特征物(8~15 nm)Ti与Y摩尔比一般在0.5~1之间,有可能为Y2Ti2O7、Y2TiO5或其混合物;3) 较小纳米特征物(<8 nm)Ti与Y摩尔比接近于1,可能以Y2Ti2O7为主[6, 14];4) 更小尺度(<1~2 nm)、特别是具有异常摩尔比(n(Ti)/n(Y)为1.5~4,n(O)/n[Ti+Y]<1)的纳米特征物,可能以原子团簇为主,但也可能存在有严重偏离化学计量比的Yi2Ti2O7。近年来,国内研究人员也迅速跟进,但研究工作主要集中在制备工艺和性能评估上。其中,中南大学、北京科技大学、上海交通大学等[15-23]先后通过机械合金化结合热致密化和形变热处理,获得了较高密度、特征尺寸为3~10 nm纳米析出结构。HRTEM表征发现,纳米相成分以 Y-Ti-O为主,具有明显长程有序结构,Y-Ti-O与基体存在共格取向。
同时,基于第一性原理的计算研究工作也在不断开展和深入,获得了对NFA合金中纳米析出结构的许多新认识。ALINGER等[24]首先基于第一性原理原子对势开展了动力学晶格蒙特卡洛的计算模拟,重现了共格Y-Ti-O原子簇团的形成。FU等[25]通过空位和原子簇团形核计算,提出了空位主导的形核机制。JIANG等[26-27]对Y-Ti-O簇团的形核驱动力首次给出了定量评估,并提出了可不依赖空位的形核机制, 进而考察了两种主要氧化物析出相(Y2Ti2O7和Y2TiO5)的晶体结构、化学成键和变形性质,并发现Y2Ti2O7与基体间的弹性变形协调性优于Y2TiO5的。两种氧化物的延展性均优于铁基体的,有利于保证合金的断裂韧性。BARNARD等[28-29]计算证实原子团簇在基体中更容易形成Y2Ti2O7的焦绿石(Pyrochlore)结构,沿位错的原子扩散是纳米氧化物相粗化的主要方式,并进一步预测1 175 K下这些氧化物在铁素体基体中可稳定存在80年以上。YANG等[30]针对不同极性Yi2Ti2O7表面的计算发现,Yi2Ti2O7具有偏离化学计量比的天性,这一倾向与铁素体基体无关。ERHART[31]计算比较了多种氧化物(Al2O3、TiO2、Y2O3、YAP、YAG、YAM、MgO、CaO、BaO、SrO)的氦捕获能力,发现氦原子都倾向于占据氧化物的填隙位,并降低其迁移能力。最近,CHOUDHURY等[32]介绍了含失配位错Fe/Y2O3界面的计算研究方法和实践,但未涉及界面相图及界面捕获氦等问题。
受以上实验和计算工作的启发,本文作者针对该合金中纳米氧化物的析出特点,提出基于第一性原理密度泛函理论,分别计算和考察铁基体、Y2Ti2O7和Y2TiO5中的各种氦捕获结构和能力,并预测Fe/Y2Ti2O7界面相图和界面结构,在此基础上进一步计算考察Fe/Y2Ti2O7界面的氦捕获。通过与氧化物体相计算结果的比较,初步揭示NFA合金中纳米氧化物及其界面与该合金的特殊氦吸收性能的相关性。
1 计算方法
第一性原理计算材料学方法的最大特点是从最基本的热力学原理出发,以计算量子力学为手段,对体系能量、晶体结构、电子结构等进行无参数的精确计算,结合合理的物理和热力学模型,可对材料的宏观物理性能、化学性能、和力学性能等进行直接的理论预测[33-37]。其计算过程由于不需要引入任何经验型参数或实验数据,计算结果依靠能量准则或原子间力准则自我收敛,故可排除一切人为因素的影响,研究结果可以做到自我支持。在此,针对本研究目标,基于第一性原理计算方法,主要开展以下几项内容的研究工作:1) 计算铁基体、Y2Ti2O7、和Y2TiO5中的各种氦缺陷结构,从能量角度评价其相对稳定性;2) 根据高分辨电镜表征结果,构建和考察Fe/Y2Ti2O7界面的不同原子结构类型,并通过界面能计算,确定界面平衡相结构,并据此计算氦在Fe/Y2Ti2O7界面上的偏聚;3) 综合铁基体、氧化物、及其界面的计算结果,探讨Y2Ti2O7颗粒的尺寸效应,并对NFAs中氦泡形成、演变和最终结局提出自己的假设。
所有计算均采用基于第一性原理密度泛函方法程序包-VASP(Vienna Ab-initio Simulation Package)[38]。其中,离子实与价电子之间的相互作用势采用投影缀加平面波方法(PAW)[39]描述。交换关联势函数的确定则根据BCC-Fe、焦绿石型Y2Ti2O7和正交Y2TiO5晶体的能量-体积数据,对普适性状态函数[40]进行拟合,通过比较实验值及前人计算值[27, 41-48]作出最优选择。所测试的交换关联势函数包括局域密度近似(LDA)[49]以及广义梯度近似(GGA)下的PW-91[50]和PBE[51]泛函。计算显示,PAW-PW91对BCC-Fe和焦绿石型Y2Ti2O7的体结构与体性质可获得最好预测(BCC-Fe: 晶格常数a=2.8303 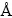 ,体模量B=174 GPa;焦绿石型Y2Ti2O7: a=10.1835
,体模量B=174 GPa;焦绿石型Y2Ti2O7: a=10.1835 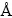 ,B=183 GPa;正交Y2TiO5: a=10.44
,B=183 GPa;正交Y2TiO5: a=10.44 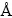 ,b=3.71
,b=3.71 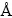 ,c=11.34
,c=11.34 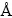 ,B=129 GPa),故选择PAW-PW91泛函进行后面所有相关计算。为平衡计算精度和计算效率,经优化计算确定,对Y2Ti2O7采用500 eV的平面波截断能,能量积分采用5×5×5的Monkhorst-Pack(M-P)网格;Y2TiO5计算中平面波截断能为520 eV,能量积分采用4×12×4的M-P网格; BCC-Fe计算则采用4×4×4超胞、350 eV的平面波截断能和7×7×7的M-P网格;界面计算则采用500 eV的平面波截断能和2×2×1的M-P网格。所有缺陷结构的原子弛豫计算,允许晶胞体积与形状同时变化,直至原子间力收敛至0.02 eV/
,B=129 GPa),故选择PAW-PW91泛函进行后面所有相关计算。为平衡计算精度和计算效率,经优化计算确定,对Y2Ti2O7采用500 eV的平面波截断能,能量积分采用5×5×5的Monkhorst-Pack(M-P)网格;Y2TiO5计算中平面波截断能为520 eV,能量积分采用4×12×4的M-P网格; BCC-Fe计算则采用4×4×4超胞、350 eV的平面波截断能和7×7×7的M-P网格;界面计算则采用500 eV的平面波截断能和2×2×1的M-P网格。所有缺陷结构的原子弛豫计算,允许晶胞体积与形状同时变化,直至原子间力收敛至0.02 eV/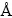 。
。
2 结果与讨论
2.1 氦在Y2Ti2O7、Y2TiO5和Fe体相中的相对稳定性
首先计算体心立方铁基体中He缺陷结构及其对应的缺陷形成能。所考察的缺陷结构可参考文献[52],具体包括单个铁空位(Vacancy)、四面体或八面体填隙的He (HeItetra或HeIocta)、占据铁空位的He (HeV,即取代位的He),以及共占一个铁空位的He团簇((2He)V或(3He)V)。缺陷形成能(Ef)按式(1)计算:
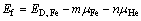 (1)
(1)
式中:ED, Fe为包含缺陷的Fe超胞的总能;μFe 和μHe分别为为Fe和He的纯态化学势;m和n分别为超胞中Fe和He原子的数量。计算结果如表1所列。
表1 计算所得铁基体中空位与各种He缺陷的形成能
Table 1 Calculated formation energies of various defects in iron
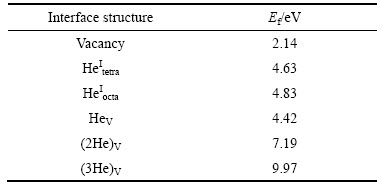
需要指出的是,表1中含有空位的He缺陷结构(HeV、(2He)V和(3He)V)的形成能,均隐含了空位形成能。在实际合金制备和服役过程中,由于热激活和辐照作用,铁基体中会产生大量的空位,即在氦捕获之前,合金中的大量空位可视为“预先存在”;材料经嬗变反应形成的各种氦缺陷结构,不应再需要额外的空位形成能。因此,若以空位作为参考能量,重新比较以上各种缺陷结构的形成能,则被“预先存在”的空位捕获而形成的HeV,实际所需能量大幅降低,只有2.28 eV,仅为填隙He缺陷(HeItetra和HeIocta)所需形成能的40%~50%。另外,由多个四面体或八面体位填隙He原子转变为相同数目的He原子同时占据一个空位而形成的各种团簇结构,如(2He)V、(3He)V等,也具有明显的能量优势。而这样的团簇结构,与相同数目的分散的单个HeV缺陷相比,所需形成能仍然更高。因此,铁基体中的氦会尽量首先分散在单个的空位中。只有辐照剂量较大,所产生的嬗变氦原子数目过多时,形成氦团簇结构才难以避免。
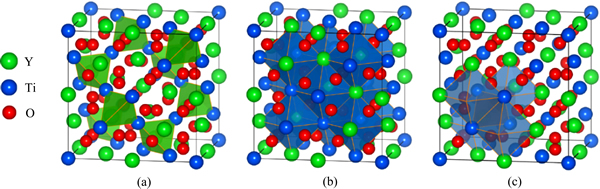
图1 Y2Ti2O7中的四面体和八面体填隙位置以及两者的几何相关性
Fig. 1 Tetrahedral(a) and octahedral(b) interstitial sites and their geometric relation(c) in Y2Ti2O7.
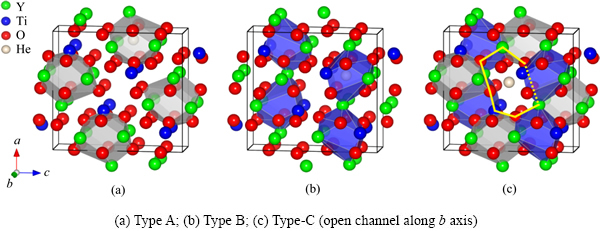
图2 Y2TiO5中A位和B位填隙位以及沿b轴方向的中空通道填隙(C位)
Fig. 2 Different interstitial sites in orthorhombic Y2TiO5
在NFAs中,主要考察两种(Y,Ti)氧化物析出相,即焦绿石结构的Y2Ti2O7和正交晶系结构的Y2TiO5相。图1所示为Y2Ti2O7中的四面体和八面体填隙位置以及两者的几何相关性。图2所示为Y2TiO5中A位和B位填隙位以及沿b轴方向的中空通道填隙(C位)。这两种复杂氧化物相均具有较为开放的晶体结构,其中Y2TiO5的层片状结构开放度更高。因此,Y2Ti2O7和Y2TiO5中的He缺陷结构只需考虑填隙型。在Y2Ti2O7中,所考察的He缺陷结构主要包括:单个He原子占据一个四面体或八面体填隙位(HeItetra或HeIocta)、两个He原子分别占据两个相邻的八面体填隙位(因相邻八面体排列方式的不同,可分为2-HeIocta (I)和2-HeIocta (II)两种)、1个2-He团簇占据1个八面体填隙位((2He)Iocta)、3个He原子分别占据3个相邻的八面体填隙位(3-HeIocta)以及1个3-He团簇占据1个八面体填隙位((3He)I)等7种情形。对于Y2TiO5,所考察的He缺陷结构主要包括:单个He原子占据A位(HeIA)、B位(HeIB)和沿b轴的通道填隙位(HeIC)以及共同占据沿b轴的通道填隙位的He团簇((2He)IC和(3He)IC)。与铁基体中He缺陷形成能相似,Y2Ti2O7和Y2TiO5中的He缺陷形成能可按式(2)计算:
 (2)
(2)
式中: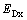 为包含缺陷的Y2Ti2O7或Y2TiO5晶胞的总能;
为包含缺陷的Y2Ti2O7或Y2TiO5晶胞的总能;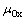 为Y2Ti2O7或Y2TiO5的纯态化学势;n为晶胞中He原子的数量。
为Y2Ti2O7或Y2TiO5的纯态化学势;n为晶胞中He原子的数量。
图3所示为Y2Ti2O7、Y2TiO5以及铁基体中He缺陷的计算结果比较。图3中,铁素体基体中所有He缺陷的形成能均以“预先存在”的空位作为参考。由图3可以看出,在Y2Ti2O7和Y2TiO5中形成填隙型He缺陷,都比在铁基体中形成的He缺陷所需能量更低。
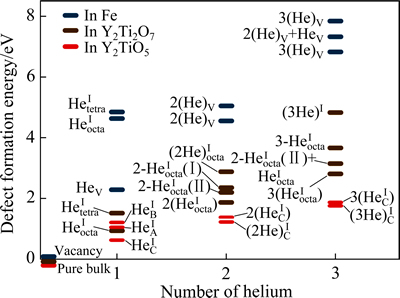
图3 Y2Ti2O7、Y2TiO5以及铁基体中氦缺陷的形成能比较
Fig. 3 Comparison of formation energies of helium defects in Y2Ti2O7 and Y2TiO5 with those in iron
特别是,Y2TiO5中He原子填隙于沿b轴方向的中空通道时,所需缺陷形成能最低。以单个He原子为例,HeIC的形成能(0.62 eV),仅为Y2Ti2O7中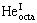 形成能(0.95 eV)的65%,或铁基体中HeV缺陷形成能(2.28 eV)的27%。在形成含多个He的缺陷团簇时,Y2TiO5也比Y2Ti2O7和铁基体更具能量优势。由此得出,铁基体中的氦具有分散到Y2Ti2O7和Y2TiO5的明显热力学趋势。在NFAs中,虽然Y2TiO5析出分数相对较小,但可能比Y2Ti2O7具有更强的优先捕获氦的能力。对比Y2TiO5、Y2Ti2O7以及铁基体中单个He缺陷的形成能,根据玻尔兹曼统计概率估算,在T=773 K下,氧化物Y2TiO5和Y2Ti2O7中捕获嬗变He的数量分别约
形成能(0.95 eV)的65%,或铁基体中HeV缺陷形成能(2.28 eV)的27%。在形成含多个He的缺陷团簇时,Y2TiO5也比Y2Ti2O7和铁基体更具能量优势。由此得出,铁基体中的氦具有分散到Y2Ti2O7和Y2TiO5的明显热力学趋势。在NFAs中,虽然Y2TiO5析出分数相对较小,但可能比Y2Ti2O7具有更强的优先捕获氦的能力。对比Y2TiO5、Y2Ti2O7以及铁基体中单个He缺陷的形成能,根据玻尔兹曼统计概率估算,在T=773 K下,氧化物Y2TiO5和Y2Ti2O7中捕获嬗变He的数量分别约
为铁基体的1010和108倍。另外,Y2Ti2O7中形成He团簇结构((2He)Iocta或(3He)I),比相同数目氦原子分散形成多个单独的填隙缺陷,如2(HeIocta)、2-HeIocta、2-HeIocta+HeIocta或3(HeIocta),所需要的能量更高。由此推断,与Y2TiO5中的情形相似,Y2Ti2O7中He-He自相互作用也具有排斥性。氦在氧化物中表现出来的这种占位特点,ERHART[31]在其他类型氧化物中也有类似发现。
综合上述分析,可以认为在铁基体中被捕获的嬗变氦原子,会尽可能地先单独占据不同的空位;而在NFAs中,氦原子会被大量、细小弥散的析出氧化物优先捕获,并且会尽可能地先单独占据氧化物中不同的填隙位置。进一步对Y2Ti2O7和Y2TiO5的氦捕获机理进行分析发现,捕获后的氦原子被活化,能够与近邻的氧原子间发生微量的电荷转移,转移的电荷数量与He-O的原子间距密切有关,此过程实质上是一种弱化学吸附反应[53-54]。
2.2 铁素体/Y2Ti2O7界面结构的确定
铁素体基体和析出的纳米氧化物相之间,存在有大量的界面,而界面作为一种典型的面缺陷形式,可能对嬗变氦的捕获发挥重要作用。为全面揭示NFA合金的氦捕获行为,有必要对铁素体/氧化物界面进行专门的计算研究。图3已清楚显示,体相Y2TiO5对氦的捕获能力比Y2Ti2O7的略强,据此推测,Fe/Y2TiO5界面可能有更强的氦捕获能力。但由于Y2TiO5在NFAs中的析出量相对较少,目前国内外对Fe/Y2TiO5界面位向关系尚未见确切研究报道,故在此暂时只考察铁素体/Y2Ti2O7界面,但这并不妨碍在此结果的基础上推断NFA合金中纳米氧化物析出相对材料整体抗辐照损伤性能的影响。
采用第一性原理计算研究界面时,为了包含界面适配位错而必须构建一个超大的模型,这在实际计算中显然是不现实的。本文作者在RIBIS等[55]“立方对立方”位向关系(即{100}<100>ferritic//{100}<100>Y2Ti2O7)的基础上,通过对BCC-Fe (100)和Y2Ti2O7 (100)表面施加应变进行界面模型的构建。在所得到的Fe(100) ( )/Y2Ti2O7(100)(
)/Y2Ti2O7(100)( )界面模型中,BCC-Fe和Y2Ti2O7受到的应变最小:在沿<100>Fe(//<100> Y2Ti2O7)的方向上,BCC-Fe晶格压缩了5.1%,Y2Ti2O7晶格拉伸了5.4%,从而构成5d{110}Fe 匹配5d{440}Y2Ti2O7的结构。尽可能地接近RIBIS的观测结果(8d{110}Fe 匹配9d{440}Y2Ti2O7的结构),计算用的界面模型采用Fe(100)/Y2Ti2O7(100)/Fe(100)三明治型模型,两侧包含5层原子层(40个Fe原子),中间氧化物区为一个Y2Ti2O7单胞(88个原子)。
)界面模型中,BCC-Fe和Y2Ti2O7受到的应变最小:在沿<100>Fe(//<100> Y2Ti2O7)的方向上,BCC-Fe晶格压缩了5.1%,Y2Ti2O7晶格拉伸了5.4%,从而构成5d{110}Fe 匹配5d{440}Y2Ti2O7的结构。尽可能地接近RIBIS的观测结果(8d{110}Fe 匹配9d{440}Y2Ti2O7的结构),计算用的界面模型采用Fe(100)/Y2Ti2O7(100)/Fe(100)三明治型模型,两侧包含5层原子层(40个Fe原子),中间氧化物区为一个Y2Ti2O7单胞(88个原子)。
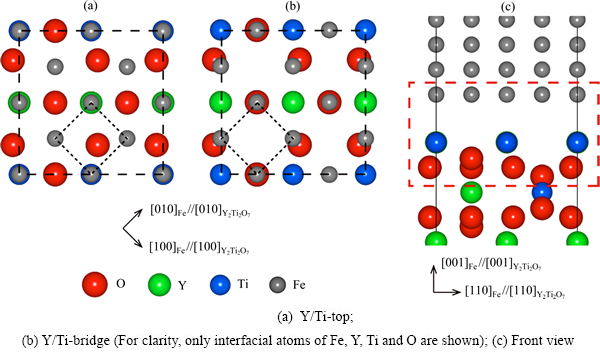
图4 Fe(100)(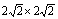 )/Y2Ti2O7(100)(
)/Y2Ti2O7(100)(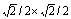 )界面的两种原子配位方式的俯视图
)界面的两种原子配位方式的俯视图
Fig. 4 Top views of Fe(100)( )/Y2Ti2O7(100)(
)/Y2Ti2O7(100)(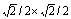 ) interfaces with two coordination types
) interfaces with two coordination types
除了位向关系,界面结构的稳定性还会受到界面化学计量比性质和原子配位的影响[56-57]。本文作者前期关于Y2Ti2O7极性表面的计算研究表明[30],对应不同的氧化学活度(即氧气分压),Y2Ti2O7自由表面会形成不同的终端结构。对应于界面处氧化学活度的不同,铁素体/Y2Ti2O7界面结构可能存在3种不同的化学计量比类型:理想化学计量比型(标记为“stoi”)、非化学计量比的富Y/Ti型(“ns-2Y2Ti”)和富O型(“ns-5O”)。针对上述每种化学计量比类型,根据界面两侧不同的相结构所作的平移操作,都可以构建出至少两种典型的界面原子配位方式(见图4),即界面层Fe原子坐落于Y和Ti原子正上方(“Y/Ti-top”)和坐落于Y和Ti原子之间的桥位上(“Y/Ti-bridge”)。后一种也可以看做是界面层Fe原子坐落于O原子上方(“O-top”)的配位方式。界面的配位方式以及化学计量比性质,共同影响着界面的局域化学环境,进而影响界面的结构与性质,比如界面形成能、界面结合强度以及界面元素偏聚效应等。这些影响在其他一些金属/氧化物界面体系(如Ni/Al2O3和Cu/Al2O3)中已经得到了理论上的阐明[56-60]。
针对上述方式构造的6种界面结构开展计算,结果表明,对于理想化学计量比型和富Y/Ti型结构,Y/Ti-bridge型配位方式相比Y/Ti-top型配位方式具有更低的形成能;而对于富O型结构,Y/Ti-top型配位方式相当不稳定,其结构在完全弛豫后自行转变为Y/Ti-bridge型。因此,在后续计算和讨论中,界面结构无论是理想化学计量比型、富Y/Ti型或富O型,其原子配位都只考察Y/Ti-bridge型方式。
NFAs典型制备工艺是对铁基预合金粉末(Fe、Cr、Ti)和Y2O3粉末进行高能球磨实现机械合金化,球磨过程中Y2O3组分被强制分解进入合金基体,在随后的高温制备工艺中与Ti组分共同析出[1, 61]。其中涉及的一系列化学反应,可以总结表达为
Y2O3+2Ti+2O2→Y2Ti2O7 (3)
为确定给定热力学条件下的界面结构,根据反应式(3)和缺陷热力学推导,可以计算热力学平衡条件下各界面结构所对应的界面能(γI)为
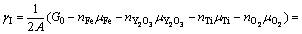
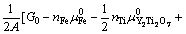
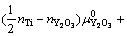
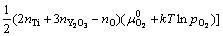 (4)
(4)
式中:A和G0分别为界面模型面积和界面模型超胞的总能。μi和ni分别是各组分i (i为 Fe、O、Y、Ti或Y2O3)的化学势及对应的原子(或分子)数目;上标“0”表示各组分的体相无缺陷态;T为温度。需要说明的是,式(4)中的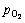 所指的氧气分压,反映的是界面附近的局域氧活度。注意到本研究中考虑的所有界面结构模型均具有一个固定的Y与Ti比,即nY=nTi,或
所指的氧气分压,反映的是界面附近的局域氧活度。注意到本研究中考虑的所有界面结构模型均具有一个固定的Y与Ti比,即nY=nTi,或
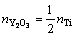 [30],因此,式(4)可以进一步简化为
[30],因此,式(4)可以进一步简化为
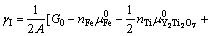
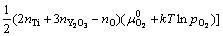 (5)
(5)
据此,根据计算所得的γI和对应的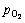 ,可以预测任何给定温度下界面相图的等温截面。
,可以预测任何给定温度下界面相图的等温截面。
图5所示为计算得到的Fe(100)/Y2Ti2O7(100)界面在900 K时的等温截面相图。由图5可见,正如式(5)所示,非化学计量比界面结构的界面能并非一固定 值,而是随环境参数(T和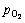 )的变化而变化。由能量最低原理可知,在该温度下,当
)的变化而变化。由能量最低原理可知,在该温度下,当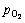 低于e-43 atm(2.14×10-14 Pa)时,富Y/Ti界面(ns-2Y2Ti)在热力学上占优;而当
低于e-43 atm(2.14×10-14 Pa)时,富Y/Ti界面(ns-2Y2Ti)在热力学上占优;而当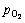 高于e-41 atm(1.58×10-13 Pa)时,富O界面(ns-5O)在热力学上占优。显然,e-43 atm(2.14×10-14 Pa)和e-41 atm(1.58×10-13 Pa)可以被视为900 K下界面发生非化学计量比-理想化学计量比结构转变的临界氧分压值。随着界面附近
高于e-41 atm(1.58×10-13 Pa)时,富O界面(ns-5O)在热力学上占优。显然,e-43 atm(2.14×10-14 Pa)和e-41 atm(1.58×10-13 Pa)可以被视为900 K下界面发生非化学计量比-理想化学计量比结构转变的临界氧分压值。随着界面附近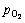 的不断增大,能量最低的界面结构,将由富Y-Ti 相(ns-2Y2Ti)逐渐过渡至理想化学计量比相,进而再转变到富O相(ns-5O)。该转变趋势与Y2Ti2O7(100)自由表面相同[30],但与后者不同的是,非化学计量比界面结构(包括富Y-Ti相和富O相)在更大的
的不断增大,能量最低的界面结构,将由富Y-Ti 相(ns-2Y2Ti)逐渐过渡至理想化学计量比相,进而再转变到富O相(ns-5O)。该转变趋势与Y2Ti2O7(100)自由表面相同[30],但与后者不同的是,非化学计量比界面结构(包括富Y-Ti相和富O相)在更大的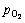 范围内能量占优,而理想化学计量比相只能在相当狭窄的
范围内能量占优,而理想化学计量比相只能在相当狭窄的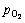 范围内(-43<ln
范围内(-43<ln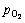 <-42)才可能优先形成。
<-42)才可能优先形成。
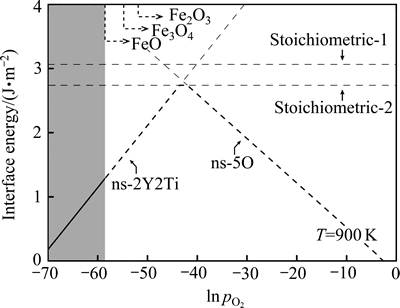
图5 计算所得Fe(100)/Y2Ti2O7(100)界面相图在900 K下的等温截面
Fig. 5 Calculated isothermal section of Fe(100)/Y2Ti2O7(100) interface phase diagram at 900 K
在确定了界面相图和界面相随环境参数变化的转变序列后,随之而来的问题是:在给定温度下(如900 K时),哪一个界面相在NFAs中最有可能出现。众所周知,在一定的氧活度条件下,铁可以直接被氧化成FeO、Fe2O3和Fe3O4等。NFA合金在制备过程中,必须确保铁基体不能被直接氧化。据此可以推断,无论采用怎样的合金制备工艺,Y2Ti2O7在铁素体基体中析出时,合金内部所对应的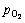 必定低于生成上述各种铁氧化物的临界值,而这些临界氧分压可以通过Fe和O之间的反应平衡计算确定。如根据化学反应
必定低于生成上述各种铁氧化物的临界值,而这些临界氧分压可以通过Fe和O之间的反应平衡计算确定。如根据化学反应
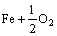 →
→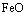 ,生成FeO的临界氧分压可由式(6)估算:
,生成FeO的临界氧分压可由式(6)估算:
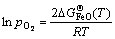 (6)
(6)
式中: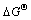 (T)为FeO在温度T下的标准吉布斯形成能;R为摩尔气体常数(R=8.314 J/(mol·K))。相似地,生成Fe2O3和Fe3O4的临界氧分压可分别由式(7)和(8)估算:
(T)为FeO在温度T下的标准吉布斯形成能;R为摩尔气体常数(R=8.314 J/(mol·K))。相似地,生成Fe2O3和Fe3O4的临界氧分压可分别由式(7)和(8)估算:
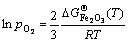 (7)
(7)
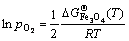 (8)
(8)
参考NIST-JANAF表[62]得知,900 K时分别对应有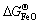 =-213.12,
=-213.12,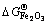 =-586.51,
=-586.51,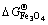 = -822.43 kJ/mol,由此可以估算,在900 K下纯铁氧化生成FeO、Fe2O3和Fe3O4需要的临界氧分压分别约为e-57、e-52、e-55 atm(1.78×10-20)、2.64×10-18和1.32×10-19 Pa)。换言之,在析出Y2Ti2O7时,NFA合金中内部氧分压必定低于e-57 atm(1.78×10-20 Pa)。将该
= -822.43 kJ/mol,由此可以估算,在900 K下纯铁氧化生成FeO、Fe2O3和Fe3O4需要的临界氧分压分别约为e-57、e-52、e-55 atm(1.78×10-20)、2.64×10-18和1.32×10-19 Pa)。换言之,在析出Y2Ti2O7时,NFA合金中内部氧分压必定低于e-57 atm(1.78×10-20 Pa)。将该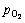 上限取值标记在界面相图的等温截面上,即图5中的灰色部分。据此可以推断,在900 K下,具有立方对立方位向关系的铁素体/ Y2Ti2O7界面只可能为富Y/Ti相结构,具体而言,该界面的典型结构应该是非化学计量比的富Y/Ti终端类型(ns-2Y2Ti),该界面结构所反映的氧化物化学组成为Y2.5Ti2.5O7,与EELS实验分析技术推断的NFAs中纳米氧化物析出相的平均化学组成(Y2.2Ti2.3O7)[63]较为吻合。
上限取值标记在界面相图的等温截面上,即图5中的灰色部分。据此可以推断,在900 K下,具有立方对立方位向关系的铁素体/ Y2Ti2O7界面只可能为富Y/Ti相结构,具体而言,该界面的典型结构应该是非化学计量比的富Y/Ti终端类型(ns-2Y2Ti),该界面结构所反映的氧化物化学组成为Y2.5Ti2.5O7,与EELS实验分析技术推断的NFAs中纳米氧化物析出相的平均化学组成(Y2.2Ti2.3O7)[63]较为吻合。
2.3 氦在Fe/Y2Ti2O7界面的偏聚和Y2Ti2O7颗粒的尺寸效应
一旦界面相结构确定,就可以进一步计算评估界面对氦的捕获。根据900 K下富Y-Ti界面相的结构特点,可以确定界面上的3种氦填隙位置(HeiA、HeiB以及HeiT)以及两种近界面的八面体填隙位置(HesA和HesB)。经完全弛豫得到的界面填隙结构如图6所示。与体相中He缺陷计算相似,界面中的He缺陷形成能可按式(9)计算:
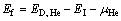 (9)
(9)
式中: 为包含He缺陷的界面超胞的总能;EI为纯净界面超胞的总能;μHe为超胞中He原子的纯态化学势。
为包含He缺陷的界面超胞的总能;EI为纯净界面超胞的总能;μHe为超胞中He原子的纯态化学势。
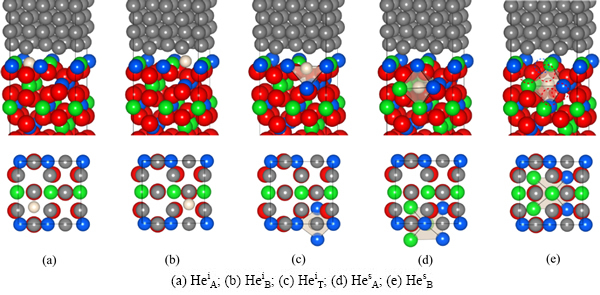
图6 富Y-Ti型界面上可能的氦填隙位置(下方显示的分别是各结构所对应的界面层俯视图)
Fig. 6 Possible interstitial sites for helium on Y/Ti-rich interface (Corresponding top views of interfacial layers are shown at the bottom)
表2 计算所得界面处各种He缺陷结构的形成能
Table 2 Calculated formation energies of various helium-trapping interface structures
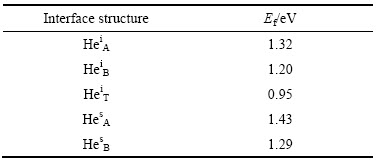
由表2可知,HeiT缺陷结构所需形成能最低(0.95 eV),与He原子占据体相Y2Ti2O7中八面体填隙位所需能量(HeIocta =0.94 eV)几乎完全相同,表明Fe/Y2Ti2O7界面对氦的捕获能力与Y2Ti2O7体相的相当,两者均明显强于铁基体的,因而,铁基体中的He有较强的向界面偏聚的趋势。另外注意到,He原子填隙于Y2Ti2O7一侧近界面处的缺陷结构(HesA和HesB),其缺陷形成能(1.43和1.29 eV)高于He原子占据体相Y2Ti2O7中八面体填隙位所需能量,这种差异可能与界面应变有关,同时也明显高于界面上的氦填隙能(HeiT)。因此,当Y2Ti2O7 内部填隙的氦达到饱和时,新捕获的氦如果足够地靠近界面,也会存在向界面偏聚的强烈趋势。
NFAs中Y2Ti2O7界面应变对Y2Ti2O7中八面体填隙能影响,显然应与Y2Ti2O7颗粒大小有关。若Y2Ti2O7相尺寸极小(约为1 nm,即一个单胞大小),被Y2Ti2O7填隙八面体捕获的氦原子,实质上均处于界面邻域,受界面应变的影响,其缺陷形成能应接近HesA和HesB,即为1.29~1.43 eV,将获得向界面偏聚的强烈趋势,并因此可减少系统总能的0.3~0.4 eV。距离界面越远,Y2Ti2O7颗粒中填隙的氦越稳定,向界面偏聚的趋势越弱。若Y2Ti2O7相尺寸较大(>5 nm),填隙在Y2Ti2O7中的氦原子大部分将远离界面一个单胞以上的距离,界面偏聚的趋势伴随界面应变效应的消除而基本消除。据此可以推断,对于较大Y2Ti2O7颗粒,氦可以在颗粒内部和界面被捕获且稳定下来;而对于低纳米尺度的Y2Ti2O7颗粒,氦则更容易被颗粒界面捕获。
综合以上所有结果,可以对NFAs中氦泡形成、演变和最终结局提出初步的假设:核反应中产生的中子辐射会在合金基体中产生大量氦原子。通常情况下,这些氦原子会被铁基体中的空位捕获,并以此逐渐团簇并长大,最终形成氦泡甚至空洞,使材料的性能退化。NFA合金中存在有大量、超高密度且分布弥散的富Y-Ti-O析出相(Y2Ti2O7和Y2TiO5),它们对氦原子的捕获能力明显强于基体中的空位。较大尺度的Y2Ti2O7相能够将氦捕获在其体内或界面上,而低纳米尺度的Y2Ti2O7相则更倾向于将氦捕获在其界面上。由于这些氧化物在铁基体中以纳米尺度大量弥散地分布,且能够优先捕获氦原子,因此有助于氦原子在合金中的分散和稳定,从而有效抑制氦原子在铁基体中形成有害氦泡的可能,使NFA合金能够获得不同于常规铁基高温合金的高抗辐照损伤特性。
3 结论
1) 相比于铁基体中的空位,氦更容易被Y2Ti2O7的八面体填隙位和Y2TiO5沿b轴方向的中空通道捕获。两种氧化物中He-He原子间均存在排斥性的相互作用,说明氦在NFAs中的分散度将在很大程度上取决于NFAs中Y2Ti2O7和Y2TiO5相的析出数量和弥散程度。
2) 在给定温度下,Fe/Y2Ti2O7界面的原子结构取决于合金内部的氧分压。随氧分压的增大,其界面可以由富Y/Ti相结构,相继向理想化学计量比相或者富O相结构转变。根据制备条件和计算所得的界面相图推断,NFAs中Fe/Y2Ti2O7界面平衡结构为且仅可能为富Y/Ti型,这符合NFAs中氧化物常常偏离理想化学计量比的实验观察。
3) 氦在Fe/Y2Ti2O7界面的偏聚能力受氧化物颗粒尺寸影响。较大尺度的Y2Ti2O7颗粒能够将氦捕获在体内或界面上,而低纳米尺度的Y2Ti2O7颗粒倾向于将氦捕获在其界面上。
4) NFA合金中氧化物以纳米尺度大量、弥散、稳定地分布,对合金中的氦捕获和氦分散起到了关键性作用,极大抑制了大尺寸氦泡的形成和长大,是该型合金能够获得高抗辐照损伤容限的重要原因。
REFERENCES
[1] ODETTE G R, ALINGER M J, WIRTH B D. Recent developments in irradiation-resistant steels[J]. Annual Review of Material Research, 2008, 38: 471-503.
[2] WU Y, HANEY E M, CUNNINGHAM N J, ODETTE G R. Transmission electron microscopy characterization of the nanofeatures in nanostructured ferritic alloy MA957[J]. Acta Materialia, 2012, 60: 3456-3468.
[3] WANG M, ZHOU Z J, SUN H Y, HU H L, LI S F. Microstructural observation and tensile properties of ODS-304 austenitic steel[J]. Materials Science and Engineering A, 2013, 559: 287-292.
[4] HOELZER D T, ODETTE G R. Regular and ODS ferritic steel as structural materials for power plant HHFCs[C]// International HHFC Workshop on Readiness to Proceed from Near Term Fusion Systems to Power Plants, La Jolla, CA: UCSD, 2008.
[5] SAKASEGAWA H, CHAFFRON L, LEGENDRE F, BOULANGER L, COZZIKA T, BROCQ M, DE CARLAN Y. Correlation between chemical composition and size of very small oxide particles in the MA957 ODS ferritic alloy[J]. Journal of Nuclear Materials, 2009, 384(2): 115-118.
[6] ALINGER M J, ODETTE G R, HOILZER D T. On the role of alloy composition and processing parameters in nanocluster formation and dispersion strengthening in nanostuctured ferritic alloys[J]. Acta Materialia, 2009, 57: 392-406.
[7] OHNUMA M, SUZUKI J, OHTSUKA S, KIM S-W, KAITO T, INOUE M, KITAZAWA H. A new method for the quantitative analysis of the scale and composition of nanosized oxide in 9Cr-ODS steel[J]. Acta Materialia, 2009, 57: 5571-5581.
[8] HIRATA A, FUJITA T, WEN Y R, SCHNEIBEL J H, LIU C T, CHEN M W. Atomic structure of nanoclusters in oxide-dispersion-strengthened steels[J]. Nature Materials, 2011, 10: 922-926.
[9] CUNNINGHAM N J, ODETTE G R, STERGAR E. Further atom probe tomography studies of nanostructured ferritic alloy MA957 in three conditions: Fusion reactor materials program semiannual progress report[R]. DOE/ER-0313/49, 2010: 11-16.
[10] ODETTE G R, HOELZER D T. Irradiation-tolerant nanostructured ferritic alloys: Transforming helium from a liability to an asset[J]. JOM, 2010, 62: 84-92.
[11] MARQUIS E A. Core/shell structures of oxygen-rich nanofeatures in oxide-dispersion strengthened Fe-Cr alloys[J]. Applied Physics Letters, 2008, 93: 181904.
[12] KLIMENKOV M, LINDAUR,  . New insights into the structure of ODS particles in the ODS-Eurofer alloy[J].Journal of Nuclear Materials, 2009, 386: 553-556.
. New insights into the structure of ODS particles in the ODS-Eurofer alloy[J].Journal of Nuclear Materials, 2009, 386: 553-556.
[13] LOZNAO-PEREZ S, DE CASTRO BERNAL V, NICHOLLS R J. Achieving sub-nanometre particle mapping with energy- filtered TEM[J]. Ultramicroscopy, 2009, 109: 1217-1228.
[14] BHATTACHARYYA D, DICKERSON P, ODETTE G R, MALOY S A, MISRA A, NASTASI M A. On the structure and chemistry of complex oxide nanofeatures in nanostructured ferritic alloy U14YWT[J]. Philosophical Magazine, 2012, 92: 2089-2107.
[15] 刘 峰. 氧化物弥散强化铁基高温合金的制备及强化机理研究[D]. 长沙: 中南大学, 2011.
LIU Feng. Study on fabrication and strenthening mechanism of oxide dispersion strengthened iron-based super alloy[D]. Changsha: Central South University, 2011.
[16] LIU F, LIU Y, LIU D, WANG Y, ZHAO D, ZHANG L. Formation of vacancies in a nanocluster-strengthened ferritic steel [J]. Materials Letters, 2011, 65(2): 253-255.
[17] LIU F, LIU Y, WEN Y, DOU Y, ZHAO D, LIU C T. Microstructures and mechanical properties of Fe-14Cr-3W- Ti-Y2O3 steel with 1wt.% Cu addition fabricated by a new method[J]. Journal of Nuclear Materials, 2011, 414: 422-425.
[18] ZHAO D, LIU Y, LIU F, WEN Y, ZHANG L, DOU Y. ODS ferritic steel engineered with bimodal grain size for high strength and ductility[J]. Materials Letters, 2011, 65: 1672-1674.
[19] 刘东华, 刘 咏, 刘祖铭, 王 斌. 球磨对雾化铁基合金14YWT粉末烧结性能的影响[J]. 粉末冶金材料科学与工程, 2010, 15: 288-293.
LIU Dong-hua, LIU Yong, LIU Zu-ming, WANG Bin. Influence of mechanical alloying on sintering characters of atomized iron-based 14YWT powder[J]. Materials Science and Engineering of Powder Metallurgy, 2010, 15: 288-293.
[20] 王 曼, 周张健, 闰志刚, 于鹏飞, 孙红英. ODS-316奥氏体钢显微结构及弥散相的TEM研究[J]. 金属学报, 2013, 49: 153-158.
WANG Man, ZHOU Zhang-jian, RUN Zhi-gang, YU Peng-hui, SUN Hong-yin. TEM characterization of dispersions and microstructure of ODS-316 austenitic steel[J]. Acta Metallurgica Sinica, 2013, 49: 153-158.
[21] LI M, ZHOU Z J, HE P, XU Y, LIAO L. Annealing behavior of 12-Cr based mechanical alloyed oxide dispersion strengthened ferritic alloys[J]. Journal of Nuclear Materials, 2011, 417: 189-192.
[22] HU H L, ZHOU Z J, LIAO L, ZHANG L F, WANG M, LI S F, GE C C. Corrosion behavior of a 14Cr-ODS steel in supercritical water[J]. Journal of Nuclear Materials, 2013, 437: 196-200.
[23] HU H L, ZHOU Z J, LI M, ZHANG L, WANG M, LI S, GE C C. Study of the corrosion behavior of a 18Cr-oxide dispersion strengthened steel in supercritical water[J]. Corrosion Science, 2012, 65: 209-213.
[24] ALINGER M J, WIRTH B D. Towards understanding the formation and stability of nanometer scale Y-Ti-O clusters in nanostructured ferritic alloys using lattice-based Monte Carlo simulations: Fusion reactor materials program semiannual progress report[R]. DOE/ER-0313/38, 2005: 124-134.
[25] FU C L,  , PAINTER G S, CHEN X Q. Vacancy mechanism of high oxygen solubility and nucleation of stable oxygen-enriched clusters in Fe[J]. Physical Review Letters, 2007, 99: 225502.
, PAINTER G S, CHEN X Q. Vacancy mechanism of high oxygen solubility and nucleation of stable oxygen-enriched clusters in Fe[J]. Physical Review Letters, 2007, 99: 225502.
[26] JIANG Y, SMITH J R, ODETTE G R. Formation of Y-Ti-O nanoclusters in nanostructured ferritic alloys: A first-principles study[J]. Physical Review B, 2009, 79: 064103.
[27] JIANG Y, SMITH J R, ODETTE G R. Prediction of structural, electronic and elastic properties of Y2Ti2O7 and Y2TiO5[J]. Acta Materialia, 2010, 58: 1536-1543.
[28] BARNARD L, ODETTE G R, SZLUFARSKA I, MORGAN D. An ab initio study of Ti-Y-O nanocluster energetic in nanostructured ferritic alloys[J]. Acta Materialia, 2012, 60: 935-947.
[29] BARNARD L, CUNNINGHAM N, ODETTE G R, SZLUFARSKA I, MORGAN D. Thermodynamic and kinetic modeling of oxide precipitation in nanostructured ferritic alloys[J]. Acta Materialia, 2015, 91: 340-354.
[30] YANG L, JIANG Y, ODETTE G R, ZHOU W C, LIU Z, LIU Y. Nonstoichiometry and relative stabilities of Y2Ti2O7 polar surfaces[J]. Acta Materialia, 2013, 61: 7260-7270.
[31] ERHART P. A first-principles study of helium storage in oxides and at oxide-iron interfaces[J]. Journal of Applied Physics, 2012, 111: 113502.
[32] CHOUDHURY S, MORGAN D, UBERUAGAL B P. Massive interfacial reconstruction at misfit dislocations in metal/oxide interfaces[J]. Scientific Reports, 2014, 4: 6533.
[33] 陶辉锦, 孙顺平, 张铖铖, 陈 图, 罗 伟, 江 勇. 金属间化合物 L10-TiAl 点缺陷浓度的第一原理计算研究[J]. 中国有色金属学报, 2014, 24(11): 2789-2797.
TAO Hui-jin, SUN Shun-ping, ZHANG Cheng-cheng, CHEN Tu, LUO Wei, JIANG Yong. First principles of point defect concentrations in L10-TiAl intermetallic composite[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(11): 2789-2797.
[34] 江 勇, 蓝国强, 王怡人, 周松松. 内氧化Cu/Al2O3界面热力学与杂质效应[J]. 中国有色金属学报, 2013, 23(11): 3154-3164.
JIANG Yong, LAN Guo-qiang, WANG Yi-ren, ZHOU Song-song. Interfacial thermodynamics and impurity effects on internally oxidized Cu/Al2O3 interface[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(11): 3154-3164.
[35] 江 勇. 基于第一性原理揭示活性元素铪对热障涂层关键界面的强化机理[J]. 中国有色金属学报, 2011, 21(6): 1463-1472.
JIANG Yong. Strengthening mechanisms of reactive-element Hf on the key interface in thermal barrier coating systems[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(6): 1463-1472.
[36] JIANG Yong, XU Can-hui, LAN Guo-qiang. First-principles thermodynamics of metal-oxide surfaces and interfaces: A case study review[J]. Transactions of Nonferrous Metals Society of China, 2013, 23(1): 180-192.
[37] JIANG Yong, LUO Kang, ZANG Bing, FU Shang, JIANG Yong, YI Dan-qing. Stress/strain aging mechanisms in Al alloys from first principles[J]. Transactions of Nonferrous Metals Society of China, 2014, 24(7): 2130-2137.
[38] KRESSE G,  J. VASP the guide[EB/OL]. http://cms.mpi.univie.ac.at/ vasp/vasp/vasp.html.
J. VASP the guide[EB/OL]. http://cms.mpi.univie.ac.at/ vasp/vasp/vasp.html.
[39] KRESSE G, JOUBERT J. From ultrasoft pseudopotentials to the projector augmented-wave method[J]. Physical Review B, 1999, 59: 1758-1775.
[40] VINETT P, ROSE J H, FERRANTE J, SMITH J R. Universal features of the equation of state of solids[J]. Journal of Physics-Condensed Matter, 1989, 1: 1941-1963.
[41] CASPERSEN K J, LEW A, ORTIZ M, CARTER E A. Importance of shear in the BCC-to-hcp transformation in iron[J]. Physical Review Letters, 2004, 93: 115501.
[42] SIMMONS G, WANG H. Single crystal elastic constants and calculated aggregate properties: A Handbook[M]. 2nd ed. Cambridge, MA: The MIT Press, 1971.
[43] LEESE J, LORD A E Jr. Elastic stiffness coefficients of single-crystal iron from room temperature to 500 ℃[J]. Journal of Applied Physics, 1968, 39: 3986-3988.
[44] DEVER D J. Temperature dependence of the elastic constants in α-iron single crystals: Relationship to spin order and diffusion anomalies[J]. Journal of Applied Physics, 1972, 43: 3293-3301.
[45] SUBRAMANIAN M A, ARAMUDAN G, SUBBA RAO G V. Oxide pyrochlores: A review[J]. Progress in Solid State Chemistry, 1983, 15: 55-143.
[46] GLERUP M, NIELSEN O F, POULSEN F W. The structural transformation from the pyrochlore structure, A2B2O7, to the fluorite structure, AO2, studied by Raman spectroscopy and defect chemistry modeling[J]. Journal of Solid State Chemistry, 2001, 160: 25-32.
[47] HAILE S, WUENSCH B J, PRINCE E. Neutron rietveld analysis of anion and cation disorder in the fast-ion conducting pyrochlore system Y2(ZrxTi1-x)2O7[J]. Materials Research Society Symposia Proceedings, 1990, 166: 81-86.
[48] HE L F, SHIRAHATA J, NAKAYAMA T, SUZUKI T, SUEMATSU H, IHARA I, BAO Y W, KOMATSU T, NIIHARA K. Mechanical properties of Y2Ti2O7[J]. Scripta Materialia, 2011, 64: 548-551.
[49] KOHN W, SHAM L J. Self-consistent equations including exchange and correlation effects[J]. Physical Review, 1965, 140: A1133.
[50] PERDEW J P, CHEVARY J A, VOSKO S H, JACKSON K A, PEDERSON M R, SINGH D J, FIOLHAIS C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation[J]. Physical Review B, 1992, 46: 6671-6687.
[51] PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple[J]. Physical Review Letters, 1996, 77: 3865-3868.
[52] SELETSKAIA T, OSETSKY Y, STOLLER R E, STOCKS G M. Magnetic interactions influence the properties of helium defects in iron[J]. Physical Review Letters, 2005, 94: 046403.
[53] YANG L, JIANG Y, ODETTE G R, YAMAMOTO T, LIU Z, LIU Y. Trapping helium in Y2Ti2O7 compared to in matrix iron: A first principles study[J]. Journal of Applied Physics, 2014, 115: 143508.
[54] JIN Y, JIANG Y, YANG L, LAN G, ODETTE G R, YAMAMOTO T, SHANG J, DANG Y. First principles assessment of helium trapping in Y2TiO5 in nano-featured ferritic alloys[J]. Journal of Applied Physics, 2014, 116: 143501.
[55] RIBIS J, DE CARLAN Y. Interfacial strained structure and orientation relationships of the nanosized oxide particles deduced from elasticity-driven morphology in oxide dispersion strengthened materials[J]. Acta Materialia, 2012, 60: 238-252.
[56] SMITH J R, JIANG Y, EVANS A G. Adhesion of the γ-Ni(Al)/ α-Al2O3 interface: a first-principles assessment[J]. International Journal of Materials Research, 2007, 98: 1214-1221.
[57] JIANG Y, SMITH J R, EVANS A G. First principles assessment of metal/oxide interface adhesion[J]. Applied Physics Letters, 2008, 92: 141918.
[58] ZHANG W, SMITH J R, WANG X G, EVANS A G. Influence of sulfur on the adhesion of the nickle/alumina interface[J]. Physical Review B, 2003, 67: 245414.
[59] LAN G, JIANG Y, YI D, LIU S. Theoretical prediction of impurity effects on the internally oxidized metal/oxide interface: The case study of S on Cu/Al2O3[J]. Physical Chemistry Chemical Physics, 2012, 14: 11178-11184.
[60] LAN G, WANG Y, JIANG Y, ZHOU H, YI D. Effects of rare-earth dopants on the thermally grown Al2O3/Ni(Al) interface: the first-principles prediction[J]. Journal of Materials Science, 2014, 49: 2640-2646.
[61] HOELZER D T, BENTLEY J, SOKOLOV M A, MILLER M K, ODETTE G R, ALINGER M J. Influence of particle dispersions on the high-temperature strength of ferritic alloys[J]. Journal of Nuclear Materials, 2007, 367/370: 166-172.
[62] CHASE M W Jr. NIST-JANAF Thermochemical tables[J]. Journal of Physical and Chemical ReferenceData, 1998: 9.
[63] KLIMIANKOU M, LINDAU R,  . Energy-filtered TEM imaging and EELS study of ODS particles and argon-filled cavities in ferritic-martensitic steels[J]. Micron, 2005, 36: 1-8.
. Energy-filtered TEM imaging and EELS study of ODS particles and argon-filled cavities in ferritic-martensitic steels[J]. Micron, 2005, 36: 1-8.
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(51471189,51171211,51471049);国家重点基础研究发展计划资助项目子课题(NC2014CB644001-2);中南大学粉末冶金国家重点实验室自主项目
收稿日期:2015-01-12;修订日期:2015-09-19
通信作者:江 勇,教授,博士;电话:0731-88830263;E-mail: yjiang@csu.edu.cn
摘 要:基于第一性原理计算研究纳米结构铁素体合金(NFAs)对中子辐照引发的氦的选择性捕获能力。结果表明:与铁基体中的空位相比,氦更容易被NFAs中Y2Ti2O7的八面体间隙和Y2TiO5沿b轴方向的中空通道捕获;被氧化物捕获的氦相互间存在排斥作用,说明氦在NFAs中的分散度将在很大程度上取决于氧化物的析出分数和弥散度;通过计算界面相图推断,铁素体/Y2Ti2O7界面应为富Y/Ti型,与实验观测到的Y2Ti2O7析出相的非化学计量比特性吻合;氦对Fe/Y2Ti2O7界面有较强的偏聚倾向,且可能对氧化物颗粒尺寸敏感。较大尺度的Y2Ti2O7能够将氦捕获在界面和体内,而低纳米尺度的Y2Ti2O7更倾向于将氦捕获在界面。
[15] 刘 峰. 氧化物弥散强化铁基高温合金的制备及强化机理研究[D]. 长沙: 中南大学, 2011.
[19] 刘东华, 刘 咏, 刘祖铭, 王 斌. 球磨对雾化铁基合金14YWT粉末烧结性能的影响[J]. 粉末冶金材料科学与工程, 2010, 15: 288-293.
[20] 王 曼, 周张健, 闰志刚, 于鹏飞, 孙红英. ODS-316奥氏体钢显微结构及弥散相的TEM研究[J]. 金属学报, 2013, 49: 153-158.
[34] 江 勇, 蓝国强, 王怡人, 周松松. 内氧化Cu/Al2O3界面热力学与杂质效应[J]. 中国有色金属学报, 2013, 23(11): 3154-3164.
[35] 江 勇. 基于第一性原理揭示活性元素铪对热障涂层关键界面的强化机理[J]. 中国有色金属学报, 2011, 21(6): 1463-1472.
[38] KRESSE G, J. VASP the guide[EB/OL]. http://cms.mpi.univie.ac.at/ vasp/vasp/vasp.html.


