
Use of ferricyanide for gold and silver cyanidation
F. Xie, D. B. Dreisinger
Department of Materials Engineering, University of British Columbia,
309-6350 Stores Road, Vancouver, BC, Canada V6T 1Z4
Received 4 December 2008; accepted 20 April 2009
Abstract:
Low gold and silver leaching kinetics has been commonly observed in traditional gold-silver cyanidation process, especially in heap leaching and in situ leaching operations. The different mineralogy of gold and silver in the ores is suspected to be the main reason, e.g., the occurrence of low solubility acanthite (Ag2S) typically results in low overall silver extraction. Due to the low solubility of oxygen in cyanide solution, the reactivity and availability of oxidant is believed to be the critical limitation for gold and silver dissolution. The use of ferricyanide as the auxiliary oxidant for gold and silver cyanidation has been examined. The rotating disc test results prove the assistant effect of ferricyanide on increasing the dissolution rate of gold and silver. The potential use of ferricyanide in gold/silver cyanidation process is proposed based on the leaching results of actual ores.
Key words:
ferricyanide; cyanidation; gold; silver;
1 Introduction
The cyanidation process has been successfully practiced in the extraction of gold and silver from their ores for the past 100 years. Historical study has revealed that cyanide and oxygen act as complex ligand and oxidant respectively and they both play important roles in the gold/silver cyanidation process. The well-known Elsner equation (1946) describes the stoichiometry of the reactions as follows[1]:
4Ag+8CN-+O2+2H2O=![]() +4OH- (1)
+4OH- (1)
4Au+8CN-+O2+2H2O=![]() +4OH- (2)
+4OH- (2)
Due to the low solubility of oxygen in cyanide solution, the availability and the reactivity of oxygen are believed to be critical, especially in heap leaching and in situ leaching operations. Moreover, conventional extraction results revealed that silver recovery was noticeably lower than that of gold under typical cyanidation conditions and sometimes silver extraction was only about half that of gold. Since silver may occur as silver sulfide minerals in the ore, e.g. acanthite (Ag2S) and argentojarosites (AgFe3[SO4]2(OH)6), the low solubility of these silver sulfides was believed to limit extraction[2-3]. The use of auxiliary oxidants including hydrogen peroxide (H2O2), calcium peroxide (CaO2), and per-manganate (KMnO4) for gold and silver cyanidation process has been proposed with the purpose of increasing gold and silver dissolution rate[4-9]. Unfortunately, none of these oxidants has been successfully practiced, probably due to their high cost and potential reactions with free cyanide.
On the other hand, the investigations on the influence of iron minerals on gold cyanidation process have confirmed that the presence of iron cyanide may potentially increase gold leaching rate. The catalytic function of ferricyanide on gold/silver cyanidation has been reported[10-11]. The half reaction between the dissolved iron-cyanide species can be expressed as
![]() +e=
+e=![]() , φ0=0.36 V (vs SHE) (3)
, φ0=0.36 V (vs SHE) (3)
Though the standard reduction potential of ![]() is slightly lower than that of oxygen in alkaline solution, the oxidation capability of ferricyanide-cyanide solution can be maintained at a relatively high level due to its high solubility in cyanide solution. In this work, the dissolution of gold and silver sulfide in ferricyanide-cyanide solution under different experimental conditions has been examined. The potential use of ferricyanide as the supplemental oxidant in the gold/silver cyanidation process has been discussed based on the leaching of actual gold-silver ore.
is slightly lower than that of oxygen in alkaline solution, the oxidation capability of ferricyanide-cyanide solution can be maintained at a relatively high level due to its high solubility in cyanide solution. In this work, the dissolution of gold and silver sulfide in ferricyanide-cyanide solution under different experimental conditions has been examined. The potential use of ferricyanide as the supplemental oxidant in the gold/silver cyanidation process has been discussed based on the leaching of actual gold-silver ore.
2 Experimental
2.1 Samples and reagents
A pure gold rod (purity of 99.95 %) with a diameter of 2.4 cm was used in the gold leaching tests. A synthetic silver sulfide powder sample (particle size D80=16 mm) was pressed under a moderate pressure (1.5 Pa) with Caver Presser (New Jersey, USA) to form a 1 cm-thick disc with a diameter of 2.4 cm. The pure silver sulfide disc was used in the silver leaching tests. Three natural gold-silver ore samples (A, B and C from Barrick Gold Co.) were also used to examine the effect of ferricyanide on gold/silver extraction. The grades of gold and silver in the three samples are shown in Table 1. X-ray fluorescence(XRF) analysis indicated the gangue in the samples mainly occurring as quartz and the possible occurrence of acanthite in these ores. All the solutions used in the tests were made with de-ionized water. The potassium hexacyanoferrate (Ⅲ) (K3Fe(CN)6), sodium cyanide (NaCN), sodium hydroxide (NaOH), and nitrogen (N2) were all certified analytical chemicals.
Table 1 Grade of gold and silver in three ore samples

2.2 Test procedures
The rotating disc tests (Au disc) were conducted in a sealed reactor. The reactor was put in a water bath in which the temperature of the leaching solution could be automatically controlled. The reactor lid had several openings that allowed the insertion of the rotating disc, reagents injection and sampling tubes. When necessary, nitrogen gas was introduced to the cell to repulse the air. No sintering process was applied before the discs were used in the leaching tests. The Au/Ag2S disc was leached in 800 mL solution under different leaching conditions up to 40 min. The solution pH was maintained at 11.5 by addition of NaOH and H2SO4 solutions. Leaching solutions were sampled at periodic intervals for subsequent analysis.
Leaching of the ore sample-A was conducted in a covered 2 L PYREX glass beaker under the room temperature. 500 g ore sample-A was leached in 1 L solution up to 48 h. Comparative leaching of ore sample-B and C (0.5 g/L Fe as ferricyanide was used where applicable) was conducted in the small columns where counter-flow circulation of 2 L of solution was used to leach 200 g solid sample. The solution pH was controlled by addition of 2 mol/L NaOH solution. Free cyanide was determined by titration with standard silver nitrate solution. Gold and silver in solution were analyzed by atomic absorption spectrometry(AAS) by Chem Met Co., a certified assayer located in Vancouver, B.C., Canada.
3 Results and discussion
3.1 Dissolution of gold disc
3.1.1 Effect of different oxidants
The rotating disc technique is used to study the gold and silver dissolution mechanisms. According to Levich equation, which shows that the flux is a function of ω1/2 [12], there is
![]() (4)
(4)
where dn/dt is the rate of reaction, mol/s; A is the surface area of the rotating disc, m2; J is the flux of reactant O, mol?m2/s; D0 is the diffusion coefficient of reactant O, m2/s; ω is the rotating speed of the disc, rad/s; υ is the kinematic viscosity of the solution, mol/m3; c0 is the bulk solution concentration of reactant O, mol/m3.
The most important feature of the rotating disc system is the “uniformly accessible surface” that means the rate of mass transport to the surface of disc is uniform. The critical condition is to maintain a laminar flow in which the Reynold’s number (Re=r2w/n) is greater than that for natural convection but less than that for turbulence (the critical value is 105). Dissolution of gold disc in different leaching conditions has been examined and the results are shown in Fig.1. Plot of gold dissolution versus leaching time exhibited near linear relationship, indicating the gold dissolution took place under the steady-state conditions during the leaching period. Gold dissolution rate under different experimental conditions can be calculated out from the slopes of the plots. The dissolution rate of gold in aerated cyanide solution in this research gives a value of 0.19 ?m/h, which is consistent with the results claimed by JEFFREY and WADSWORTH, but is lower than that claimed by GUZMAN et al[13-15]. Some factors including impurities in gold disc and leaching solutions, surface treatment, and leaching and measurement system may lead to the different results. Some researchers suggested to heat the gold plate before the experiment in order to remove hydrocarbons absorbed on the gold surface[15]. Limited by the coating material, this process was not applied in this work. However, under the same experimental conditions, the gold dissolution rate in ferricyanide-cyanide solution (0.5 g/L Fe as ![]() gives the value of 0.74 ?m/h, much greater than that in aerated cyanide solution. The addition of ferrocyanide
gives the value of 0.74 ?m/h, much greater than that in aerated cyanide solution. The addition of ferrocyanide![]() was not effective, indicating that the function of ferricyanide was critical. In ferricyanide- cyanide solution, the dissolution of gold can be deduced as follows:
was not effective, indicating that the function of ferricyanide was critical. In ferricyanide- cyanide solution, the dissolution of gold can be deduced as follows:
Au+![]() +2CN-=
+2CN-=![]() +
+![]() (5)
(5)
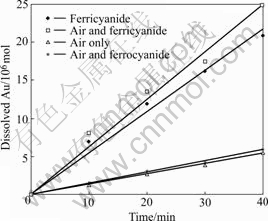
Fig.1 Dissolution of gold disc under different conditions (0.5 g/L NaCN, pH=11.5, 25 ℃, 600 r/min)
3.1.2 Effect of rotating speed and temperature
The effect of rotating speed on the dissolution rate of Au disc in the ferricyanide-cyanide leaching system is shown in Fig.2. According to Eq.(4), the dissolution rate as a function of the square root of rotating speed should be linear under the diffusion controlled conditions. The plot of the dissolution rate of Au disc versus square root of rotating speed shows a near linear relationship. So under the experimental conditions the dissolution of Ag2S with ferricyanide-cyanide solution is a diffusion- controlled process. The effect of temperature on the dissolution rate of Au disc was investigated in the temperature range of 25-55 ℃. The results are shown in Fig.3. The activation energy was calculated from the slope of the Arrhenius plot to be 6.8 kJ/mol. A typical mass transfer controlled process is thus confirmed.
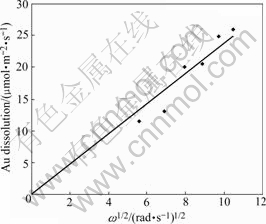
Fig.2 Effect of rotating speed on dissolution rate of Au disc (0.5 g/L NaCN, 0.5 g/L Fe as ![]() pH=11.5, 25 ℃, N2)
pH=11.5, 25 ℃, N2)
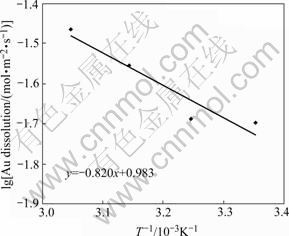
Fig.3 Effect of temperature on dissolution rate of Au disc (0.5 g/L NaCN, 0.5 g/L Fe as ![]() pH=11.5, N2, 600 r/min, N2)
pH=11.5, N2, 600 r/min, N2)
3.2 Dissolution of Ag2S disc
The dissolution of silver sulfide under different leaching conditions has been examined and the results are shown in Fig.4. In the absence of any oxidant, dissolution of silver sulfide in cyanide solution is only dependent on the following equilibrium reaction:
Ag2S+4CN-=![]() +S2- (6)
+S2- (6)
The equilibrium constant for this reaction is so small (lg K=-21 at 25 ℃, 105 Pa) that the dissolution of silver sulfide can be neglected compared with that in the presence of natural air or ferricyanide. Fig.4 shows that the dissolution rate of Ag2S disc in ferricyanide-cyanide solution (0.5 g/L Fe as ![]() gives the value of 0.78 μm/h, which is much larger than that in aerated cyanide solution (0.48 μm/h). The addition of ferrocyanide
gives the value of 0.78 μm/h, which is much larger than that in aerated cyanide solution (0.48 μm/h). The addition of ferrocyanide![]() is proved ineffective to increasing the leaching rate. The examination of sulfur speciation in the leachate shows the dominant sulfur species, thiocyanate (SCN-), accounts for more than 95% of total sulfur. These results all seem to support the view of the direct attack of silver sulfide by the ferricyanide in cyanide solution:
is proved ineffective to increasing the leaching rate. The examination of sulfur speciation in the leachate shows the dominant sulfur species, thiocyanate (SCN-), accounts for more than 95% of total sulfur. These results all seem to support the view of the direct attack of silver sulfide by the ferricyanide in cyanide solution:
Ag2S+![]() +5CN-=
+5CN-=
![]() +
+![]() +S(CN)- (7)
+S(CN)- (7)
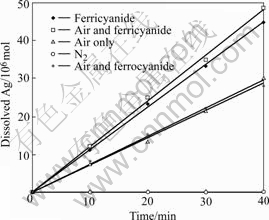
Fig.4 Dissolution of silver sulfide disc under different conditions (0.5 g/L NaCN, pH=11.5, 25 ℃, 600 r/min)
The possible formation of ![]() or
or ![]() will depend on the activity of free cyanide in the leaching solution. Further examination on the effect of rotating speed and temperature shows that the dissolution of Ag2S disc in ferricyanide-cyanide solution is also a diffusion-controlled process with the apparent activation energy of 7.8 kJ/mol[11].
will depend on the activity of free cyanide in the leaching solution. Further examination on the effect of rotating speed and temperature shows that the dissolution of Ag2S disc in ferricyanide-cyanide solution is also a diffusion-controlled process with the apparent activation energy of 7.8 kJ/mol[11].
3.3 Leaching of gold and silver ore
The gold and silver leaching results with and without the addition of ferricyanide for ore sample-A are shown in Fig.5. It is shown that both the dissolution rates of gold and silver in the ferricyanide-cyanide solution show obvious increase over that without the presence of ferricyanide. In aerated cyanide solution, the gold and silver extractions reach 62% and 24% respectively after 1 h leaching, compared with 87% and 47% in the presence of ferricyanide (0.5 g/L Fe as K3Fe(CN)6) after the same leaching time. The final gold extractions in the two cases are almost the same. The final silver extraction with the addition of ferricyanide is slightly higher than that without ferricyanide. The column leaching results for ore samples B and C are shown in Figs.6 and 7. For sample-B, the dissolution rate of silver shows a moderate increase in the presence of ferricyanide over the natural air. For sample-C, little difference on silver leaching rate is observed. It seems that the efficiency of ferricyanide is ore-specific. For the three samples, the silver extractions are all relatively low (all below 60%). The mineralogy analysis of the ore samples shows that a significant amount of fine silver particles is locked in silica, which renders the cyanidation process ineffective, even with the addition of ferricyanide. The presence of non-silver sulfide minerals such as pyrrhotite and chalcopyrite can also compete with gold/silver minerals to consume ferricyanide. It is noticed that the consumption of cyanide and alkali in ferricyanide leaching system is slightly higher than that without the addition of ferricyanide, probably due to the side reactions between these minerals and ferricyanide.
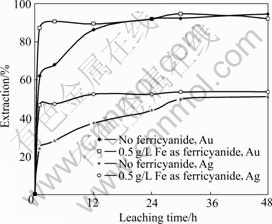
Fig.5 Plot of Au and Ag extraction from sample-A vs leaching time (0.5 g/L NaCN, pH=11.5, room temperature)
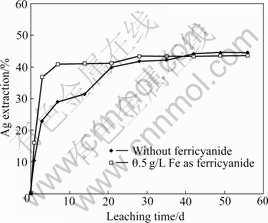
Fig.6 Plot of Ag extraction from sample-B vs leaching time (0.5 g/L NaCN, pH=11.5, room temperature)
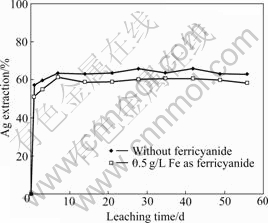
Fig.7 Plot of Ag extraction from sample-C vs leaching time (0.5 g/L NaCN, pH=11.5, room temperature)
4 Conclusions and recommendations
The leaching of pure gold and silver sulfide discs and the actual gold-silver ore with ferricyanide-cyanide solution has been examined. Ferricyanide is proves to be a powerful oxidant and both Ag2S and Au dissolution rates show significant increase in the ferricyanide- cyanide solution compared with the aerated cyanide solution. The effect of rotating speed and temperature shows that gold dissolution in ferricyanide-cyanide solution is a typical diffusion controlled process with the activation energy of 6.8 kJ/mol. The comparison leaching of gold-silver ores shows that the function of ferricyanide is ore-specific. For some ores, both the gold and silver leaching kinetics have been improved with the addition of ferricyanide and for others little effect is determined.
Ferricyanide may be potentially used as an auxiliary oxidant in the gold/silver cyanidation process, especially in heap leaching or in-situ leaching operations where the availability and reactivity of the oxidant is critical since ferricyanide has a relatively high solubility in cyanide solution. Another advantage is that ferricyanide is stable and does not react with free cyanide in alkaline cyanide solution. Ferricyanide may also find its way in treating those ores with high content of silver sulfide minerals. According to Eq.(7), theoretically, about 6 kg potassium ferricyanide is needed to recover 1 kg silver from pure silver sulfide. However, the presence of sulfide minerals such as pyrrhotite and chalcopyrite may potentially react with ferricyanide and result in high reagent cost. For those traditional cyanidation-zinc cementation operations, more work may need to be conducted to confirm the potential effect of iron-cyanide on the cementation process due to the possible formation of insoluble ferricyanide/ferrocyanide precipitates.
Acknowledgement
The authors wish to thank Dr. Jianming LU and Dr. Berend WASSINK for their kind help in the research and Barrick Gold for sponsoring the project.
References
[1] Marsden J, House I. The chemistry of gold extraction [M]. London, UK: Ellis Horwood Ltd, 1992: 230-264.
[2] Hiskey J B. Current status of U.S. gold and silver heap leaching operations [C]// Hiskey J B. Au & Ag Heap and Dump Leaching Practice. Colorado, US: AIME, 1983: 1-7.
[3] Gasparrini C. The mineralogy of silver and its significance in metal extraction [J]. CIM Bulletin, 1984, 77(86): 99-110.
[4] Cruells M, Roca A, Pati?o F, Salinas E, Rivera I. Cyanidation kinetics of argentian jarosite in alkaline media [J]. Hydrometallurgy, 2000, 55(2): 153-165.
[5] Luna R M, Lapidus G T. Cyanidation kinetics of silver sulfide [J]. Hydrometallurgy, 2000, 56(2): 171-188.
[6] Cruz R, Luna-Sánchez R M, Lapidus G T, González I, Monroy M. An experimental strategy to determine galvanic interactions affecting the reactivity of sulfide mineral concentrates [J]. Hydrometallurgy, 2005, 78(2): 198-208.
[7] Loroesch J, Knorre H, Griffiths A. Developments in gold leaching using hydrogen peroxide [J]. Mining Engineering, 1989, 41(9): 963-965.
[8] Dutrizac J E. The leaching of silver sulfides in ferric ion media [J]. Hydrometallurgy, 1994, 35(3): 275-292.
[9] Nugent A, Brackenbury K, Kinner J. AuPLUS systems for the treatment of gold ores using hydrogen peroxide and calcium peroxide [C]// World Gold’91. Queensland: AIMMEM, 1991: 173-176.
[10] Deschenes G, Rousseaub M, Tardifc J, Prud'hommea P J H. Effect of the composition of some sulphide minerals on cyanidation and use of lead nitrate and oxygen to alleviate their impact [J]. Hydrometallurgy, 1998, 50(2): 201-205.
[11] Xie F, Dreisinger D. Leaching of silver sulfide with ferricyanide-cyanide solution [J]. Hydrometallurgy, 2007, 88(1/4): 98-108.
[12] Levich V G. Physicochemical hydrodynamics [M]. Englewood Cliffs, New Jersey: Prentice Hall, 1962: 688-689.
[13] Jeffrey M I, Ritchie I M. The leaching and electrochemistry of gold in high purity cyanide solutions [J]. Journal of Electrochemical Society, 2001, 148(4): D29-D36.
[14] Wadsworth M E. Surface processes in silver and gold cyanidation [J]. International Journal of Minerals Processing, 2000, 58(1/4): 351-368.
[15] Guzman L, Segarra M, Chimenos J M, Cabot P L, Espiell F. Electrochemistry of conventional gold cyanidation [J]. Electrochimica Acta, 1999, 44(16): 2625-2632.
Corresponding author: F. XIE; Fax: +1-604-822-3619; E-mail: xiefeng@interchange.ubc.ca
DOI: 10.1016/S1003-6326(08)60338-6
(Edited by YANG Bing)


