文章编号:1004-0609(2013)07-1914-09
2A97铝锂合金等离子电解氧化膜的制备和耐蚀性能
薛志刚1,程英亮1,吴湘权1,吴有伍1,李劲风2
(1. 湖南大学 材料科学与工程学院,长沙 410082;
2. 中南大学 材料科学与工程学院,长沙 410083)
摘 要:
摘 要:研究2A97铝锂合金在硅酸盐电解液中等离子电解氧化膜的生长规律,分析直流条件下电流密度、氧化时间等电参数对氧化陶瓷膜的表面和截面形貌、成分和相组成以及耐蚀性的影响。结果表明:等离子电解氧化处理提高了铝锂合金的耐腐蚀性能,但处理时间过长,膜层微观结构中缺陷增多,对膜层的耐蚀性带来不利影响,提高电流密度对膜层的耐蚀性也没有明显的影响。铝锂合金的等离子电解氧化膜微观结构中存在两类不同放电类型导致的特征结构,其中来源于基体/膜层界面的火花放电生成的结构含有较多的Al,而氧化膜表层或浅层的火花放电主要在膜层表面生成含有较多电解液成分的菜花状结构。膜层的相结构主要为γ-Al2O3。
关键词:
中图分类号:TG174. 2 文献标志码:A
Preparation and corrosion resistance of plasma electrolytic oxidation coatings on 2A97 aluminium-lithium alloy
XUE Zhi-gang1, CHENG Ying-liang1, WU Xiang-quan1, WU You-wu1, LI jing-feng2
(1. College of Materials Science and Engineering, Hunan University, Changsha 410082, China;
2. School of Materials Science and Engineering, Central south University, Changsha 410083, China)
Abstract: The plasma electrolytic oxidation(PEO) processes of 2A97 aluminium-lithium alloy in silicate electrolyte were studied. The PEO processes were carried out with DC regime, and the factors such as current density and treatment time on the morphologies of surface and the cross section of the coatings and the corrosion resistance of coatings were analyzed. The results show that the PEO processing enhances the corrosion resistance of the aluminium-lithium alloy, however, the corrosion resistance will be deteriorated by long processing time, due to more defects developed in the coatings, and the corrosion resistance of the coatings is also little affected by increasing the current density. There are two types of microstructures existing in the PEO coatings of aluminium-lithium alloy, which are resulted from the different types of discharging. The microstructure resulted from the discharge originated at substrate/coating interface contains more content of Al, while the discharge happening on the surface or shallow depth of PEO coating causes the formation of the cauliflower-like structure with electrolyte species on the surface of the PEO coatings. The main phase composion of the coatings is γ-Al2O3.
Key words: aluminium-lithium alloy; plasma electrolytic oxidation; corrosion resistance
2A97铝锂合金是我国自主研发的Al-Cu-Li系铝合金,具有低密度、高比模量、高比强度和高比刚度等良好的综合性能,与一般铝合金相比,采用铝锂合金可使构件质量减轻10%~15%,刚度提高15%~20%[1],因此,在航空航天等领域具有广阔的应用前景。但由于锂是一种非常活泼的元素,铝锂合金在潮湿和盐雾等环境中极易发生腐蚀,从而导致力学性能下降和使用寿命缩短[2-3],有关研究表明,高的晶间腐蚀敏感性与由表面引起的剥离腐蚀是铝锂合金发生腐蚀的主要原因[4-5],所以有必要采用适当的工艺技术克服这方面的不足。
等离子电解氧化技术(Plasma electrolytic oxidation,PEO),又称微弧氧化或阳极火花沉积,可以在特定的电解液中,通过等离子放电和电化学过程的共同作用,在铝[6-12]、镁[13-17]、钛[18-23]、锆[24-28]等有色金属及其合金材料表面原位生长氧化物陶瓷膜,赋予金属表面耐磨、耐蚀、以及生物相容性等一系列性能。等离子电解氧化过程工作在金属表面氧化物的击穿电位(400~600 V)之上,该技术起源于100多年前金属在电解液中施加阳极电流时表面火花放电现象的发现,但直到20世纪70—80年代,前苏联和德国等的科学家才将其扩展到工业方面的应用,如提高镁、铝合金的耐磨性与耐腐蚀性能[29]。VOEVODIN等[6]研究了主要合金成分为锌、镁、铜的B95铝合金在硅酸盐溶液中的PEO行为,得到了Al-Si-O为主要成分的PEO膜层,由于硅的参与,膜层由硬度高的Al2O3相和较软的Al2O3/SiO2混合相组成,膜层的耐磨性能得到了较大的提高。根据YEROKHIN等[29]的说法,铝合金上的PEO膜层通常分为三层:主要由非晶相组成的多孔的外层、受高温影响而形成的致密的内层以及很薄的一层界面层。又如MONFORT等[30]研究了纯铝在Na2CO3/KOH电解液体系的直流PEO氧化膜,发现膜层大致分为两层,而且膜层相结构、形貌等受电解液中硅酸盐的含量影响很大。
虽然目前有关铝合金的等离子电解氧化的研究已较多[6-12, 29-30],但由于等离子电解氧化技术的复杂性,关于等离子放电过程的机理及膜层的生长机理、微观结构的研究远未完善[30]。而且,具体到铝锂合金的等离子氧化技术,国内外这方面的工作还有待开展,只有很少的文献报道[1, 31]。
本文作者利用直流等离子电解氧化技术,成功在硅酸钠溶液中制得2A97铝锂合金PEO膜层,并对膜层的微观结构、相组成及耐腐蚀性能进行了初步的研究。
1 实验
本研究工作所采用材料为2A97铝锂合金板材(名义成分为(质量分数):0.15%Si、0.15%Fe、2.0%~3.2%Cu、0.20%~0.6%Mn、0.25%~0.50%Mg、0.17%~1.0%Zn、0.001%~0.10%Be、0.8%~2.3%Li、0.001%~0.10%Ti、0.08%~0.20%Zr,GB/T3190—2008),板材经热处理(520 ℃固溶处理2 h,淬火;165 ℃时效24 h,空冷)。将板材切割成尺寸为10 mm×10 mm×4 mm的试样,试样的一面与铜导线相连,然后用环氧树脂进行包封,制成面积为1 cm2的电极。电极经金相砂纸逐级打磨,最后用蒸馏水及酒精清洗,吹干后置于干燥器中备用。PEO处理采用由8 g/L的Na2SiO3·9H2O与1 g/L的KOH混合的电解液,由蒸馏水配制。实验电源采用DYY-6C直流电源(规格600 V,400 mA)。试样作为阳极,不锈钢板(面积约为80 cm2) 作为阴极,放在电解液中,通上电源进行实验。实验过程中,用恒温磁力搅拌器对电解液进行搅拌,以利于散热,并用冷却水对电解液进行冷却,控制电解液的温度低于50 ℃。实验完成后,将电极取出,用蒸馏水冲洗、吹干后备用。
采用TT260覆层测厚仪测量PEO膜层的厚度,测试12次,记录平均值和标准差。膜层的表面和横截面微观形貌及成分采用环境扫描电子显微镜(QUANTA 2000,FEI,USA)观察和EDS能谱仪进行表征。膜层的相结构采用SIMENS D5000型X射线衍射仪进行分析。
2A97铝锂合金PEO膜层的耐蚀性通过动电位极化曲线来评价,将其与基体合金的数据进行比较。电化学测试在CHI660C电化学工作站上进行, 采用三电极体系,将2A97铝锂合金或经PEO处理的试样作为工作电极,铂片为辅助电极,饱和甘汞电极(SCE)为参比电极, 在3.5%中性NaCl溶液中进行测试,试验中先将电极浸泡10 min,待体系稳定后进行极化曲线测量,从开路电位-500 mV向正向扫描,扫描速度为1 mV/s。
2 结果与分析
2.1 2A97铝锂合金PEO过程槽电压—时间曲线
图1所示为2A97 铝锂合金在100 mA/cm2电流密度下进行PEO处理的槽电压和时间的关系。从图1可看出,在PEO开始的20 s 内,电压以将近20 V/s的线性关系急速上升,根据相关文献的观点,该过程对应于常规的阳极氧化膜生长过程[32]。当电位达到一定的值后,电极表面出现火花(在本实验中观察到火花出现的时间是5 s,到15 s时火花剧烈),这是等离子放电的结果,对应于氧化膜的介电击穿。在20 s以后,电位上升速度大幅度衰减,在1 800 s试验终止时的电位是503 V,在20~1 800 s期间,电位上升的速度为0.056 7 V/s。
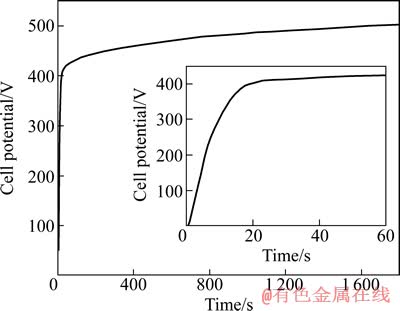
图1 2A97铝锂合金在100 mA/cm2电流密度下PEO过程的时间电位曲线
Fig. 1 Voltage—time curve during PEO process of 2A97 aluminium-lithium alloy at current density of 100 mA/cm2
2.2 PEO膜层生长的动力学曲线
分别在不同的电流密度下测量了膜层的生长动力学曲线,结果如图2所示。从图2可看出,电流密度为200 mA/cm2时,膜层的生长速度较快,处理10 min 时,PEO膜层的厚度为23.5 μm,而电流密度为100 mA/cm2时,PEO膜层的厚度为13.3 μm。
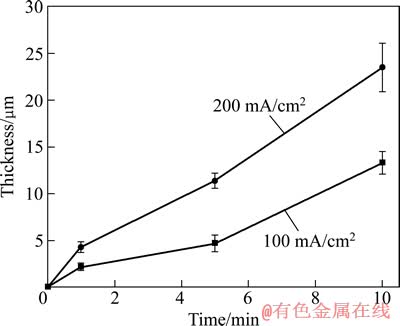
图2 2A97铝锂合金PEO过程的膜层生长动力学曲线
Fig. 2 Growth kinetics of PEO coatings of 2A97 aluminium-lithium alloy
2.3 2A97铝锂合金PEO膜层极化曲线测试结果
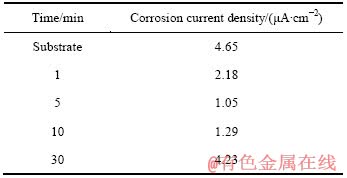
将100 mA/cm2 的电流密度下处理不同时间(1、5、10和30 min)的试样以及未经PEO处理的2A97铝锂合金在3.5% NaCl溶液中进行极化曲线测试,结果如图3所示。从图3可看出,铝锂合金经PEO处理后,腐蚀电位正移,阳极极化曲线分支受抑制,表明经处理后合金的耐蚀性能得到了提高。但是,不同处理时间的极化曲线和未经处理的基体合金都比较接近,说明耐蚀性能的提高并不是很明显。利用塔菲尔外推法对图3的极化曲线数据进行处理,得到不同试样的腐蚀电流密度数据,结果如表1所列。

图3 2A97铝锂合金在100 mA/cm2电流密度下经过不同处理时间的PEO膜层的极化曲线
Fig. 3 Potentiodynamic polarization curves of 2A97 aluminium-lithium alloy substrate and PEO coatings with different treatment times at current density of 100 mA/cm2
表1 对应图3的不同试样的腐蚀电流密度
Table 1 Free corrosion current densities of substrate and PEO coatings corresponding to Fig. 3
为考察不同电流密度对PEO膜层的耐蚀性的影响,对在200 mA/cm2下制得的膜层也进行了电化学测试,其结果如图4所示,腐蚀电流密度列于表2中。从表2可看出,在200 mA/cm2制得的膜层的耐蚀性同在100 mA/cm2制得的膜层的耐蚀性相近,而且随着处理时间的延长,膜层的耐蚀性有下降的趋势,这种耐蚀性的下降可能与膜层中缺陷(如微裂纹、孔洞等)的增多有关。
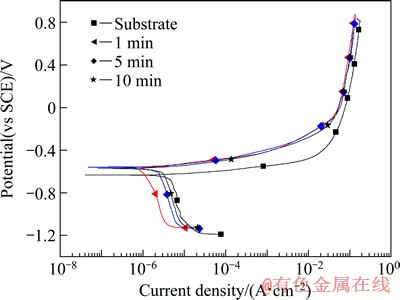
图4 2A97铝锂合金基体及在200 mA/cm2电流密度下经过不同处理时间的PEO膜层的极化曲线
Fig. 4 Potentiodynamic polarization curves of 2A97 aluminium-lithium alloy substrate and PEO coatings underwent for different treatment times at constant current density of 200 mA/cm2
2.4 2A97铝锂合金PEO膜层微观结构
图5所示为在100 mA/cm2 的电流密度下,分别处理1、5、10和30 min 的2A97 铝锂合金PEO膜层的截面形貌。从图5(a)可看出,处理1 min 后, 膜层的厚度约为1.2 μm。随着处理时间的延长,膜层的厚度增加,与处理5 min和10 min的膜层相比,处理30 min的膜层厚度有大幅度的提高,厚度将近40 μm,然而与处理时间短的膜层相比,其微观结构中的裂纹明显增多。这也就可以解释表1中腐蚀测试的结果:由于处理30 min的试样微观缺陷的增多,导致其腐蚀电流密度的增加。
表2 对应图4的不同试样的腐蚀电流密度
Table 2 Free corrosion current densities of substrate and PEO coatings corresponding to Fig. 4

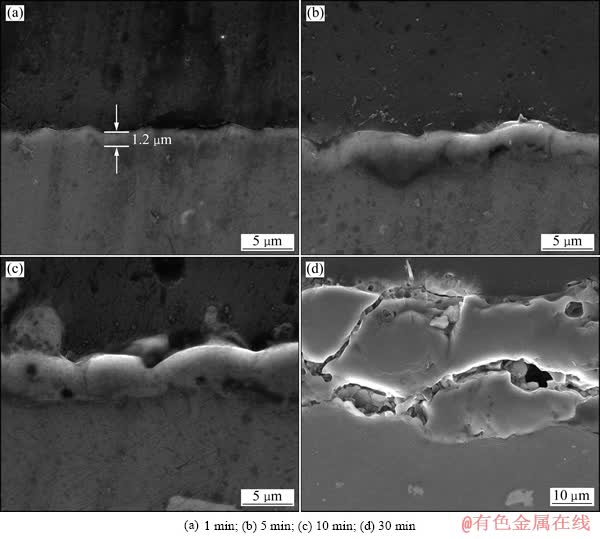
图5 不同处理时间的铝锂合金PEO膜层的截面二次电子像
Fig. 5 Second electron images of cross-sections of 2A97 aluminium-lithium alloy PEO coatings with different treatment times
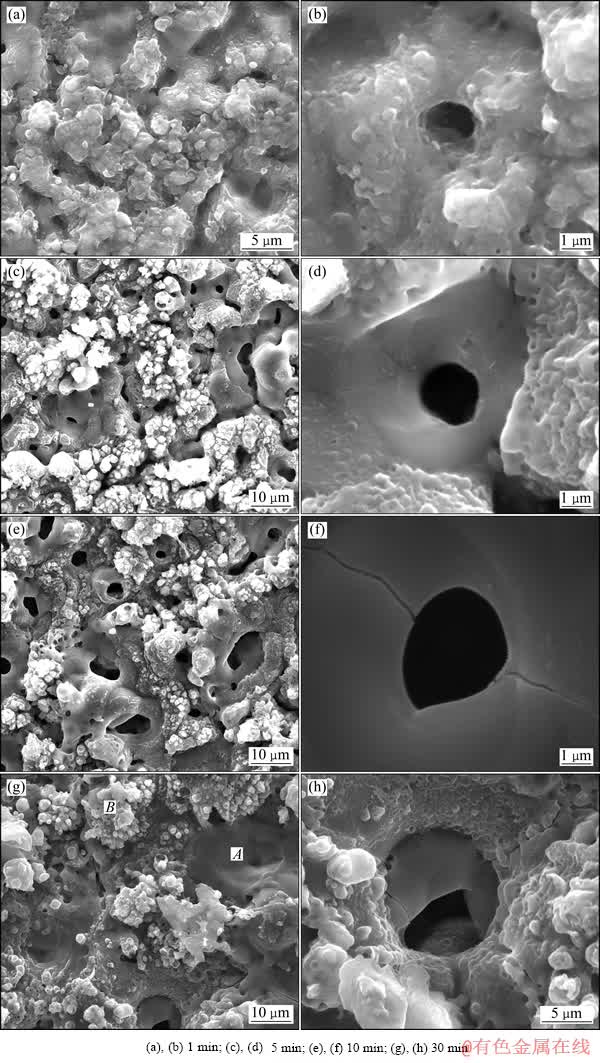
图6 不同处理时间的铝锂合金PEO膜层微观形貌
Fig. 6 Surface morphologies of 2A97 aluminium-lithium alloy PEO coatings with different treatment times
图6所示为不同处理时间的铝锂合金PEO膜层的表面形貌。从图6(a)可知,处理1 min的膜层表面微孔较少,但从微观上看,表面并不平整,存在很多高低起伏。从图6(b)可知,膜中存在微小孔洞,其尺寸在1 μm左右,这些微孔很可能是等离子氧化过程中气体的通道。在等离子氧化过程中阳极上产生的气体主要是氧气,而且氧的生成量超过了阳极上法拉第过程所产生的氧量[33]。在等离子氧化过程中,阳极电位在100 V以上,水分子可以在电极表面依靠电化学过程失电子分解生成氧气分子。此外,等离子体的温度可达4 000~7 000 K[34],等离子体极高的温度甚至可将水分子直接分解成氢和氧(水在2 700和3 700 ℃时,分解成氧气的量分别可达1.4%和15%),因此,水的热分解可能是电极上产生过量氧气的原因[33]。从图6 (d)、(f)和(h)可知,在不同处理时间的PEO膜上均有微孔结构,从图6(f)和(h)可知,膜层表面存在贯穿微孔的裂纹,这些裂纹是凝固过程热应力释放的结果。从图6(c)和(e)可知,随着处理时间从5 min延长到10 min,PEO膜表面的微孔尺寸增加,而处理30 min的试样的表面微孔数目有所减少。从图6(g)可知,处理30 min 的试样表面存在如A点所示凝固结构及如B点所示的突出表面的不规则菜花状结构。这两种结构在处理时间短的试样,如处理5和10 min的试样的表面也同样存在。对A点和B点所示结构的成分进行EDS能谱分析,结果表明,A点所示结构的成分为(摩尔分数,%):O 56.65,Al 39.13,Si 4.22。而B点所示的菜花状结构的成分为(摩尔分数,%):C 38.73,O 45.59,Na 2.46,Al 0.85,Si 11.62,K 0.74(C含量很明显是样品污染的结果)。从能谱分析的结果可知,A点和B点在成分上的差别主要体现在铝和硅的含量上,A点的铝含量非常高而硅含量较低,而B点的铝含量和硅含量恰好与其相反。这种现象和HUSSEIN等[35]对纯铝在硅酸盐溶液中的PEO膜层的研究中观察到的现象一致。根据HUSSEIN等[35]的研究,PEO过程存在3种不同类型的火花放电:第一种为从金属-氧化膜界面开始,穿过氧化膜直到膜层-电解液界面进行放电,命名为B型火花放电;第二种和第三种分别是在靠近电极表面或相对较深的微孔中的放电,分别称为的A和C放电类型。B型放电强度最大,温度最高,由于它发源于金属基体和氧化膜的界面,因此由其产生的表面凝固组织中基体金属的含量很高。而A和C型放电发生于膜层的上部或表层,由其产生的凝固组织中来自电解液的成分较多。这个模型很好地解释了本实验中观察到的实验结果,即图6(g)中的A点所示的结构很明显是B型火花放电的结果,含铝量非常高,而 B点所示的菜花状结构应是A或C型火花放电的结果,含有较多的来自电解液的硅成分。
2.5 膜层的相结构
对100 mA/cm2下处理10 min的试样进行了XRD测试,其结果如图7所示。
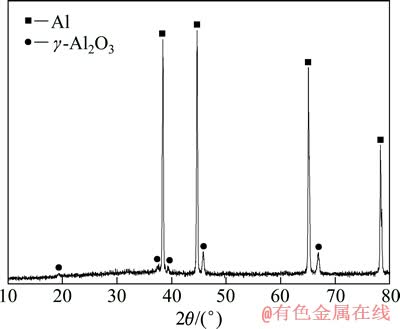
图7 在100 mA/cm2的电流密度下处理10 min的铝锂合金PEO膜层的XRD谱
Fig. 7 XRD patterns of aluminium-lithium alloy PEO coatings with treatment time of 10 min and current density of 100 mA/cm2
对于纯铝在硅酸盐溶液中的直流PEO膜层相结构,根据MONFORT等[30]的研究,其膜层主要相为γ-Al2O3和α-Al2O3,其中γ-Al2O3占据主导地位,此外,当PEO过程的电压升高到470 V 以上时,膜层中还会出现少量的δ-Al2O3。当电解液中硅酸盐的浓度提 高到一定的程度,膜层中非晶相(可能是SiO2)成分增加,XRD衍射曲线会出现明显的非晶包,此外,可能会出现Al2SiO5或Al6Si2O13 相。Na2SiO3在PEO过程的作用是可以提高膜层的生长速度,同时还可以抑制铝在碱性溶液中的活性溶解[36]。此外,提高Na2SiO3浓度还可能生成以莫来石(3Al2O3-2SiO2)为主要成分的膜层,莫来石具有良好的热和化学稳定性[36]。
从图7可知,与MONFORT等[30]的研究类似,本研究中膜层主要相结构是γ-Al2O3。膜层中没有出现莫来石、Al2SiO5或Al6Si2O13 等相,其原因应是本研究所采用的电解液中硅酸盐含量较低,当然,膜层中可能存在一定量的含硅的非晶相,因为EDS能谱结果表明膜中存在硅,而在图7中没有体现。此外,由于EDS的局限,能谱分析中不能检测到锂,而且图7的XRD也没有显示锂的氧化物的存在,这是因为合金中的锂的含量毕竟较少,没有达到XRD能检测到的精度值,但并不能否认在铝锂合金的PEO膜层中有锂的氧化物存在。关于膜层中相的问题,需要深入进行讨论,通常γ-Al2O3 是一种不稳定相,它的形成和PEO过程的放电和冷却特征有关。在PEO过程中,在单一的火花放电熄灭后,放电过程的熔融物质和电解质接触,将会遭遇高达108 K/s 的冷却速度[29]。作为这种激冷的结果,将在放电通道的靠近表面的部位、靠近基体的位置以及在放电通道的内壁形成非晶相和如γ-Al2O3这种非稳定相。然而,当这些非稳定相形成后,在其附近的膜层中的后续放电、击穿过程中(指穿过氧化膜的B型放电),热效应将会使这些非稳态γ-Al2O3转变为热力学稳定的α-Al2O3。此外,PEO过程中还存在着表面气体中的放电或氧化膜局部厚度区间的放电(相当于前面提到的A和C型放电)。在这两种情况下,都将会促使PEO膜层发生晶化、退火和烧结过程,同时导致γ-Al2O3向α-Al2O3的转变[36]。本实验的XRD结果表明,铝锂合金直流PEO膜层中主要为不稳定的γ-Al2O3相,这一点和文献[36]的结果不同,其原因可能为本实验处理的时间较短,膜层较薄(图7中对应的处理10 min的PEO膜层厚度为13.3 μm),膜层更容易受到激冷的影响形成非稳态的γ-Al2O3,而文献[36]中PEO膜层的处理时间是350 min,膜层厚度约为120 μm,膜层因经历了更多的持续放电,受到热效应的影响而形成α-Al2O3。
3 结论
1) 采用直流等离子电解氧化技术,在硅酸盐电解液中制得2A97铝锂合金PEO膜层。在100 mA/cm2条件下,处理30 min时所得PEO膜层的厚度达到40 μm左右。
2) 电化学极化曲线结果表明,PEO处理提高了铝锂合金的耐蚀性,但处理时间过长,对膜层的耐蚀性不利,而且提高电流密度到200 mA/cm2时,制得的膜层的耐蚀性也没有明显的区别。处理时间长的试样的耐蚀性下降是膜层微观结构中缺陷增多的结果。
3) 铝锂合金的等离子电解氧化膜微观结构中存在两类不同放电类型导致的特征结构,B型放电生成的结构含有较多的Al,而A和C型放电在膜层的表面生成还有较多电解液成分硅的菜花状结构。XRD结果表明,膜层的相结构主要为γ-Al2O3。
REFERENCES
[1] 刘一洋, 赵志龙, 阎光明, 侯军占. 铝锂合金微弧氧化陶瓷膜层特性的研究[J]. 电镀与环保, 2007, 27(2): 25-27.
LIU Yi-yang, ZHAO Zhi-long, YAN Guang-ming, HOU Jun-zhan. Properties research of ceramic coating on Al-Li alloy by micro-arc oxidation[J]. Electroplating and Pollution Control, 2007, 27(2): 25-27.
[2] 梁文杰, 潘清林, 李运春, 何运斌, 李文斌, 周迎春, 路聪阁. 新型Al-Cu-Li-(Sc+Zr)合金的剥蚀行为[J]. 中国腐蚀与防护学报, 2009, 29(1): 30-34.
LIANG Wen-jie, PAN Qing-lin, LI Yun-chun, HE Yun-bin, LI Wen-bin, ZHOU Ying-chun, LU Cong-ge. Exfollation corrosion behaviors of Al-Cu-Li-(Sc+Zr) alloy[J]. Journal of Chinese Society for Corrosion and Protection, 2009, 29(1): 30-34.
[3] 黄兰萍, 陈康华, 李 松, 宋 旼. 高温预析出后7055铝合金局部腐蚀性能和时效硬[J]. 稀有金属材料与工程, 2007, 36(9): 1628-1633.
HUANG Lan-ping, CHEN Kang-hua, LI Song, SONG Min. Influence of high-temperature pre-precipitation on local corrosion behaviors and ageing harden of 7055 aluminum alloy[J]. Rare Metal Materials and Engineering, 2007, 36(9): 1628-1633.
[4] 李劲风, 张 昭, 张鉴清, 曹楚南. 铝-锂合金腐蚀性能研究综述[J]. 中国腐蚀与防护学报, 2003, 23(5): 316-320.
LI Jin-feng, ZHANG Zhao, ZHANG Jian-qing, CAO Chu-nan. Review of research on corrosion behavior of Al-Li alloys[J]. Journal of Chinese Society for Corrosion and Protection, 2003, 23(5): 316-320.
[5] 李劲风, 郑子樵, 李世晨, 谭澄宇, 梁 英, 于利军.2195铝-锂合金晶间腐蚀及剥蚀行为研究[J]. 材料科学与工程学报, 2004, 22(5): 640-643.
LI Jin-feng, ZHENG Zi-qiao, LI Shi-chen, TAN Cheng-yu, LIANG Ying, YU Li-jun. Study on intergranular corrosion and exfoliation corrosion behaviors of 2195 Al-Li alloy[J]. Journal of Materials Science and Engineering, 2004, 22(5): 640-643.
[6] VOEVODIN A A, YEROKHIN A L, LYUBIMOV V V, DONLEY M S, ZABINSKI J S. Characterization of wear protective Al-Si-O coatings formed on Al-based alloys by micro-arc discharge treatment[J]. Surface and Coating Technology, 1996, 86/87(2): 516-521.
[7] RUDNEV V S, LYSENKO A E, NEDOZOROV P M, YAROVAYA T P, MINAEV A N. Thermal behaviour of an oxide layer on aluminum[J]. Protection of Metals, 2007, 43(5): 465-469.
[8] LUKIYANCHUK I V, RUDNEV V S, KURYAVYI V G., BOGUTA D L, BULANOVA S B, GORDIENKO P S. Surface morphology, composition and thermal behaviour of tungsten containing anodic spark coatings on aluminium alloy[J]. Thin Solid Films, 2004, 446(1): 54-60.
[9] RAMA KRISHNA L, SOMARAJU K R C, SUNDARARAJAN G. The tribological performance of ultra-hard ceramic composite coatings obtained through microarc oxidation[J]. Surface and Coating Technology, 2003, 163/164: 484-490.
[10] CURRAN J A, CLYNE T W. The thermal conductivity of plasma electrolytic oxide coatings on aluminium and magnesium[J]. Surface and Coating Technology, 2005, 199(2): 177-183.
[11] 杨 巍, 蒋百灵, 时惠英. LY12铝合金微弧氧化膜层的形成与生长机制[J]. 中国有色金属学报, 2010, 20(10): 1949-1954.
YANG Wei, JIANG Bai-ling, SHI Hui-ying. Formation and growth mechanism of microarc oxidation coating on LY12 aluminium alloy[J]. The Chinese Journal of Nonferrous Metals, 2010, 20(10): 1949-1954.
[12] 杨 巍, 蒋百灵, 鲜林云, 时惠英. 溶质离子在铝合金微弧氧化陶瓷膜形成过程中的作用机理[J]. 中国有色金属学报, 2009, 19(3): 464-468.
YANG Wei, JIANG Bai-ling, XIAN Lin-yun, SHI Hui-ying. Action mechanism of solute ions on forming process of microarc oxidation coatings on aluminium alloy[J]. The Chinese Journal of Nonferrous Metals, 2009, 19(3): 464-468.
[13] 韩晓光, 雷明凯, 朱小鹏, 单英春, 徐久军. 强流脉冲离子束辐照镁合金微弧氧化膜的耐腐蚀性能[J]. 中国有色金属学报, 2012, 22(2): 337-342.
HAN Xiao-guang, LEI Ming-kai, ZHU Xiao-peng, SHAN Ying-chun, XU Jiu-jun. Anti-corrosion properties of micro-arc oxidation films on AZ31 magnesium alloy irradiated by high-intensity pulsed ion beam[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(2): 337-342.
[14] 蒋永锋, 杨华山, 包晔峰, 刘益阳. 镁合金微弧氧化陶瓷层表面的电泳成膜机理[J]. 中国有色金属学报, 2011, 21(7): 1562-1569.
JIANG Yong-feng, YANG Hua-shan, BAO Ye-feng, LIU Yi-yang. Formation mechanism of electrophoretically deposited organic layer on PEO film of magnesium alloy[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(7): 1562-1569.
[15] ZHANG L J, FAN J J, Zhang Z, CAO F H, ZHANG J Q, CAO C N. Study on the anodic film formation process of AZ91D magnesium alloy[J]. Electrochimica Acta, 2007, 52(17): 5325-5333.
[16] WU C S, ZHANG Z , CAO F H, ZHANG L J , ZHANG J Q, CAO C N. Study on the anodizing of AZ31 magnesium alloys in alkaline borate solutions[J]. Applied Surface Science, 2007, 253(8): 3893-3898.
[17] WU Hai-lan, CHENG Ying-liang, CHEN Zhen-hua, WANG Hui-min, ZHANG Zhao. The anodization of ZK60 magnesium alloy in alkaline solution containing silicate and corrosion properties of the anodized films[J]. Applied Surface Science, 2007, 253(24): 9387-9394.
[18] 王艳玲, 惠松骁, 叶文君, 米绪军. 医用钛合金的微弧氧化膜层组织及其磨损性能[J]. 中国有色金属学报, 2010, 20(S1): s188-s192.
WANG Yan-ling, HUI Song-xiao, YE Wen-jun, MI Xu-jun. Microstructure and its wear property of micro-arc oxidation coating on biomedical titanium alloy[J]. The Chinese Journal of Nonferrous Metals, 2010, 20(S1): s188-s192.
[19] LIU X Y, CHU P K, DING C X. Surface modification of titanium, titanium alloys, and related materials for biomediacal applications[J]. Material Science and Engineering A, 2004, 47(3/4): 49-121.
[20] MARTINI C, CESCHINI L, TARTERINI F, PAILLARD J M, CURRAN J A. PEO layers obtained from mixed aluminate- phosphate baths on Ti-6Al-4V: Dry sliding behaviour and influence of a PTFE topcoat[J]. Wear, 2010, 269(11/12): 747-756.
[21] KHORASANIAN M, DEHGHAN A, SHARIAT M H, BAHROLOLOOM M E, JAVADPOUR S. Microstructure and wear resistance of oxide coatings on Ti-6Al-4V produced by plasma electrolytic oxidation in an inexpensive electrolyte[J]. Surface and Coating Technology, 2011, 206(6): 1495-1502.
[22] WANG Y M, JIA D C, GUO L X, LEI T Q, JIANG B L. Effect of discharge pulsating on microarc oxidation coatings formed on Ti6Al4V alloy[J]. Material Chemistry and Physics, 2005, 90(1): 128-133.
[23] YEROKHIN A L, NIE X, LEYLAND A, MATTHEWS A. Characterization of oxide films produced by plasma electrolytic oxidation of a Ti-6Al-4V alloy[J]. Surface and Coating Technology, 2000, 130(2/3): 195-206.
[24] CHENG Ying-liang, WU Fan. Plasma electrolytic oxidation of Zircaloy-4 alloy with DC regime and properties of coatings[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(7): 1638-1646.
[25] CHENG Y, MATYKINA E, SKELDON P, THOMPSON G. Characterization of plasma electrolytic oxidation coatings on Zircaloy-4 formed in different electrolytes with AC current regime[J]. Electrochimica Acta, 2011, 56(24): 8467-8476.
[26] CHENG Y, MATYKINA E , ARRABAL R, SKELDON P, THOMPSON G E. Plasma electrolytic oxidation and corrosion protection of Zircaloy-4[J]. Surface and Coating Technology, 2012, 206(14): 3230-3239.
[27] CHENG Y, WU F, MATYKINA E, SKELDON P, THOMPSON G E. The influences of microdischarge types and silicate on the morphologies and phase compositions of plasma electrolytic oxidation coatings on Zircaloy-2[J]. Corrosion Science, 2012, 59: 307-315.
[28] 程英亮, 伍 帆. Cu2+及等离子电解氧化处理对Zr-4合金腐蚀性能的影响[J]. 中国有色金属学报, 2012, 22(6): 1616-1623.
CHENG Ying-liang, WU Fan. Effects of Cu2+ and plasma electrolytic oxidation on corrosion properties of Zircaloy-4 alloy[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(6): 1616-1623.
[29] YEROKHIN A L, NIE X, LEYLAND A, MATTHEWS S J, DOWEY S J. Plasma electrolysis for surface engineering[J]. Surface and Coatings Technology, 1999, 122(2/3): 73-93.
[30] MONFORT F, BERKANI A, MATYKINA E, SKELDON P, THOMPSON G E, HABAZAKI H, SHIMIZU K, Development of anodic coatings on aluminium under sparking conditions in silicate electrolyte[J]. Corrosion Science, 2007, 49(2): 672-693.
[31] 赵志龙, 刘一洋, 阎光明. 2090铝-锂合金微弧氧化陶瓷膜层特性的研究[J]. 电镀与环保, 2008, 28(5): 36-38.
ZHAO Zhi-long, LIU Ui-yang, YAN Guang-ming. Research on properties of ceramic coating on 2090 Al-Li alloy by micro-arc oxidation[J]. Electroplating and Pollution Control, 2008, 28(5): 36-38.
[32] DUAN H, YAN C, WANG F. Growth process of plasma electrolytic oxidation films formed on magnesium alloy AZ91D in silicate solution[J]. Electrochimica Acta, 2007, 52(15): 5002-5009.
[33] SNIZHKO L O, YEROKIN A L, PILKINGTON A, GUREVINA N L, MISNYANKIN D O, LEYLAND A, MATTHEWS A. Anodic processes in plasma electrolytic oxidation of aluminium in alkaline solutions[J]. Electrochimica Acta, 2004, 49(13): 2085-2095.
[34] HUSSEIN R O, NIE X, NORTHWOOD D O. Influence of process parameters on electrolytic plasma discharging behaviour and aluminum oxide coating microstructure[J]. Surface and Coating Technology, 2010, 205(6): 1659-1667.
[35] HUSSEIN R O, NIE X, NORTHWOOD D O, YEROKIN A, MATTHEWS A. Spectroscopic study of electrolytic plasma and discharging behaviour during the plasma electrolytic oxidation(PEO) process[J]. Journal of Physics D: Applied Physics, 2010, 43(10): 105203.
[36] KALKANCI H, KURNAZ S C. The effect of process parameters on mullite-based plasma electrolytic oxide coatings[J]. Surface and Coatings Technology, 2008, 203(1/2): 15-22.
(编辑 何学锋)
基金项目:国家自然科学基金资助项目(51071066);教育部新世纪人才支持计划资助项目(NCET-12-0172);湖南大学“青年教师成长计划”资助项目(531107040029)
收稿日期:2012-09-25;修订日期:2012-12-27
通信作者:程英亮,副教授,博士;电话:13036798588;E-mail:chengyingliang@hnu.edu.cn
[1] 刘一洋, 赵志龙, 阎光明, 侯军占. 铝锂合金微弧氧化陶瓷膜层特性的研究[J]. 电镀与环保, 2007, 27(2): 25-27.
[2] 梁文杰, 潘清林, 李运春, 何运斌, 李文斌, 周迎春, 路聪阁. 新型Al-Cu-Li-(Sc+Zr)合金的剥蚀行为[J]. 中国腐蚀与防护学报, 2009, 29(1): 30-34.
[3] 黄兰萍, 陈康华, 李 松, 宋 旼. 高温预析出后7055铝合金局部腐蚀性能和时效硬[J]. 稀有金属材料与工程, 2007, 36(9): 1628-1633.
[4] 李劲风, 张 昭, 张鉴清, 曹楚南. 铝-锂合金腐蚀性能研究综述[J]. 中国腐蚀与防护学报, 2003, 23(5): 316-320.
[5] 李劲风, 郑子樵, 李世晨, 谭澄宇, 梁 英, 于利军.2195铝-锂合金晶间腐蚀及剥蚀行为研究[J]. 材料科学与工程学报, 2004, 22(5): 640-643.
[11] 杨 巍, 蒋百灵, 时惠英. LY12铝合金微弧氧化膜层的形成与生长机制[J]. 中国有色金属学报, 2010, 20(10): 1949-1954.
[12] 杨 巍, 蒋百灵, 鲜林云, 时惠英. 溶质离子在铝合金微弧氧化陶瓷膜形成过程中的作用机理[J]. 中国有色金属学报, 2009, 19(3): 464-468.
[13] 韩晓光, 雷明凯, 朱小鹏, 单英春, 徐久军. 强流脉冲离子束辐照镁合金微弧氧化膜的耐腐蚀性能[J]. 中国有色金属学报, 2012, 22(2): 337-342.
[14] 蒋永锋, 杨华山, 包晔峰, 刘益阳. 镁合金微弧氧化陶瓷层表面的电泳成膜机理[J]. 中国有色金属学报, 2011, 21(7): 1562-1569.
[18] 王艳玲, 惠松骁, 叶文君, 米绪军. 医用钛合金的微弧氧化膜层组织及其磨损性能[J]. 中国有色金属学报, 2010, 20(S1): s188-s192.
[28] 程英亮, 伍 帆. Cu2+及等离子电解氧化处理对Zr-4合金腐蚀性能的影响[J]. 中国有色金属学报, 2012, 22(6): 1616-1623.
[31] 赵志龙, 刘一洋, 阎光明. 2090铝-锂合金微弧氧化陶瓷膜层特性的研究[J]. 电镀与环保, 2008, 28(5): 36-38.


