文章编号:1004-0609(2010)05-0903-05
LiF对Na3AlF6-K3AlF6-AlF3熔体初晶温度和Al2O3溶解度的影响
黄有国1, 2,田忠良1,赖延清1,李 劼1,刘业翔1
(1. 中南大学 冶金科学与工程学院,长沙 410083;2 广西师范大学 化学化工学院,桂林 541004)
摘 要:
采用步冷曲线法测定Na3AlF6-K3AlF6-AlF3-LiF熔体的初晶温度,采用旋转刚玉片质量损失法测定Al2O3在Na3AlF6-K3AlF6-AlF3-LiF熔体中的溶解度。结果表明:当LiF添加量在0~4%范围内时,每添加1% LiF使Na3AlF6-K3AlF6-AlF3熔体的初晶温度降低3~4.7 ℃,小于Na3AlF6-AlF3熔体中添加LiF后初晶温度的影响;在0~3%的范围内,每添加1% LiF将使 Al2O3在Na3AlF6-K3AlF6-AlF3熔体中的溶解度降低0.23%~0.55%。
关键词:
Al2O3;LiF;Na3AlF6-K3AlF6-AlF3熔体;初晶温度;溶解度;
中图分类号:TF821 文献标志码:A
Effect of LiF on liquidus temperature and solubility of Al2O3 in Na3AlF6-K3AlF6-AlF3 melts
HUANG You-guo1, 2, TIAN Zhong-liang1, LAI Yan-qing1, LI Jie1, LIU Ye-xiang1
(1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. School of Chemistry and Chemical Engineering, Guangxi Normal University, Guilin 541004, China)
Abstract: The liquidus temperatures of Na3AlF6-K3AlF6-AlF3-LiF melts were tested by cooling curve, and the solubilities of Al2O3 in Na3AlF6-K3AlF6-AlF3-LiF melts were determined by the mass loss of rotating sintered corundum disc. The results show that the liquidus temperature decreases by 3-4.7 ℃ with increasing LiF by 1% in the content range of 0-4%, the increment is less than that in Na3AlF6 melts. The solubility of Al2O3 in Na3AlF6-K3AlF6-AlF3 melts decreases by 0.23%-0.55% with increasing LiF by 1% in the content range of 0-3%.
Key words: Al2O3; LiF; K3AlF6-K3AlF6-AlF3 melt; liquidus temperature; solubility
低温电解铝由于能提高电流效率、降低能耗以及延长现行槽的寿命,并能为惰性电极提供相对友好的工作环境而成为研究的热点[1-4]。开发出具有低温、高Al2O3溶解度与快溶解速度以及高电导率特性的电解质是实现低温电解铝的基础[5-8]。
周传华等[9]报道,在低温Na3AlF6-AlF3熔体中,Al2O3的溶解性能无法满足铝电解工业的要求。在相同条件下,K3AlF6具有比Na3AlF6更好的Al2O3溶解性能[10]。因此,往低温Na3AlF6-AlF3熔体中添加一定量的K3AlF6可极大地提高Al2O3在熔体中的溶解度。如KF+NaF与AlF的摩尔比为1.5的K3AlF6-Na3AlF6- AlF3熔体中,当温度的850 ℃时,Al2O3的溶解度为8.83%[9](质量分数,下同);而当KF-AlF3(即K3AlF6-AlF3)熔体的温度为700 ℃时,Al2O3的溶解度为6.0 %[11]。然而,K3AlF6的添加导致在相同条件下电解质的导电性能下降[12]。
众所周知,在Na3AlF6-AlF3熔体中添加LiF,能够改善熔体的导电性能,但同时将降低电解质对Al2O3的溶解能力[13]。为开发出具有低温、高Al2O3溶解能力和高电导的低温电解质,本文作者在前期研究工作的基础上[14-15],研究LiF的添加对Na3AlF6- K3AlF6-AlF3熔体初晶温度及Al2O3溶解度的影响。
1 实验
试验所用原料Na3AlF6、K3AlF6、LiF均为分析纯;AlF3纯度为99.9%;Al2O3溶解度测定用旋转刚玉片由纯度高于99%的Al2O3制备。所有原料实验前均置于200 ℃的真空干燥箱中恒温20 h以上。
熔体初晶温度的测定采用步冷曲线法,相关细节参考文献[14,16]。
Al2O3溶解度的测定采用旋转刚玉片质量损失 法[11, 17],其实验装置如图1所示。将装有一定量充分混合的电解质的高纯石墨坩锅置于试验电阻炉中,并升温至目标温度(过热度为40 ℃)。当电解质充分熔化后,将带有刚玉片的不锈钢杆浸入并搅拌,一段时间后取出,用30%的AlCl3热溶液清洗,烘干后称量。反复上述过程,直到浸入电解质中的刚玉片在实验前、后质量不再发生变化为止。实验条件下,Al2O3在熔体中的溶解度为所有刚玉片在实验过程中质量损失的和。在此过程中没有考虑由于电解质挥发而导致的误差。
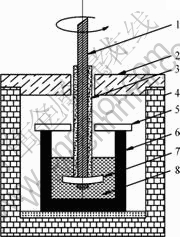
图1 测定Al2O3溶解度实验的示意图
Fig.1 Schematic diagram for Al2O3 solubility measurement: 1—Steel shaft; 2—Furnace lid; 3—Graphite sleeve; 4—Furnace; 5—Sintercorundum lid; 6—Graphite crucible; 7—Sintercorundum disc; 8—Melt
2 结果与讨论
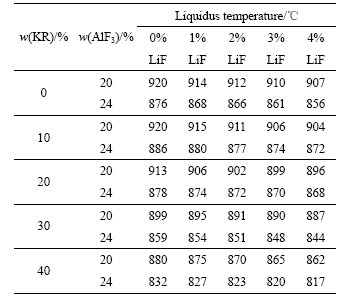
表1所列为不同LiF含量的Na3AlF6-K3AlF6-AlF3熔体的初晶温度。从表1所列数据来看,在0~4%的范围内,随着LiF添加量的增加,Na3AlF6-K3AlF6-AlF3熔体的初晶温度降低。对于K3AlF6(KR)的含量为0%(质量分数),AlF3含量为20%的 Na3AlF6-AlF3熔体,其初晶温度为 920 ℃。然而,当熔体中添加的LiF为1%、2%、3%和4%(质量分数)后,其初晶温度分别为914、912、910和907 ℃。同样,当向KR为20%,AlF3为24%的Na3AlF6-K3AlF6-AlF3熔体中添加2%的LiF时,其初晶温度由878 ℃降低至 872 ℃。
将表1中数据与文献[18]报道的结果相比,在0~4%的范围内,LiF添加量对Na3AlF6-K3AlF6-AlF3熔体初晶温度的影响幅度要小于相同条件下对Na3AlF6的影响。如当Na3AlF6-K3AlF6-AlF3熔体KR的含量分别为0、10%、20%、30%和40%,AlF3含量为20%时,LiF的添加量每添加1%,其初晶温度分别降低约3、4.1、4.1、2.9和4.6 ℃;当AlF3含量为24%时,对应熔体的初晶温度分别降低约4.7、3.4、2.4、3.6和3.7 ℃。而在Na3AlF6熔体中,LiF的添加量每添加1%,熔体初晶温度降低约7.8 ℃[18]。
表1 不同LiF含量时Na3AlF6-K3AlF6-AlF3熔体的初晶温度
Table 1 Liquidus temperatures of Na3AlF6-K3AlF6-AlF3 melts with different mass fractions of LiF
此外,将表1所列熔体的初晶温度数据与KR的含量的关系作图(见图2)。从图2可知,当KR的含量从0提高到10%时,Na3AlF6-K3AlF6-AlF3-LiF熔体的初晶温度变化不大;而当KR的含量从10%提高到40%时,熔体初晶温度随KR含量的提高而急剧下降。当熔体中LiF添加量为1%,KR的含量为0和10%时,熔体初晶温度分别为914和915 ℃;而当KR的含量从10%提高到40%时,熔体初晶温度由915 ℃降低到875 ℃。同时,从图2中也可看出,LiF的添加并没有改变KR的含量对熔体初晶温度的影响规律。
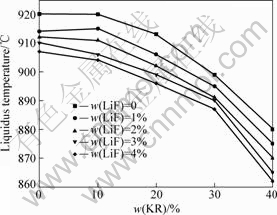
图2 Na3AlF6-K3AlF6-AlF3-LiF熔体的初晶温度与KR含量的关系
Fig.2 Relationship between KR content and liquidus temperature of Na3AlF6-K3AlF6-AlF3-LiF melt
表2所列为Al2O3在不同LiF含量的Na3AlF6- K3AlF6-AlF3熔体中的溶解度。从表2可看出,LiF 的添加将降低 Al2O3在Na3AlF6-K3AlF6-AlF3熔体中的溶解度。如在KR含量为10%,AlF3含量为20%的Na3AlF6-K3AlF6-AlF3熔体中,当LiF添加量为0%、1%和3%时,Al2O3在熔体中的溶解度分别为6.55%、5.50%和4.96%;而后KR含量为20%,AlF3含量为24%的Na3AlF6-K3AlF6-AlF3熔体中添加3%的LiF时,Al2O3的溶解度由5.39%降低至4.00%。产生这种现现象的原因可能在于:Al2O3在MF-AlF3类冰晶石熔体中的溶解,主要是由于Al2O3与熔体中的AlF63-反应生成[Al2O2F4]2-和Al2OF84-而溶解[19]。熔体中AlF63-的数量越多,则Al2O3的溶解度越大。而在LiF-AlF3、NaF-AlF3和KF-AlF3熔体中,AlF63-存在以下的分解反应:

LiF-AlF3熔体中反应(1)和(2)的分解常数在MF-AlF3类冰晶石熔体中是最大的,而KF-AlF3熔体中反应(1)和(2)是最小的[19]。所以,在熔体中添加LiF,熔体中AlF63-的数量降低,从而使Al2O3的溶解度 降低。
在0~3%的范围内,LiF添加量每增加1%,Al2O3在Na3AlF6-K3AlF6-AlF3熔体中的溶解度降低0.23%~
表2 不同LiF含量的Na3AlF6-K3AlF6-AlF3熔体中Al2O3的溶解度
Table 2 Solubility of Al2O3 in Na3AlF6-K3AlF6-AlF3 melts with different mass fractions of LiF
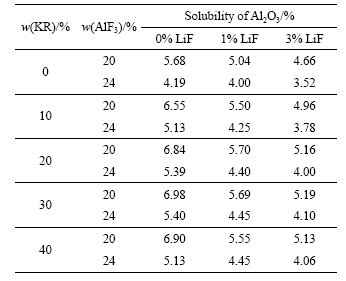
0.55%。对于KR的含量分别为0、10%、20%、30%和40%的熔体,当AlF3含量为20%时,LiF添加量每增加1%,Al2O3溶解度分别降低约0.32%、0.49%、0.52%、0.55%和0.54%;而当AlF3含量为24%时,Al2O3溶解度则分别降低约0.23%、0.42%、0.43%、0.40%和0.34%。YANG等[11]通过对Al2O3在AlF3含量为33%、700 ℃的K3AlF6熔体中的溶解度测定后认为,平均每添加1%LiF后,Al2O3的溶解度降低约0.37%。
从Al2O3在Na3AlF6-K3AlF6-AlF3-LiF熔体中的溶解度与KR的含量的关系(见图4)来看,随着KR含量的升高,Al2O3的溶解度逐渐增大,但当KR含量增加到一定程度后,Al2O3溶解度反而下降。也就是说,对于Na3AlF6-K3AlF6-AlF3-LiF熔体,当KR含量为20%~ 30%时,Al2O3的溶解度存在最大值。对于AlF3含量为20%的Na3AlF6-K3AlF6-AlF3的熔体,当KR含量从0提高到30%时,Al2O3溶解度从5.68%增加到6.98%;而当KR含量继续提高到40%时,Al2O3溶解度则开始下降(6.90%)。往该熔体中添加1% LiF后,当KR含量分别为0%、20%和40%时,Al2O3在对应熔体中的溶解度分别为5.04%、5.70%和5.55%。正如文献[19]提到的,在KF-AlF3熔体中存在较多的AlF63-,Al2O3在熔体中的溶解度随着KR含量的增加而增大。KR含量的增加降低了熔体的初晶温度,因而在过热度不变的情况下,当KR含量增大时,熔体的温度随之降低,所以,KR含量增加到一定程度后,Al2O3溶解度略微降低。在熔体中添加LiF后,熔体中AlF63-数量减小,所以增加相同量的KR,Al2O3溶解
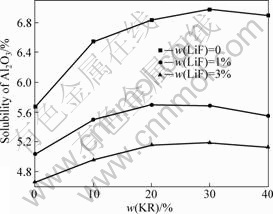
图3 含有20%AlF3的Na3AlF6-K3AlF6-AlF3-LiF熔体中Al2O3的溶解度与KR含量的关系
Fig.3 Relationship between KR content and solubility of Al2O3 in Na3AlF6-K3AlF6-AlF3-LiF melts with 20% AlF3
度增加幅度减小。
3 结论
1) 在0~4%的范围内,随着LiF添加量的增加,Na3AlF6-K3AlF6-AlF3熔体初晶温度降低,与对Na3AlF6熔体初晶温度的影响类似,但其影响程度要小些;LiF添加量每增加1%,Na3AlF6- K3AlF6-AlF3熔体初晶温度降低3℃~4.7℃,且LiF的添加不改变KR对Na3AlF6-K3AlF6-AlF3-LiF熔体初晶温度的影响规律。
2) LiF的添加将降低 Al2O3在Na3AlF6-K3AlF6- AlF3熔体中的溶解度。在0~3%的范围内,LiF添加量每增加1%,Al2O3在Na3AlF6-K3AlF6-AlF3熔体中的溶解度降低0.23%~0.55%。Al2O3在Na3AlF6-K3AlF6- AlF3-LiF熔体中的溶解度随KR含量的增加先提高后下降。
REFERENCES
[1] YANG J H, HRYN J N, DAVIS R D, ROY A, KRUMDICK G K, POMYKALA J A. New opportunities for aluminum electrolysis with metal anodes in a low temperature electrolyte system[C]// TABEREAUX A T. Light Metals 2004. Warrendale PA: TMS, 2004: 321-326.
[2] TIAN Zhong-liang, LAI Yan-qing, LI Jie, LI Xin-zheng, LIU Ye-xiang. Low temperature electrolysis of alumina with NiFe2O4 based cermet inert anodes[J]. JOM, 2004, 56(11): 285.
[3] ZAIKOV Y, KHRAMOV A, KOVROV V, KRYUKOVSKY V, APISAROV A, TKACHEVA O, CHEMESOV O, SHUROV N. Electrolysis of aluminum in the low melting electrolytes based on potassium cryolite[C]//DEYOUNG D H. Light Metals 2008. Warrendale PA: TMS, 2008: 505-508.
[4] TIAN Zhong-liang, LAI Yan-qing, LI Jie, LIU Ye-xiang. Effect of Ni content on corrosion behavior of Ni/(10NiO-90NiFe2O4) cermet inert anode[J]. Trans Nonferrous Met Soc China, 2008, 18(2): 361-365.
[5] WANG Jia-wei, LAI Yan-qing, TIAN Zhong-liang, LI Jie, LIU Ye-xiang. Investigation of 5Cu-(10NiO-NiFe2O4) inert anode corrosion during low-temperature aluminum electrolysis[C]// S?RLIE M. Light Metals 2007. Warrendale PA: TMS, 2007: 525-530.
[6] BALARAJU J N, ANANTH V, SEN U. Studies on low temperature Al electrolysis using composite anodes in NaF-KCl bath electrolyte[J]. Journal of the Electrochemical Society, 1995, 142(2): 439-444.
[7] YANG J H, HRYN J N, KRUMDICK G K. Aluminum electrolysis tests with inert anodes in KF-AlF3-based electrolytes[C]//GALLOWAY T J. Light Metals 2006. Warrendale PA: TMS, 2006: 421-424.
[8] REDKIN A, TKATCHEVA O, ZAIKOV Y, APISAROV A. Modeling of cryolite-alumina melts properties and experimental investigation of low melting electrolytes[C]//S?RLIE M. Light Metals 2007. Warrendale PA:TMS, 2007: 513-517.
[9] 周传华, 马淑兰, 李国勋, 沈剑韵. 新型低温铝电解质体系的研究——氧化铝的溶解度与溶解速度[J]. 有色金属. 1998(2): 81-84.
ZHOU Chuan-hua, MA Shu-lan, LI Guo-xun, SHEN Jian-yun. Studied on the new type low temperature electrolyte for aluminum electrolysis—Solubility and dissolution rate of alumina[J]. Nonferrous Metals, 1998(2): 81-84.
[10] FEDKIN A, THATCHEVA O, ZAIKOV Y. Modeling of cryolite-alumina melts properties and experimental investigation of low melting electrolytes[C]//BOHNER H O. Light Metals 1985. Warrendale PA: TMS, 1985: 501-506.
[11] YANG J H, GRACZYK D G, WUNSCH C, HRYN J N. Alumina solubility in KF-AlF3-based low-temperature electrolyte system[C]//S?RLIE M. Light Metals 2007. Warrendale PA: TMS, 2007: 537-541.
[12] APISAROV A P, KRYUKOVSKII V A, ZAIKOV Y P, RED’KIN A A, TKACHEVA O Y, KHOKHLOV V A. Conductivity of low-temperature KF-AlF3 electrolytes containing lithium fluoride and alumina[J]. Russian Journal of Electrochemistry, 2007, 43(8): 870-874.
[13] FROLOV A V, GUSEV A O, ZAIKOV Y P, KHRAMOV A P, SHUROV N I, TKACHEVA O Y, APISAROV A P, KOVROV V A. Modified alumina-cryolite bath with high electrical conductivity and dissolution rate of alumina[C]//S?RLIE M. Light Metals 2007. Warrendale PA: TMS, 2007: 571-576.
[14] WANG Jia-wei, LAI Yan-qing, TIAN Zhong-liang, LI Jie, LIU Ye-xiang. Temperature of primary crystallization in party of system Na3AlF6-K3AlF6-AlF3[C]//DEYOUNG D H. Light Metals 2008. Warrendale PA: TMS, 2008: 513-518.
[15] HUANG You-guo, LAI Yan-qing, TIAN Zhong-liang, LI Jie, LIU Ye-xiang, LI Qing-yu. Electrical conductivity of (Na3AlF6-40wt%K3AlF6)-AlF3 wt% melts[C]//DEYOUNG D H. Light Metals 2008. Warrendale PA: TMS, 2008: 519-521.
[16] SCHMID-FETZER R, OHNO M, MIRKOVIC D. Liquidus and solidus temperatures of Mg-rich Mg-Al-Mn-Zn alloys[J]. Acta Materialia, 2006, 54(15): 3883-3891.
[17] SKYBAKMOEN E, SOLHEIM A, STERTEN A. Alumina solubility in molten salt systems of interest for aluminum electrolysis and related phase diagram data[J]. Metallurgical and Materials Transactions B: Process Metallurgy and Materials Processing Science. 1997, 28(1): 81-86.
[18] 邱竹贤, 张明杰, 何鸣鸿. 低温铝电解的研究[J]. 轻金属, 1984, (6): 33-36.
QIU Zhu-xian, ZHANG Ming-jie, HE Ming-hong. Progress on the low temperature electrolysis for aluminum reduction[J]. Light Metals, 1984(6): 33-36.
[19] XU Qian, MA Yi-ming, QIU Zhu-xian. Calculation of thermodynamic properties of LiF-AlF3, NaF-AlF3 and KF-AlF3[J]. Calphad: Computer Coupling of Phase Diagrams and Thermochemistry, 2001, 25(1): 31-42.
(编辑 李艳红)
基金项目:国家重点基础研究发展计划资助项目(2005CB623703);国家高技术研究发展计划资助项目(2008AA030503)
收稿日期:2009-03-13;修订日期:2009-12-13
通信作者:田忠良,副教授,博士;电话:0731-88830649;E-mail:tianzhongliang@126.com


