Trans. Nonferrous Met. Soc. China 29(2019) 1346-1352
Removal of Co(II) from aqueous solution by complexation-ultrafiltration and shear stability of PAA-Co complex
Jing GAO1, Yun-ren QIU1, Mao-lin LI2, Hui-shang LE1
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Changsha Research Institute of Mining and Metallurgy Co., Ltd., Changsha 410012, China
Received 6 August 2018; accepted 14 January 2019
Abstract:
Removal of Co(II) from aqueous solutions by complexation-ultrafiltration was investigated using polyacrylic acid sodium (PAAS) as complexing agent with the help of rotating disk membrane, and the shear ability of PAA-Co complex was studied. The effects of the mass ratio of PAAS to Co(II) (P/M) and pH on the rejection of Co(II) were studied, and the optimum conditions were P/M=8 and pH=7. The rejection of Co(II) was over 97% when the rotating speed of the disk (n) was less than 710 r/min at the optimum P/M and pH. The distribution of the forms of cobalt on the membrane surface was established by the membrane partition model, and the critical shear rate, the smallest shear rate at which the PAA-Co complex begins to dissociate, was calculated to be 1.4×104 s-1, and the corresponding rotating speed was 710 r/min. The PAA-Co complex dissociated when the shear rate was greater than the critical one. The regeneration of PAAS and recovery of Co(II) were achieved by shear-induced dissociation and ultrafiltration.
Key words:
shear stability; critical shear rate; PAA-Co complex; shear-induced dissociation; complexation-ultrafiltration;
1 Introduction
Cobalt is widely used in the lithium batteries, alloys, industrial catalysts, dyes and other fields [1]. Cobalt- containing wastewater treatment is of great significance to environmental protection and metal reuse. Treatment methods of cobalt-containing wastewater include chemical precipitation-flotation [2], adsorption [3], ion exchange [4] and complexation-ultrafiltration [5]. Among them, complexation-ultrafiltration has the advantages of high separation efficiency, low energy consumption and easy operation [5]. Complexation- ultrafiltration, also called polymer enhanced ultrafiltration (PEUF), has been extensively used in the separation and concentration of metal-loaded water effluents [6]. In this process, the metals are bound to complexing agent to form macromolecular complex and rejected by the membrane, whereas unbound metals pass through the membrane.
Polyacrylate (PAA) is usually used as complexing agent to bond with the heavy metal elements [7-10] and rare elements [11]. The stability of polymer-metal complex is one of the important factors of complexation-ultrafiltration. In our previous research, the critical shear rates of PAA-Ni complex and PAA-Cu complex have been obtained [7,8]. In this study, removal of Co(II) from aqueous solutions by complexation-ultrafiltration was investigated using polyacrylic acid sodium (PAAS) as complexing agent with the help of rotating disk membrane, and the shear ability of PAA-Co complex was also studied. Furthermore, the recovery of Co(II) and the regeneration of PAAS were achieved by shear-induced dissociation and ultrafiltration.
2 Experimental
2.1 Materials
Sodium polyacrylate (PAAS) with the relative molecular mass of 5×105 was used as complexing agent (Tianjin Guangfu Fine Chemical Research Institute, China), and the small molecular substance was eliminated by hollow fiber ultrafiltration membrane with the relative molecular mass cut-off (MWCO) of 2×104 (Tianjin Aisheng Membrane Filtration Technology Co., Ltd., China) [10]. A PES flat ultrafiltration membrane with the relative molecular mass cut-off of 3×104 (SEPRO, USA) was used for complexation- ultrafiltration. Co(NO3)2·6H2O, HCl and NaOH (analytical grade, Sinopharm Chemical Reagent Co., Ltd., China) was used.
A series of solutions containing different concentrations of PAAS and 10 mg/L Co(II) were prepared which were magnetically stirred for 2 h at 35 °C before the experiments. pH was adjusted by adding hydrochloric acid or sodium hydroxide. All the solutions in the experiment were prepared with deionized water.
2.2 Ultrafiltration process and rotating disk membrane module
The diagram of ultrafiltration process is shown in Fig. 1. In Fig. 1(a), a flat membrane, with an effective area of 0.0253 m2 (inner radius (rin) is 0.005 m, and outer radius (rout) is 0.088 m), was fixed on the cover of the cylindrical housing. A rotating disk with four radial vans (shown in Fig. 1(b)) is faced to the membrane, which can rotate at adjustable speed ranging from 0 to 3000 r/min to generate shear on the membrane. It avoids increasing the shear rate by providing large flow or high pressure, and can significantly reduce the energy consumption while strengthening the effect of shearing [12].
The feed was pumped from a thermostatic and stirred tank to the rotating disk membrane module, and the feed flow was 12 L/h. The permeate was collected for analyses when the permeate flux became stable after changing hydraulic conditions.
The cobalt concentration in the feed and permeate was determined by ICP-OES. The Co(II) rejection (R) was calculated as follows:
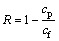 (1)
(1)
where cp and cf are the cobalt concentrations in the permeate and feed, respectively.
2.3 Calculation of critical radius and critical shear rate of PAA-Co complex
The shear is generated on the membrane surface by the rotating disk. BOUZERAR et al [13] has obtained the formulas of shear rate by solving the axisymmetric Navier-Stokes equations:
 (2)
(2)
where υ is kinematic viscosity of the fluid; ω is the rotating speed of the disk; r is the radius; γml and γmt represent the shear rates generated by the rotating disk at laminar and turbulent state, respectively; k is the velocity factor, which is defined as the rotating speed ratio of the fluid to the rotating disk at the same radius and its value is only related to the geometry of the device, which is less than 1 and always independent of radius [14]. Here, k is 0.54, calculated from pressure measurements inside the housing at different locations and speeds [7].
According to Eq. (2), the shear rate distribution map on the membrane surface at the corresponding rotating speeds can be calculated as Fig. 2.
From Fig. 2, the shear rate increases with the radius as well as the rotating speed, and the shear rate on the membrane surface can arrive at the level of 105 s-1. A large shear rate is very likely to break the bond of PAA-Co complex, and leads to the dissociation of PAA-Co complex.
We define the smallest shear rate at which PAA-Co complex begins to dissociate as the critical shear rate (γc). A partition model is proposed to calculate the critical shear rate (γc). The membrane is divided into innumerable small rings, as shown in Fig. 3.
According to Darcy’s law, the permeate flux can be expressed as the following formula:
 (3)
(3)
where Ji is the permeate flux through the ith rin (m3·m-2·s-1), μ is the dynamic viscosity of the solution (Pa·s), Rt is the total resistance (m-1), Rm is the membrane resistance (m-1), Rf is the membrane fouling resistance (m-1), Rp is the concentration polarization resistance (m-1) and Pi is pressure (kPa) on the ith ring.
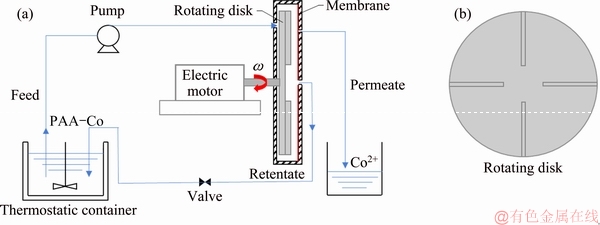
Fig. 1 Schematic diagram of ultrafiltration process (a) and rotating disk with four radial vans (b)

Fig. 2 Shear rate distribution on membrane surface
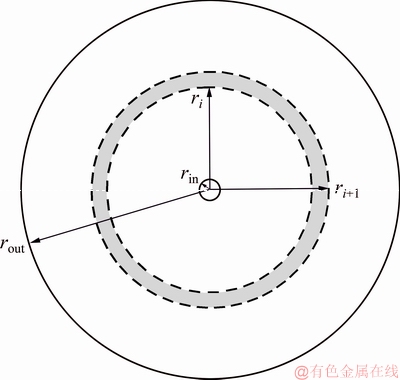
Fig. 3 Membrane surface segmentation diagram
In order to determine the membrane resistance, we studied the effects of the mass ratio of PAAS to Co(II) (P/M) on the relative viscosity (μr) and membrane intrinsic resistance (Rc), where μr is the ratio of the viscosity of the solution to the pure water, and Rc is the ratio of total resistance to the membrane resistance. The result is shown in Fig. 4.
The result indicated that the polarization layer resistance and fouling resistance can be ignored in this operation condition.
Pi in Eq. (3) can be calculated as [14]
Pi=P0+1/2ρ(kωri)2 (4)
where P0 is the peripheral pressure at rest (n=0 r/min) (kPa); ρ is the density of the solution (kg/m3); ω is the angular velocity of the disk (rad/s). k here is the same as Eq. (1).

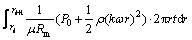 (5)
(5)
where Vi is the permeate volume through the ith ring of the membrane.
The radial location of the critical shear rate is defined as the critical radius (rc). The critical radius divides the membrane into complexation region and dissociation region, as shown in Fig. 5.

Fig. 4 Effect of P/M on relative viscosity and membrane intrinsic resistance
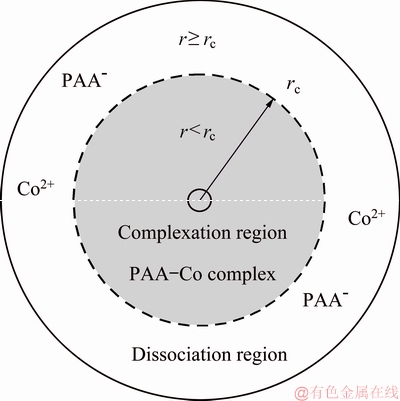
Fig. 5 Cobalt morphology distribution on membrane surface in shear field
When the radius is greater than rc, PAA-Co complex begins to dissociate and free Co(II) can pass though the membrane. While the radius is less than rc, PAA-Co complex is stable, and can be rejected by the membrane. Thus, permeate volume of complexation region and dissociation region can be expressed as
Complexation region:
 rin≤r<>c (6)
rin≤r<>c (6)
Dissociation region:
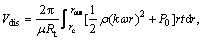 rc≤r≤rout (7)
rc≤r≤rout (7)
Thus, the total volume of permeate can be expressed as
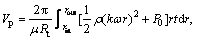 rin≤r≤rout (8)
rin≤r≤rout (8)
where rin and rout are the inner radius and outer radius of the membrane, respectively; Vp, Vcom and Vdis are the permeate volumes through the whole membrane, complexation region and dissociation region, respectively.
According to the mass balance,
cpVp=ccomVcom+cdisVdis (9)
where cp, ccom and cdis are the permeate concentrations through the whole membrane, complexation region and dissociation region, respectively.
Equation (10) can be obtained by simplifying Eqs. (6)-(9):

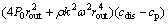 (10)
(10)
In Eq. (10), cp can be directly measured. cdis is identical to the cobalt concentration of feed. ccom is equal to the cobalt concentration in the permeate of static filtration under the same condition. By testing the cobalt concentration in permeate at a certain speed, the critical radius (rc) at the corresponding rotating speeds can be calculated. Then, the critical shear rate (γc) of PAA-Co complex can be obtained by Eq. (1).
It is worth noting that Eq. (10) only applies at cp>ccom. When cp=ccom, it indicates that PAA-Co complex has not yet been dissociated in this shear field, and the whole membrane surface is in complexation region. That is to say, the shear rate at the membrane surface is smaller than the critical shear rate, and the critical radius (rc) is greater than the outer radius of membrane (rout).
3 Results and discussion
3.1 Effect of P/M on permeate flux and Co(II) rejection
On the surface of the membrane, the greater the radius, the greater the pressure. Thus, it is necessary to introduce the permeation coefficient F, which is defined as the permeate flux per unit pressure. The variations of Co(II) rejection and F with P/M are shown in Fig. 6.
From Fig. 6, F is hardly affected by P/M at P/M<35. Co(II) rejection increases at 0
6. In the industry, in order to obtain the good rejection, a slightly excess P/M 7-8 is chosen.
3.2 Effect of pH on Co(II) rejection
pH is the important parameter governing the metal rejection because of its influences on the solution chemistry and charge of membrane surface [15,16]. Almost all sites of polymer-carboxylic groups are protonated (—COOH) at pH<3, and metal-polymer complex can be hardly formed [17]. Co(II) starts to form precipitate and causes a decrease of permeate flux at pH>9 [18]. In this study, Co(II) rejection at pH 3-8 is discussed in Fig. 7.

Fig. 6 Variations of permeation coefficient and Co(II) rejection with P/M

Fig. 7 Variations of Co(II) rejection with pH under different P/M
It can be seen from Fig. 7 that the Co(II) rejections increase with increasing pH. —COO- increases with increasing pH, which promotes the formation of PAA-Co complex [19]. Co(II) rejection is above 97% at pH 6-8. Considering the simple operation and friendly environment, pH=7 is chosen.
3.3 Effect of rotating speed on Co(II) rejection
The variations of Co(II) rejection with rotating speed of disk are shown in Fig. 8 and Fig. 9.
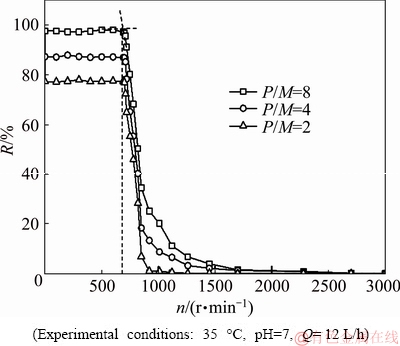
Fig. 8 Variations of Co(II) rejection with rotating rate under different P/M

Fig. 9 Variations of Co(II) rejection with rotating rate under different pH
It can be seen from Fig. 8 and Fig. 9 that PAA-Co complex can be dissociated when the rotating speed of disk is high enough. Co(II) rejections are more than 97% at 0
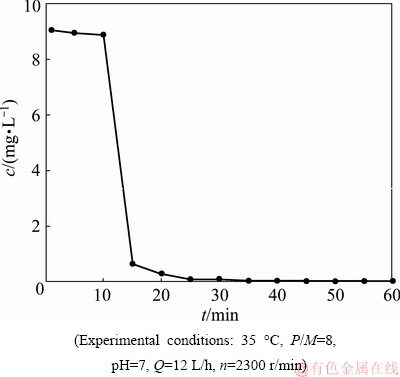
To further study the reason for the decline of Co(II) rejection, experiments of the stability of PAAS in a shear field were performed. PAAS rejections keep constant at 0 Co(II) rejections are remarkably decreased at n> 710 r/min no matter whatever P/M is. As the rotating speed continues to increase, the dissociation degree of PAA-Co complex increases. Therefore, by adjusting the rotating speed of disk, the removal of cobalt can be achieved. In Fig. 9, at the same rotating speed, the lower the pH is, the higher the dissociation degree of PAA-Co complex is. The Co(II) rejection remains constant at pH 7 and n<710 r/min. There is no constant value at pH 6 and 5 even at low speed. Therefore, high rotating speed and low pH are beneficial to the dissociation of PAA-Co complex. PAA-Cu complex is stable at pH 7, 6 and 5 at low rotating speed [8]. Unlike PAA-Cu complex or PAA-Ni complex [7], PAA-Co complex is stable at pH 7 only at very low rotating speed. This shows that PAA-Cu complex is more stable than PAA-Ni complex or PAA-Co complex. The result is consistent with CHEN et al [20]. 3.4 Critical shear rate and critical radius of PAA-Co complex According to Eq. (10) and the experimental results of Section 3.3, rc at the certain rotating speed was calculated at pH 7, as shown in Fig. 10. Fig. 10 Critical radius at corresponding rotating speed of disk It can be seen from Fig. 10 that the critical radius decreases with the increase of rotating speed of disk, meaning the extension of dissociation region from the periphery to the center of the membrane. The critical shear rates (γc) of PAA-Co complex at different rotating speeds of disk are calculated by Eq. (1). Interestingly, the critical shear rates (γc) of PAA-Co complex at different rotating speeds of disk remain constant, which is 1.4×104 s-1. By controlling the rotating speed of disk, when the shear rate is less than the critical shear rate, the cobalt can be removed from aqueous solution. In addition, the regeneration of PAAS can be obtained. 3.5 Regeneration of PAAS Shear-induced dissociation and ultrafiltration can achieve the separation of cobalt and recovery of PAAS. The rotating speed of 2300 r/min was chosen to make PAA-Co complex dissociate. Free Co(II) passed through the membrane, while the complexing agent was rejected by membrane and collected into the bank. When the feed was almost exhausted, ultrapure water was added to continue ultrafiltration until Co(II) was not detected in the permeate. Then, the collected polymer was concentrated, and the regenerated PAAS was got. The variation of cobalt concentration in permeate with ultrafiltration time is shown in Fig. 11. Fig. 11 Variations of Co(II) concentration in permeate with ultrafiltration time In Fig. 11, during the first 10 min, the feed was treated by ultrafiltration, and the cobalt dissociation rate was about 90%. After 30 min, the cobalt concentration in permeate was reduced to below 0.1 mg/L. The regenerated PAAS was used to treat the solution containing cobalt under the optimal condition, and Co(II) rejection is 97%. This means that the performance of regenerated PAAS is good. 4 Conclusions (1) The shear ability of PAA-Co complex was studied, and the critical shear rate of PAA-Co complex is 1.4×104 s-1 at pH 7. (2) The mechanism of shear instability of PAA-Co complex is revealed by complexation-dissociation segmentation model, and the critical radius of membrane at the certain rotating speed of disk is calculated. The higher the rotating speed of disk is, the smaller the critical radius of membrane is. That is to say, the higher the rotating speed of disk is, the smaller the stability region area of PAA-Co complex is on the membrane surface. It can give guidance for separating metal by complexation-ultrafiltration. (3) A new efficient, green and recycle process for the recovery of cobalt and polymer under neutral conditions was established. The Co(II) can be removed more than 97% at n<710 r/min, pH=7 and P/M=8. When n≥710 r/min, pH=7 and P/M=8, PAAS can be successfully regenerated by shear induced dissociation. References [1] WANG Yan, CHEN Ze-hua, HUANG Jing, LI Gao-jie, CAO Jian-liang, ZHANG Bo, CHEN Xing-ying , ZHANG Huo-li, JIA Lei. Preparation and catalytic behavior of reduced graphene oxide supported cobalt oxide hybrid nanocatalysts for CO oxidation [J]. Transactions of Nonferrous Metals Society of China, 2018, 28: 2265-2273. [2] ZHU Shu-guang, HE Wen-zhi, LI Guang-ming. Recovery of Co and Li from spent lithium-ion batteries by combination method of acid leaching and chemical precipitation [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 2274-2281. [3] ZHANG Lei, YANG Wen-bin, WU Xiao-yuan, HUO Miao, CHEN Wen-zhe. A polyhedron-based cobalt-organic framework for gas adsorption and separation [J]. Inorganic Chemistry Communications, 2016, 67: 10-13. [4] WOLOWICZ A, HUBICKI Z. Comparison of ion-exchange resins for efficient cobalt(II) removal from acidic streams [J]. Chemical Engineering Communications, 2018, 205(9): 1207-1225. [5] SHAO Jia-hui, QIN Shu, LI wen-xi. Simultaneous recovery of nickel and cobalt from aqueous solutions using complexation-ultrafiltration process [J]. Separation Science & Technology, 2013, 48(18): 2735-2740. [6] ZHANG Xue-jun, ZENG Jian-xian, ZHANG Peng, SHEN Shao-hua. Treatment of wastewater containing high concentration nickel ions with precipitation-microfiltration-complexation-ultrafiltration processes [J]. China Environmental Science, 2016, 36(4): 1106-1111. [7] GAO Jing, QIU Yun-ren, HOU Ben, ZHANG Qiang. Treatment of wastewater containing nickel by complexation-ultrafiltration using sodium polyacrylate and the stability of PAA-Ni complex in the shear field [J]. Chemical Engineering Journal, 2018, 334: 1878-1885. [8] TANG Shu-yun, QIU Yun-ren. Removal of copper(II) ions from aqueous solutions by complexation–ultrafiltration using rotating disk membrane and the shear stability of PAA-Cu complex [J]. Chemical Engineering Research and Design, 2018, 136: 712-720. [9] SHAO Jia-hui, QIN Shu, DAVIDSON J. Recovery of nickel from aqueous solutions by complexation-ultrafiltration process with sodium polyacrylate and polyethylenimine [J]. Journal of Hazardous Materials, 2013, 244-245: 472-477. [10] XU Jing-yuan, TANG Shu-yun, QIU Yun-ren. Pretreatment of poly(acrylic acid) sodium by continuous diafiltration and time revolution of filtration potential [J]. Journal of Central South University, 2019, 26: 577-586. [11] PESTOV A V, PRIVAR Y O, USTINOV A Y. Effect of polymer backbone chemical structure on metal ions binding by imidazolylmethyl derivatives [J]. Chemical Engineering Journal, 2016, 283: 323-329. [12] KUHN M, BRIESEN H. Dynamic modeling of filter-aid filtration including surface- and depth-filtration effects [J]. Chemical Engineering & Technology, 2016, 39(3): 425-434. [13] BOUZERAR R, DING L, JAFFRIN M Y. Local permeate flux-shear-pressure relationships in a rotating disk microfiltration module: Implications for global performance [J]. Journal of Membrane Science, 2000, 170: 127-141. [14] BOUZERAR R, JAFFRIN M Y, LEFEVRE A, PAULLIER P. Concentration of ferric hydroxide suspensions in saline medium by dynamic cross-flow filtration [J]. Journal of Membrane Science, 2000, 165: 111-123. [15] GUAN Qing-jun, SUN Wei , ZHOU Gui-ying , LIU Jia-peng, YIN Zhi-gang. Recovery of cobalt and nickel in the presence of magnesium and calcium from sulfate solutions by Versatic 10 and mixtures of Versatic 10 and Cyanex 301 [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 865-873. [16] ZHANG Wen-xiang, DING Lu-hui, LUO Jian-quan. Membrane fouling in photocatalytic membrane reactors (PMRs) for water and wastewater treatment: A critical review [J]. Chemical Engineering Journal, 2016, 302: 446-458. [17] QIU Yun-ren, MAO Lian-jun, WANG Wei-hua. Removal of manganese from waste water by complexation-ultrafiltration using copolymer of maleic acid and acrylic acid [J]. Transactions of Nonferrous Metals Society of China, 2014, 24: 1196-1201. [18] CHOI H, KAI Z, DIONYSIOU D D. Effect of permeate flux and tangential flow on membrane fouling for wastewater treatment [J]. Separation & Purification Technology, 2015, 45(1): 68-78. [19] QIU Yun-ren, MAO Lian-jun. Removal of heavy metal ions from aqueous solution by ultrafiltration assisted with copolymer of maleic acid and acrylic acid [J]. Desalination, 2013, 329: 78-85. [20] CHEN Ming, SHAFER-PELTIER K E, RANDTKE S J, PELTIER E. Competitive association of cations with poly(sodium 4-styrenesulfonate) (PSS) and heavy metal removal from water by PSS-assisted ultrafiltration [J]. Chemical Engineering Journal, 2018, 344: 155-164. 高 静1,邱运仁1, 李茂林2 , 乐恢赏1 1. 中南大学 化学化工学院,长沙 410083; 2. 长沙矿冶研究院有限责任公司,长沙 410012 摘 要:采用聚丙烯酸钠(PAAS)作络合剂,研究旋转盘膜络合-超滤处理含钴稀溶液及PAA-Co络合物的剪切稳定性。研究聚合物/金属离子质量比(P/M)及pH对钴截留率的影响,并得到较佳的P/M及pH条件为P/M=8和 pH=7。在此条件下,当旋转盘转速小于710 r/min时,PAA-Co络合物的截留率达97%以上。采用络合-解离分区模型研究钴在膜面的存在形态,并得到PAA-Co络合物解络的临界剪切速率(PAA-Co络合物解络的最小剪切速率)。在pH=7条件下,PAA-Co络合物的临界剪切速率为1.4×104 s-1,对应的旋转盘临界转速为710 r/min。当剪切速率大于1.4×104 s-1时,PAA-Co络合物解络,可通过剪切诱导解络-超滤实现络合剂和Co(II)的分别回收。 关键词:剪切稳定性;临界剪切速率;PAA-Co络合物;剪切诱导解络;络合-超滤 (Edited by Bing YANG) Foundation item: Project (24176265) supported by the National Natural Science Foundation of China Corresponding author: Yun-ren QIU; Tel: +86-13507479124; E-mail: csu_tian@csu.edu.cn DOI: 10.1016/S1003-6326(19)65041-7
络合-超滤处理含钴稀溶液及PAA-Co络合物的剪切稳定性
Abstract: Removal of Co(II) from aqueous solutions by complexation-ultrafiltration was investigated using polyacrylic acid sodium (PAAS) as complexing agent with the help of rotating disk membrane, and the shear ability of PAA-Co complex was studied. The effects of the mass ratio of PAAS to Co(II) (P/M) and pH on the rejection of Co(II) were studied, and the optimum conditions were P/M=8 and pH=7. The rejection of Co(II) was over 97% when the rotating speed of the disk (n) was less than 710 r/min at the optimum P/M and pH. The distribution of the forms of cobalt on the membrane surface was established by the membrane partition model, and the critical shear rate, the smallest shear rate at which the PAA-Co complex begins to dissociate, was calculated to be 1.4×104 s-1, and the corresponding rotating speed was 710 r/min. The PAA-Co complex dissociated when the shear rate was greater than the critical one. The regeneration of PAAS and recovery of Co(II) were achieved by shear-induced dissociation and ultrafiltration.


