DOI:10.19476/j.ysxb.1004.0609.2018.01.22
粉煤灰中锗的高温火法二次富集工艺
刘丽霞1, 2,李文挺1, 2,彭 军2,张 磊2,蔡长焜2,安胜利1, 2
(1. 北京科技大学 冶金与生态工程学院,北京 100083;
2. 内蒙古科技大学 材料与冶金学院,包头 014010)
摘 要:
采用高温火法二次富集工艺对含锗粉煤灰中的锗进行富集,以锗含量为0.35%(质量分数)的粉煤灰为原料,研究温度、C含量、碱度、保温时间等工艺参数,对高温火法二次富集工艺锗的富集的影响。结果表明:当粉煤灰中碳含量为3%,碱度为1.0,在1600 ℃下保温1 h可以取得较好的锗富集效果。富集物中锗含量可达到16.65%,富集物中锗的收得率达到89.55%。对富集后的富集物和渣进行XRD物相分析,富集后锗以锗酸盐(Ca2GeO4和Ca2Ge7O16)和FeGe形式在富集物中存在,渣呈玻璃相,有利于锗的提取。
关键词:
文章编号:1004-0609(2018)-01-0183-06 中图分类号:TF843 文献标志码:A
锗是一种典型的稀有分散元素,广泛分布在自然界中,约占地壳的6.7×10-4%[1-2]。锗及其化合物常作为半导体材料、光学材料和催化剂材料等,被广泛应用于光纤通讯技术[3-8]、红外光学材料[9-12]、太阳能光伏电池[13-18]、有机物催化剂[19-20]等领域。锗具有亲石、亲铁、亲硫、亲有机物等化学性质,所以锗不能单独成矿,主要伴生在其他矿中[21-22],如铅锌矿和煤矿。对铅锌矿中锗的提取主要采用湿法为主[1],而对煤中锗的提取主要采用火法为主。
目前,褐煤燃烧发电同时收集富集的含锗粉煤灰是含锗褐煤综合利用的最佳方式,但这也只是锗的初步富集,其他富集手段包括冶金富集和化学提纯。1) 冶金富集:氯化蒸馏,即在二氧化锰和盐酸溶液中,粉煤灰经浸出、氧化、蒸馏,得GeCl4精矿[23]。2) 化学提纯:酸浸—萃取—反萃取—沉淀,得初级纯GeO2;酸浸—萃取—反萃取—氯化蒸馏—水解,得高纯GeO2[24]。但冶金富集和化学提纯存在的共同问题是:1) 原料中锗品位低,富集度不够。不管是富锗粉煤灰/煤渣,还是预富集的锗精矿,都存在这个问题。因此,火法路线需要消耗大量的碱和氯化铵,湿法路线需要消耗大量的浸酸、萃取剂、反萃取剂,使工艺成本大幅上升。2) 湿法路线流程长,浸出时间长,效率低,对酸的消耗特别大。3) 锗的损失较高,回收率通常只有60%~70%。因此,在控制工艺成本和环保因素的前提下,尽量提高这些路线锗的回收率,是目前技术研究的关键。
本文作者提出采用高温火法二次富集的工艺,对含锗粉煤灰进一步富集,研究温度、碱度、C含量,保温时间等条件对锗二次富集的影响,提高湿法处理原料的锗品位,降低后期处理盐酸用量和杂质量,减小废渣、废液的排放量,提高锗的回收率。
1 实验
1.1 实验原料
本实验选用的原料是内蒙古锡林郭勒蒙东锗业科技有限公司的含锗粉煤灰,其锗含量仅为0.35%(即3.5 kg/t)。采用ICP方法测得粉煤灰的主要成分(质量分数),结果如表1所列;并用XRD对含锗粉煤灰进行物相分析,XRD谱如图1所示。
由表1可见,含锗粉煤灰中主要成分是SiO2,铁氧化物,Al2O3和CaO,还有一定量的MgO和C,其他成分含量较低。通过图1的XRD谱分析,粉煤灰中铁氧化物主要是以Fe2O3存在,除了SiO2、Fe2O3外,其他氧化物组成复合氧化物,其中锗以锗酸盐(Mg3Fe2GeO8)的形式存在。
表1 含锗粉煤灰主要成分
Table 1 Composition of ash containing germanium (mass fraction, %)

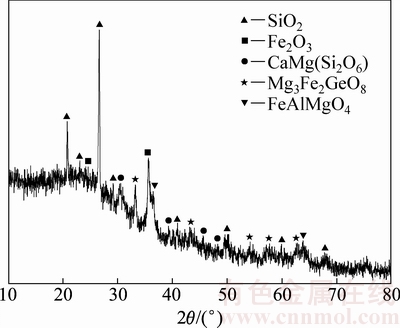
图1 含锗粉煤灰XRD谱
Fig. 1 XRD spectrum of ash containing Ge
由于粉煤灰中酸性氧化物SiO2含量比碱性氧化物CaO、K2O、MgO和Na2O总量要高,该粉煤灰属于酸性渣。粉煤灰中含有一定的碳,煤的燃烧残余的碳量不稳定,碳有很强的还原性,可以将粉煤灰中的GeO2还原为GeO,有利于锗的再次挥发富集,但也会将粉煤灰中的铁氧化物还原为铁,同时将Ge氧化物还原为金属锗,不利于锗的后期富集或提取。
1.2 实验流程图和实验方法
高温火法二次富集新工艺富集含锗粉煤灰中锗的流程图如图2所示。在粉煤灰中加入CaO和C,调节粉煤灰的碱度和氧化还原性。将配好的粉煤灰进行造球,放入高温管式电阻炉中煅烧,收集挥发分,即锗的富集物,对富集物进行收集分析。
高温火法二次富集工艺富集含锗粉煤灰中锗的实验步骤如下:
1) 配料:将一定比例的CaO和碳粉加入到粉煤灰中,利用球磨机将原料混匀。
2) 造球:由于粉煤灰较轻,堆密度较小,影响其装入量,通过圆盘造球机造球,提高粉煤灰的堆密度。
3) 煅烧:称取一定量粉煤灰放入刚玉坩埚(内径50 mm,外径55 mm,高120 mm)内,外套石墨坩埚(保护作用),放入高温管式炉中,从室温升至指定温度,实验过程中通氮气,保温一段时间,收集富集物(挥发分)和残渣,进行分析。
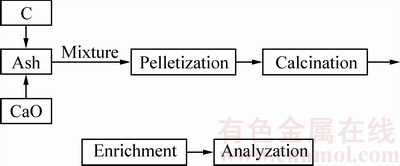
图2 实验流程图
Fig. 2 Flow chart of experiment
2 结果及分析
通过实验分析,温度、C含量、碱度、保温时间等工艺参数,对二次富集锗的回收率的有一定的影响,因此需进行实验,得到最优工艺。
2.1 碱度对粉煤灰中锗富集的影响
粉煤灰中SiO2含量较高,属于酸性渣,为了增强粉煤灰熔化后的流动性、导电性能并减轻炉渣对碱性炉衬的侵蚀,需要配入一定量的CaO。本实验按(CaO+MgO)/SiO2计算粉煤灰碱度,通过加入CaO调整其碱度,使其碱度为0.25、0.5、1.0和1.5等4个因数,其他条件固定:C含量选取初始C含量,试验温度选取1550 ℃,保温时间选取2 h,对富集后的富集物和渣进行检测。
为精确确定富集物和渣中锗含量,采用滴定法对富集物和渣中锗含量进行了测定,图3给出了不同碱度下富集后富集物和渣中锗含量。图3(a)反应出当碱度为1.0时,富集物中锗含量最高,开始随着CaO的增加富集物中锗含量增加,当碱度达到1.0时,挥发分中锗含量达到最大值,继续提高碱度会使富集物锗含量降低。从图3(b)可以看出当碱度为1.0时,高温火法二次富集后,渣中残余的锗含量最低。综上所述碱度为1.0时,最有利于粉煤灰中锗的二次富集,且该碱度下石灰添加量也较少,经济效益最高。
2.2 C含量对粉煤灰中锗富集的影响
粉煤灰中C含量并不稳定,在研究C含量对粉煤灰中锗富集的影响前,先进行脱碳处理。将粉煤灰在温度为550 ℃的马弗炉中保温3 h,当粉煤灰中存在极少火星时,取出空冷。将冷却的粉煤灰进行C含量和锗含量分析,表2给出了脱C前后粉煤灰中C和Ge含量的变化。通过表2可以看出经过脱C处理后粉煤灰中的C几乎全部被处理掉,Ge的变化并不大,说明脱C处理对粉煤灰锗含量影响不大,工艺合理。
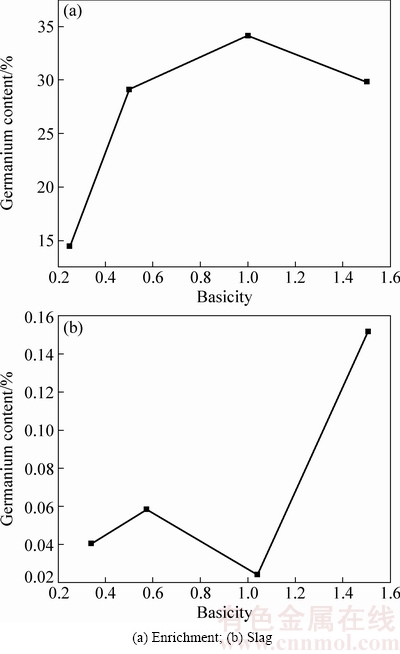
图3 不同碱度下富集物和渣中锗含量
Fig. 3 Germanium content of the Enrichment and slags at different basicities
表2 脱C前后粉煤灰中C和Ge含量
Table 2 Content of C and Ge of ash after decarbonizing

高温电炉中,称量100 g C含量为0.21%、1%、2%、3%、3.5%的粉煤灰(质量分数)分别放入Al2O3坩埚中,在1600 ℃实验温度下放入加好料的坩埚保温1 h,取出空冷,对冷却后的残余渣料锗含量进行分析。图4所示为C含量对粉煤灰富集后渣中残余锗含量的影响。由图4可以看出,C含量对粉煤灰中锗富集有一定影响。C在实验过程中,既还原铁氧化物又还原GeO2,当C含量过高的情况下,不仅铁氧化物被还原,锗也被还原熔于渣中,不利于锗的挥发富集。当C含量为3%时,粉煤灰中锗富集后的残余渣中锗含量最低,说明此时挥发出的锗量最大,最有利于锗的富集。
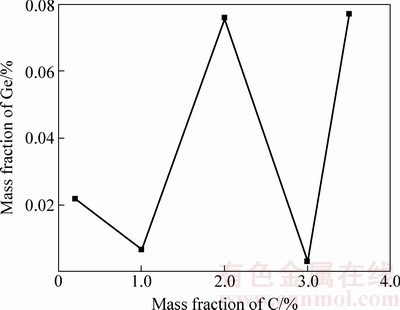
图4 不同C含量渣中残余锗含量
Fig. 4 Ge content of slags at different C contents
2.3 温度对粉煤灰中锗富集的影响
称量100 g C含量为3%,碱度为1.0的粉煤灰分别放入Al2O3坩埚中,分别在1450、1500、1550和1600 ℃的管式高温炉中保温1 h,取出空冷,对冷却后的残余渣料进行分析,图5给出了不同温度对粉煤灰富集后渣中残余锗含量的影响。从图5可以看出当温度为1600 ℃时,粉煤灰中锗富集后的残余渣中锗含量最低,渣中锗含量达到了0.0062%较低的水平,而1500 ℃时残渣中锗含量为0.0266%,1550 ℃时为0.0196%,都相对较高,说明1600 ℃时挥发出的锗量最大,最有利于锗的富集。
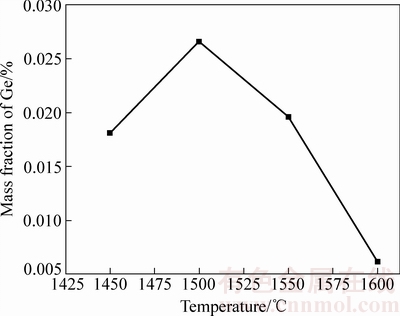
图5 不同温度下渣中残余锗含量
Fig. 5 Ge content of slags at different temperatures
2.4 保温时间对粉煤灰中锗富集的影响
称量100 g C含量为3%,碱度为1.0的粉煤灰分别放入Al2O3坩埚中,放入管式高温炉中,在温度1600 ℃条件下分别保温10 min、20 min、40 min和1 h,取出空冷,对冷却后的残余渣料进行分析,图6所示为不同保温时间对粉煤灰富集后渣中残余锗含量的影响。图6结果显示保温1 h后,渣中的锗含量已经非常低达到0.011%,满足了工业生产要求。
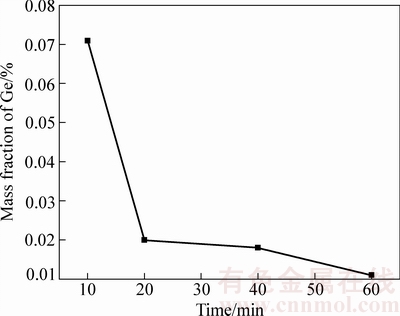
图6 不同保温时间下渣中残余锗含量
Fig. 6 Ge content of slags at different holding time
2.5 锗富集平衡实验
通过以上对温度、C含量、熔渣碱度、保温时间等工艺参数对粉煤灰中锗富集影响的研究,调整粉煤灰中碳含量为3%,碱度为1.0,在温度1600 ℃下保温1 h条件下最有利于锗的富集。经过前期实验粉煤灰中锗富集后的的品位得到了提高,为进一步确定锗的收得率,进行了锗的平衡实验,即高温火法二次富集之后分析富集物中和渣中锗含量,通过质量守恒定律确定锗在其中的分配。
锗富集的平衡实验在管式炉中进行,将C含量3%,碱度为1.0的锗尘放入Al2O3坩埚中,外套石墨坩埚起保护作用。1000 ℃下采用5 ℃/min,1000 ℃到1300 ℃采用4 ℃/min,1300 ℃以后采用3 ℃/min的升温速度升温到1600 ℃实验温度,放入盛料的坩埚在实验温度1600 ℃下保温1 h,同时收集挥发物,冷却后对富集物和渣中锗含量进行滴定分析。表3所列为锗富集实验平衡计算。
表3 锗富集实验平衡计算
Table 3 Balance computation of germanium content experiment
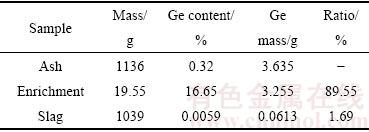
通过表3可以看出,绝大部分锗都富集到富集物中,而残渣中剩余锗含量非常低,说明锗挥发较为完全。由平衡计算结果可以看出,在实验室条件下所能收集的富集物中锗含量占原料中锗总量的89.55%,而残渣中锗含量仅为所用原料中酸溶锗的1.69%,说明锗的富集率比较高。锗总的平衡达到91.24%,后期研究发现损失的少量锗通过出气孔流失。富集物总质量是初始粉煤灰质量的17.2%,而且锗含量达到16.65%,可以大量减少后期提取盐酸用量,很大程度上节约了成本。
2.6 富集物和渣的XRD物相分析
为进一步研究高温火法二次富集工艺后锗的存在形态,对锗富集物和渣进行XRD物相分析,结果如图7所示。
通过富集物的XRD物相分析(见图7(a))可以看出,高温火法二次富集后富集物中主要物相是As2O3和一些锗酸盐还有FeGe,不存在铁氧化物和二氧化硅,减少了后期提取时盐酸的消耗。通过富集后渣的XRD物相分析可知,渣呈是玻璃相,说明渣熔化良好,有利于排渣。
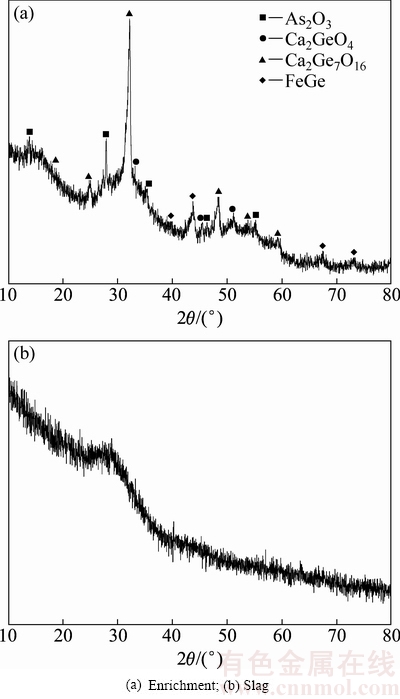
图7 富集物和渣的XRD物相分析
Fig. 7 XRD phase analysis of enrichment and slags
3 结论
1) 含锗粉煤灰中主要成分是SiO2,铁氧化物,Al2O3和CaO,还有一定量的MgO和C,其他成分含量较低,锗以以锗酸盐(Mg3Fe2GeO8)的形式存在。
2) 高温火法二次富集含锗粉煤灰中的锗的最优工艺参数是,通过添加C和CaO,使粉煤灰中碳含量为3%左右,碱度为1.0,在1600 ℃下高温煅烧1 h,富集物中锗含量可达到16.65%,锗的回收率达到89%以上。此工艺不仅提高了粉煤灰的锗品位而且减少了后期盐酸消耗。
3) 高温火法二次富集后锗主要以锗酸盐和FeGe形式存在,渣呈玻璃相,易后期排渣。
REFERENCES
[1] ZHOU Zhao-an, CHU Guang, GAN Hong-xiang, YANG Tian-zu, CHEN Lin. Ge and Cu recovery from precipitating vitriol supernatant in zinc plant[J]. Transactions of Nonferrous Metals Society of China, 2013, 23(5): 1506-1511.
[2] SCOYER J, GUISLANIN H, WOLF H U. Germanium and Germanium compounds[M]. Weinheim: Wiley-VCH Verlag GmbH & Co., 1987: 523.
[3] 褚乃林. 锗在信息高速传输主体-光导纤维中的应用前景[J]. 稀有金属, 1998, 22(5): 369-374.
CHU Nai-lin. Application prospect of germanium in optical fibres as principal part of high velocity information transmission optierl fibres[J]. Chinese Journal of Rare Metals, 1998, 22(5): 369-374.
[4] OGUAMA F A, JOHNSON A M, REED W A. Measurement of the nonlinear coefficient of telecommunication fibers as a function of Er, Al, and Ge doping profiles by using the photorefractive beam-coupling technique[J]. JOSA B, 2005, 22(8): 1600-1604.
[5] YATSENKO Y P, PRYAMIKOV A D, MASHINSKY V M, LIKHACHEV M E, MAVRITSKY A O, DIANOV E M, GURYANOV A N, KHOPIN V F, SALGANSKY M Y. Four-wave mixing with large Stokes shifts in heavily Ge-doped silica fibers[J]. Optics Letters, 2005, 30(15): 1932-1934.
[6] 黄绍艳, 肖志刚, 刘敏波, 盛江坤, 王祖军, 何宝平, 姚志斌, 唐本奇. 掺锗石英光纤的稳态和瞬态γ辐射效应研究[J]. 原子能科学技术, 2015(12): 2288-2292.
HUANG Shao-yan, XIAO Zhi-gang, LIU-Min-bo, SHENG Jiang-kun, WANG Zu-jun, HE Bao-ping, YAO Zhi-bin, TANG Ben-qi. Effect of steady-state and transient γ radiation on ge-doped quartz optical fiber[J]. Atomic Energy Science and Technology, 2015(12): 2288-2292.
[7] 孙婷婷, 王 志, 开桂云, 袁树忠, 董孝义. 掺锗微结构光纤的非线性特性研究[J]. 激光与红外, 2007(12): 1311-1314.
SUN Ting-ting, WANG Zhi, KAI Gui-yun, YUAN Shu-zhong, DONG Xiao-yi. Study on nonlinearity of microstmcture fiber with germanium doping in the core region[J]. Laser & Infrared, 2007(12): 1311-1314.
[8] YAKIMOV A I, DVURECHENSKII A V, KIRIENKO V V, STEPINA N P, NIKIFOROV A I, UL’YANOV V V, CHAIKOVSKII S V, VOLODIN V A, EFREMOV M D, SEKSENBAEV M S, SHAMIRZAEV T S, ZHURAVLEV K S. Ge/Si waveguide photodiodes with built-in layers of Ge quantum dots for fiber-optic communication lines[J]. Semiconductors, 2004, 38(10): 1225-1229.
[9] XU Hui-juan, HE Yu-ju, WANG Xun-si, NIE Qiu-hua, ZHANG Pei-quan, XU Tie-feng, DAI Shi-xun, ZHANG Xiang-hua, TAO Guang-ming. Preparation of low-loss Ge15Ga10Te75 chalcogenide glass for far-IR optics applications[J]. Infrared Physics & Technology, 2014, 65: 77-82.
[10] YIN Wen-long, FENG Kai, HE Ran, MEI Da-jiang, LIN Zhe-shuai, YAO Ji-yong, WU Yi-cheng. BaGa2MQ6 (M=Si, Ge; Q=S, Se): A new series of promising IR nonlinear optical materials[J]. Dalton Transactions, 2012, 41(18): 5653-5661.
[11] YIN Wen-long, FENG Kai, HAO Wen-yu, YAO Ji-yong, WU Yi-cheng. Synthesis, structure, and properties of Li2In2MQ6 (M=Si, Ge; Q=S, Se): A new series of IR nonlinear optical materials[J]. Inorganic Chemistry, 2012, 51(10): 5839-5843.
[12] 林正怀, 张 培, 王加贤. 纳米锗镶嵌二氧化硅薄膜的光学性质及其应用的研究[J]. 量子光学学报, 2012(4): 377-381.
LIN Zheng-huai, ZHANG Pei, WANG Jia-xian. Investigation on optical properties and application of nc-Ge/SiO2 film[J]. Journal of Quantum Optics, 2012(4): 377-381.
[13] HUDAIT M K, MODAK P, HARDIKAR S, RAO K S R K, KRUPANIDHI S B. Comparative studies of Si-doped n-type MOVPE GaAs on Ge and GaAs substrates[J]. Material Science and Engineering B, 1998, 55(1): 53-67.
[14] HUDAIT M K, KRUPANIDHI S B. Growth, optical, and electron transport studies across isotype n-GaAs/n-Ge heterojunctions[J]. Journal of Vacuum Science & Technology B, 1999, 17(3): 1003-1010.
[15] LI Jia-jia, ZHAO Xiang-yu, DU Wei, YANG Meng, MA Li-qun, DING Yi, SHEN Xiao-dong. Effect of germanium on electrochemical performance of chain-like Co-P anode material for Ni/Co rechargeable batteries[J]. Transactions of Nonferrous Metals Society of China, 2013, 23(7): 2060-2065.
[16] 孟祥东, 曲晓慧, 于兆亮, 胡 悦, 孙 萌. 硅锗光子晶体复合电极对非晶硅电池性能的影响[J]. 吉林师范大学学报(自然科学版), 2016(3): 36-40.
MENG Xiang-dong, QU Xiao-hui, YU Zhao-liang, HU Yue, SUN Meng. Properties of amorphous silicon solar cell with silicon-germanium photonic crystal back-reflector electrode[J]. Jilin Normal University Journal(Natural Science Edition), 2016(3): 36-40.
[17] DERLUYN J, DESSEIN K, FLAMAND G, MOLS Y, POORTMANS J, BORGHS G. Comparison of MOVPE grown GaAs solar cells using different substrates and group-V precursors[J]. Journal of Crystal Growth, 2003, 247(3): 237-244.
[18] FLAMAND G, MOERMAN I, DERLUYN J, DESSEIN K, VAN BAVEL M. A safer, efficient MOCVD process for GaAs solar cell growth[J]. Photovoltaics Bulletin, 2002, 8: 7-9.
[19] SARKAR S, JANA R, WAGHMARE U V, KUPPAN B, SAMPATH S, PETER S C. Ordered Pd2Ge intermetallic nanoparticles as highly efficient and robust catalyst for ethanol oxidation[J]. Chemistry of Materials, 2015, 27(21): 7459-7467.
[20] TONG Xin-li, WANG Yan-hua, NIE Guang-xia, YAN Yong-tao. Selective dehydration of fructose and sucrose to 5-hydroxymethyl-2-furfural with Heterogeneous Ge (IV) catalysts[J]. Environmental Progress & Sustainable Energy, 2015, 34(1): 207-210.
[21] BERNSTEIN L R. Germanium geochemistry and mineralogy[J]. Geochimica et Cosmochimica Acta, 1985, 49(11): 2409-2422.
[22] 雷 霆, 张玉林, 王少龙. 锗的提取方法[M]. 北京: 冶金工业出版社, 2006: 66-71.
LEI Ting, ZHANG Yu-lin, WANG Shao-long. The extraction methods of germanium[M]. Beijing: Metallurgical Industry Press, 2006: 66-71.
[23] 刘福财, 袁 琴, 王铁艳. 煤烟尘制取四氯化锗的研究[J]. 稀有金属, 2011(4): 623-626.
LIU Fu-cai, YUAN Qin, WANG Tie-yan. Preparation of germanium tetrachloride with soot[J]. Chinese Journal of Rare Metals, 2011(4): 623-626.
[24] 蒋 伟, 蒋开喜, 王海北, 蒋训雄, 张邦胜. 一种从锗精矿制备高纯二氧化锗的新工艺[J]. 矿冶, 2007(3): 26-28.
JIANG Wei, JIANG Kai-xi, WANG Hai-bei, JIANG Xun-xiong, ZHANG Bang-sheng. A new process for the preparation of high purity germanium dioxide from germanium concentrate[J]. Mining & Metallurgy, 2007(3): 26-28.
Germanium enrichment from ash by second enrichment process at high temperature
LIU Li-xia1, 2, LI Wen-ting1, 2, PENG Jun2, ZHANG Lei2, CAI Chang-kun2, AN Sheng-li1, 2
(1. School of Metallurgical and Ecological Engineering, University of Science and Technology Beijing, Beijing 100083, China;
2. Material and Metallurgy School, Inner Mongolia University of Science and Technology, Baotou 014010, China)
Abstract: The enrichment of germanium from the ash was adopted by new method of second enrichment technology at high temperature in this experiment. The ash with 0.35% germanium was used as raw material. Effects of the parameters, such as temperature, C content, basicity and soaking time, on second enrichment of germanium were investigated. The results show that the effect of germanium enrichment is better when the carbon content is 3% in the ash, the basicity is 1, and it is insulated for 1 h at 1600 ℃. The germanium content in the enrichment is 16.65%, and the yield rate of the germanium is 89.55%. The enrichment and slag were analyzed by X-ray diffraction (XRD), the results show that the germanium in the enrichment exists as germanates and FeGe, and the slags exists as glass phase, which is propitious to the extraction of germanium.
Key words: high temperature; germanium; enrichment; coal ash
Foundation item: Project(51564039) supported by the National Natural Science Foundation of China; Project (2015MS0553) supported by Inner Mongolia Science Foundation, China
Received date: 2016-11-29; Accepted date: 2017-06-05
Corresponding author: PENG Jun; Tel: +86-472-5951536; E-mail: pengjun75@163.com
(编辑 王 超)
基金项目:国家自然科学基金资助项目(51564039);内蒙古自然科学基金资助项目(2015MS0553)
收稿日期:2016-11-29;修订日期:2017-06-05
通信作者:彭 军,副教授,博士;电话:0472-5951536;E-mail:pengjun75@163.com
摘 要:采用高温火法二次富集工艺对含锗粉煤灰中的锗进行富集,以锗含量为0.35%(质量分数)的粉煤灰为原料,研究温度、C含量、碱度、保温时间等工艺参数,对高温火法二次富集工艺锗的富集的影响。结果表明:当粉煤灰中碳含量为3%,碱度为1.0,在1600 ℃下保温1 h可以取得较好的锗富集效果。富集物中锗含量可达到16.65%,富集物中锗的收得率达到89.55%。对富集后的富集物和渣进行XRD物相分析,富集后锗以锗酸盐(Ca2GeO4和Ca2Ge7O16)和FeGe形式在富集物中存在,渣呈玻璃相,有利于锗的提取。
[3] 褚乃林. 锗在信息高速传输主体-光导纤维中的应用前景[J]. 稀有金属, 1998, 22(5): 369-374.
[6] 黄绍艳, 肖志刚, 刘敏波, 盛江坤, 王祖军, 何宝平, 姚志斌, 唐本奇. 掺锗石英光纤的稳态和瞬态γ辐射效应研究[J]. 原子能科学技术, 2015(12): 2288-2292.
[7] 孙婷婷, 王 志, 开桂云, 袁树忠, 董孝义. 掺锗微结构光纤的非线性特性研究[J]. 激光与红外, 2007(12): 1311-1314.
[12] 林正怀, 张 培, 王加贤. 纳米锗镶嵌二氧化硅薄膜的光学性质及其应用的研究[J]. 量子光学学报, 2012(4): 377-381.
[16] 孟祥东, 曲晓慧, 于兆亮, 胡 悦, 孙 萌. 硅锗光子晶体复合电极对非晶硅电池性能的影响[J]. 吉林师范大学学报(自然科学版), 2016(3): 36-40.
[22] 雷 霆, 张玉林, 王少龙. 锗的提取方法[M]. 北京: 冶金工业出版社, 2006: 66-71.
[23] 刘福财, 袁 琴, 王铁艳. 煤烟尘制取四氯化锗的研究[J]. 稀有金属, 2011(4): 623-626.
[24] 蒋 伟, 蒋开喜, 王海北, 蒋训雄, 张邦胜. 一种从锗精矿制备高纯二氧化锗的新工艺[J]. 矿冶, 2007(3): 26-28.


