- Abstract:
- 1 Introduction▲
- 2 Experimental▲
- 3 Results and discus...▲
- 4 Conclusions▲
- References
- Figure
- Figure 1 (a) XRD patterns of pure NiCoP and Se-doped NiCoP; (b) XPS survey spectra; (c) Co 2p, (d) Ni 2p and (e) P 2p of NiCoP/CC and Se-NiCoP-2/CC; (f) Se 3d of Se-NiCoP-2/CC
- Figure 2 (a, b) SEM images; (c) TEM images; (d) HRTEM images; (e) EDS elemental mapping of Se-NiCoP-2
- Figure 3 (a) HER polarization curve; (b) Tafel slope plots; (c) Cdl values of obtained samples; (d) Electrochemical impedance spectroscopy; (e) Polarization curves of Se-NiCoP-2/CC electrode initially and after CV cycles; (f) Chronoamperometry J–t curve
- Figure 4 (a) OER polarization curve; (b) Tafel slope plots; (c) Polarization curves of overall water splitting;(d) Chronoamperometry curve

J. Cent. South Univ. (2021) 28: 2345-2359
DOI: https://doi.org/10.1007/s11771-021-4774-y

3D Se-doped NiCoP nanoarrays on carbon cloth for efficient alkaline hydrogen evolution
LIU Zi-xuan(刘紫轩), WANG Xiao-long(王晓龙), HU Ai-ping(胡爱平),TANG Qun-li(唐群力), XU Ya-li(徐亚利), CHEN Xiao-hua(陈小华)
College of Materials Science and Engineering, Hunan University, Changsha 410082, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Abstract:
The exploration of stable and highly efficient alkaline hydrogen evolution reaction (HER) electrocatalysts is imperative for alkaline water splitting. Herein, Se-doped NiCoP with hierarchical nanoarray structures directly grown on carbon cloth (Se-NiCoP/CC) was prepared by hydrothermal reaction and phosphorization/selenization process. The experimental results reveal that Se doping could increase the electrochemical active sites and alter the electronic structure of NiCoP. The optimized Se-NiCoP/CC electrode exhibits outstanding HER activity in alkaline electrolyte, which only needs a low overpotential of 79 mV at the current density of 10 mA/cm2. When serving as anode and cathode electrode simultaneously, the Se-NiCoP/CC electrodes achieve current density of 50 mA/cm2 at a low voltage of only 1.62 V. This work provides a feasible way to rationally design high active HER electrocatalysts.
Key words:
Cite this article as:
LIU Zi-xuan, WANG Xiao-long, HU Ai-ping, TANG Qun-li, XU Ya-li, CHEN Xiao-hua. 3D Se-doped NiCoP nanoarrays on carbon cloth for efficient alkaline hydrogen evolution [J]. Journal of Central South University, 2021, 28(8): 2345-2359.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-021-4774-y1 Introduction
Water electrolysis is acknowledged as a promising pathway to produce high pure hydrogen [1, 2]. However, electrolysis of water requires high energy consumption, so efficient catalysts are needed to speed up the reaction rate and improve the overall efficiency [3, 4]. Noble metals such as Pt have excellent catalytic activity, but their cost and resource restrict them for massive scale applications [5]. To date, considerable nonprecious metal-based HER electrocatalysts under acidic media have been developed as the substitutes for Pt-based catalysts [6, 7]. However, the harsh acidic environment and the lack of efficient catalysts for oxygen evolution reaction (OER) in acidic media make it urgent to explore efficient non-noble metal alkaline HER electrocatalysts [8-10].
Recently, bimetallic phosphides, such as FexCo1-xP, MoWP and CoMoP@C, have been demonstrated superior HER activity than their relative monometallic phosphide counterparts [11-14]. KIBSGAARD et al [15] found that Fe0.5Co0.5P has a more thermo-neutral Gibbs free energy of H adsorption (△GH*) and exhibits higher activity than FeP or CoP. In addition, previous researches have pointed out that the composition greatly affects the hydrogen evolution performance [16-20]. For example, CAO et al [21] reported that the NixCo2-xP@NC NA/NF shows the best electrochemical performance when the ratio of Ni/Co is 1:1. Nevertheless, the hydrogen binding energy of NiCoP is far from the zero-energy level [16, 22]. Therefore, it is still a challenge to further improve the HER performance of NiCoP.
Heteroatom doping is considered one of the effective strategies to enhance the HER activity of transition metal phosphides (TMPs) [1, 23-26]. It could modulate the electronic structure and increase the active sites exposure so that the optimized hydrogen adsorption free energy and an enlarged electrochemical active surface area can be achieved [27]. For example, LIN et al [16] successfully developed S-doped NiCoP nanosheet arrays and it only requires 88 mV to obtain a current density of 10 mA/cm2, which is substantially superior to NiCoP (151 mV). LIU et al [17] found that oxygen doping can optimize electrical conductivity and hydrogen adsorption Gibbs free-energy of NiCoP, which is good for HER activity. Inspired by the previous works, we consider that selenium doping into NiCoP is expected to enhance the HER performance based on the reasons as follows: 1) Due to the electronegativity of Se greater than P, similar as S and O, Se doping in NiCoP can tune the electronic state of NiCoP. In addition, its more metallic nature is beneficial to the improvement of electronic conductivity [19, 28]. 2) The larger atomic radius of selenium atom can lead to lattice defects after doping into the crystal structure of NiCoP and provides additional active sites for electrochemical reaction. For instance, HU and co-workers [29] reported that the incorporation of Se in (NiCo)S/OH nanosheets introduced structural defect and lattice distortion, which exhibited high HER performance with current density 10 mA/cm2 at 103 mV potential.
In this work, three-dimensional Se-doped NiCoP nanorod arrays on carbon cloth were constructed by hydrothermal and selenization/phosphorization process and their alkaline HER electrocatalytic performance were further investigated. The optimal Se-NiCoP/CC catalyst exhibits excellent HER activity in alkaline solution owing to several favorable advantages as follows: 1) the direct growth of the electrocatalysts on conductive substrate can not only increase the electronic conductivity of the catalyst, but also avoid the use of polymer binders, which is beneficial for increasing active sites exposure; 2) the open network and multi-level array structure facilitate the contact with electrolyte and the rapid release of gas; 3) Se doping can alter the electronic structure of NiCoP and increase active sites exposure. Thus, the reaction kinetics can be accelerated and the electrochemical active surface area can be enlarged. To further prove the competitive advantage of the Se-NiCoP/CC for practical applications, the performance for overall water splitting by applying Se-NiCoP-2/CC electrode as both cathode and anode was measured. The Se-NiCoP-2/CC||Se-NiCoP-2/CC pair only needs a low voltage of 1.62 V with 50 mA/cm2 to drive the overall water splitting.
2 Experimental
2.1 Synthesis of NiCoP/CC
Carbon cloth was firstly immersed in nitric acid (35%) at 80 °C for 12 h [30], and then cleaned with acetone, deionized water and ethanol, respectively to increase its hydrophilicity. In a typical synthetic route, CoCl2·6H2O (1.5 mmol) and NiCl2·6H2O (1.5 mmol) were dissolved in 30 mL of deionized water under vigorous stirring. Then, NH4F (8 mmol) and urea (15 mmol) were dispersed in the above solution to form a clear pink solution. The treated carbon cloth (1 cm×2 cm) was fixed on the autoclave wall through waterproof adhesive tape. The above solution was then poured into hydrothermal kettle and kept for 6 h at 120 °C. After natural cooling, the obtained NiCo-precursor was washed with DI water and dried overnight. To synthesize NiCoP/CC, the NiCo precursor was placed at the center of the tube furnace, and the NaH2PO2·H2O (0.5 g) was placed on the upstream position. Then, the quartz tube was heated to 300 °C for 2 h under Ar atmosphere. After cooling naturally, the NiCoP/CC was obtained.
2.2 Synthesis of Se-NiCoP/CC
The NiCo precursor was placed at the center of the tube furnace, and the mixture of 0.5 g NaH2PO2·H2O and 0.2 mmol selenium powder was placed in the upstream position. First, the Ar gas is pumped into the tube for 10 min to remove the air. Then, the device was heated to 300 °C (5 °C/min) for 2 h. After cooling naturally, the Se-NiCoP-2/CC was obtained. To optimize the Se doping, the amount of Se powder was adjusted to 0.1, 0.3 and 0.4 mmol, and the obtained samples were recorded as Se-NiCoP-x/CC (x=1, 3, 4), respectively.
2.3 Preparation of Pt/C(20 wt%) on carbon cloth
2 mg commercial Pt/C (20 wt%) was dissolved in a mixed solution (0.5 mL ethanol and 25 μL 5% Nafion) to form a uniform suspension. The suspension was then dropped onto carbon cloth (1 cm×1 cm) and dried at room temperature.
2.4 Materials characterization
D8 Advance X-ray diffractometer (Cu-Kα, λ=0.15418 nm), ESCALAB 250 Xi (Thermo Fisher Scientific, UK), S-4800 field emission microscope (Hitachi, japan) and JEM-2100F TEM (JEOL, Japan) were used.
2.5 Electrochemical measurements
The electrochemical performance of samples was measured on a CHI 660E electrochemical station in a three-electrode system. The Se-NiCoP-x/CC (1 cm×2 cm) was applied as the working electrode, and Hg/HgO electrode and graphite rod acted as reference electrode and counter electrode, respectively. In the electrochemical test, the area of the working electrode immersed in the electrolyte was 1 cm×1 cm. The potentials mentioned in this work were calibrated with reference to a reversible hydrogen electrode (RHE) by adding a value of (0.098+0.059pH) V. The linear sweep voltammogram (LSV) curves were conducted with a sweep rate of 5 mV/s. Cyclic voltammogram (CV) scans were performed in the above potential ranges with a sweeping rate of 100 mV/s to test the durability of samples. The long-term stability was measured through chronoamperometry under controlled potentials. Electrochemical impedance spectroscopy (EIS) test was conducted at the potentiostatic mode in the frequency range from 105 to 0.1 Hz. All electrochemical measurements were IR compensated.
3 Results and discussion
The fabrication process for Se-NiCoP/CC nanorod arrays is depicted in Scheme 1. Firstly, NiCo-precursor arrays were synthesized on carbon cloth via a hydrothermal reaction. Then, the incorporation of Se and phosphidation treatment were simultaneously realized by employing Se- powder and NaH2PO2.H2O as the Se and P resources at 300 °C under Ar environment, respectively. In this process, the pink NiCo-precursor turns to black Se doped NiCoP sample. The SEM images of NiCoP/CC and Se-NiCoP-x/CC (x=1, 3, 4) are shown in Figures S1-S4 in Supporting information display similar morphologies, which demonstrates that the structure of NiCoP is basically consistent after Se doping.
To analyze the phase structures of obtained catalysts, the X-ray diffraction (XRD) patterns of NiCoP and Se-NiCoP-x (x=1, 2, 3, 4) were carried out (Figure 1(a)). The diffraction peaks of samples at 30.6°, 35.5°, 41.0°, 44.9°, 47.6° are in good coincidence with the (110), (200), (111), (201), (210) and (300) planes of the NiCoP (JCPDS card No. 71-2336), respectively [16, 22]. In addition, no peaks about selenide-based phase were detected. The (210) peaks of Se-NiCoP-x (x=1, 2, 3, 4) samples have a slightly negative shift compared to that of NiCoP, which is attributed to the lattice expansion caused by the substitution of P by Se with larger ratomic radius [31]. It suggests that Se atoms have been successfully incorporated into the crystal structure of NiCoP.
To further determine the roles of Se doping, the XPS measurements were carried out. The survey spectra reveal that the Se-NiCoP/CC sample contains Ni, Co, P, Se and O elements (Figure 1(b)). The O element may be caused by the oxidation of samples surface in contact with air [17]. From the high-resolution Co 2p spectra (Figure 1(c)), the peaks at 778.69 eV and 793.6 eV correspond to Co-P bonds, and the two peaks at 781.79 eV and 797.93 eV are related to Co—O bonds [32]. Other peaks at 786.47 eV and 803.25 eV can be ascribed to the satellite peaks [33]. In Figure 1(d), the peaks at 853.45 eV and 870.96 eV originate from Ni-P bonds, and the peaks at 856.51 eV and 874.2 eV can be ascribed to oxidized Ni species [34]. The peaks at 861.42 eV and 880.23 eV belong to the satellite peaks [16]. In the spectra of P 2p (Figure 1(e)), the peaks at 129.71 eV and 130.64 eV are assigned to phosphide [33], while the peak at 133.91 eV is related to metal oxidized phosphorus [35]. The peak at 137.5 eV corresponds to the Auger line of the Se element. In the spectrum of the Se 3d in Figure 1(f), the peaks at 54.4 eV and 55.0 eV correspond to Se 3d5/2 and Se 3d3/2, corresponding to metal-selenium bonds [31]. Notably, compared with pure NiCoP, the peaks of Ni 2p, Co 2p and P 2p in Se-NiCoP are all shifted to higher energy region, which suggest that Se doping has a strong influence on electronic interactions [19]. The increased binding energies of Ni 2p, Co 2p and P 2p in Se-NiCoP demonstrate the electrons transfer from Ni, Co and P to Se, owing to greater electronegativity of Se atom than P atom [17]. As a result, fewer electrons in Co and Ni atoms can interact with H. The binding interaction between Co, Ni atoms and H is weakened, which is beneficial for the HER process [33].
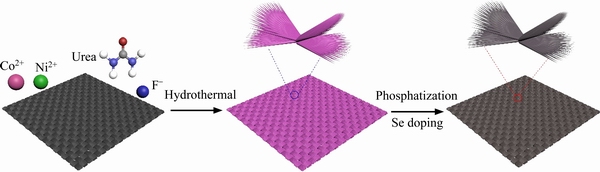
Scheme 1 Synthesis process of Se doped NiCoP nanorod arrays on carbon cloth

Figure 1 (a) XRD patterns of pure NiCoP and Se-doped NiCoP; (b) XPS survey spectra; (c) Co 2p, (d) Ni 2p and (e) P 2p of NiCoP/CC and Se-NiCoP-2/CC; (f) Se 3d of Se-NiCoP-2/CC
The SEM images of Se-NiCoP-2/CC are shown in Figures 2(a) and (b). It displays an open network and interlaced nanosheets architecture. The carbon cloth substrate is fully covered by Se-NiCoP nanosheets arrays. These 400-500 nm thick nanosheets arrays are composed of nanorods, which grow vertically and interconnect with each other on carbon cloth. Such highly open networks and multi-level array structure not only facilitate the contact between the electrode and electrolyte, but also improve the transmission rates of electroactive component and the diffusion rate of bubbles. Its TEM images (Figure 2(c)) show that the nanorods with a diameter of 50-60 nm and a length of 600- 700 nm. Moreover, there are many pores with different sizes distribution on the surface of nanorods. The high-resolution TEM (HRTEM) image (Figure 2(d)) shows the lattice fringes of 0.22 nm, attributing to the (111) plane of NiCoP [21]. Notably, in selected white-dashed areas of Se-NiCoP-2/CC, a large number of lattice mismatch were observed. It demonstrates that the incorporation of Se in NiCoP nanorods can induce lattice distortion, which may provide additional active sites for electrochemical reaction. The corresponding elemental mapping of Se-NiCoP-2/CC reveals the uniform distribution of Ni, Co, P and Se elements, which further confirms the successful incorporation of Se into the sample (Figure 2(e)). The EDS spectra of Se-NiCoP-x/CC (x=1, 2, 3, 4) show that the Se contents in the samples are 2.81 at%, 5.91 at%, 9.78 at% and 11.70 at%, respectively (Figure S5).
The electrocatalytic HER performance of all samples was measured in 1.0 mol/L KOH solution. From the polarization curves in Figure 3(a), the commercial Pt/C electrocatalyst shows the best HER activity and the pure carbon cloth has almost no activity for the HER. The overpotential of Se-NiCoP-1/CC, Se-NiCoP-2/CC, Se-NiCoP-3/CC and Se-NiCoP-4/CC at 10 mA/cm2 is 87, 79, 84 and 90 mV, respectively, which is less than that of NiCoP/CC (99 mV), indicating that Se doping can effectively improve the HER activity of NiCoP/CC. Obviously, among Se-NiCoP/CC electrodes, the Se-NiCoP-2/CC shows the best HER electrocatalytic activity. It also displays a superior performance than the reported HER catalysts in Table S1 (Supporting information). As shown in Table S2, at a large current density of 100 mA/cm2, the overpotential of Se-NiCoP-2/CC is 158 mV, which is also lower than those of NiCoP/CC (178 mV), Se-NiCoP-1/CC (168 mV), Se-NiCoP-3/CC (160 mV), Se-NiCoP-4/CC (174 mV), respectively, which further suggests the outstanding HER activity of Se-NiCoP-2/CC electrode. The reaction kinetics in the HER process were analyzed by Tafel plots (Figure 3(b)). The Tafel slope of Se-NiCoP-2/CC is 81.6 mV/dec, indicating that its hydrogen evolution process may be followed by the Volmer-Heyrovsky mechanism, and the electrochemical desorption is rate determined step [28, 36, 37]. In comparison, the Tafel slope of NiCoP/CC is 118.8 mV/dec, significantly higher than that of Se-NiCoP-1/CC (105 mV/dec),Se-NiCoP-2/CC (81.6 mV/dec), Se-NiCoP-3/CC (86 mV/dec) and Se-NiCoP-4/CC (108 mV/dec). The small Tafel slope of Se-NiCoP-2/CC is more beneficial for practical applications because it can significantly increase HER rate and reduce overpotential [22, 38].

Figure 2 (a, b) SEM images; (c) TEM images; (d) HRTEM images; (e) EDS elemental mapping of Se-NiCoP-2

Figure 3 (a) HER polarization curve; (b) Tafel slope plots; (c) Cdl values of obtained samples; (d) Electrochemical impedance spectroscopy; (e) Polarization curves of Se-NiCoP-2/CC electrode initially and after CV cycles; (f) Chronoamperometry J–t curve
The electrochemical active surface area (ECSA) of catalyst is often used to evaluate intrinsic catalytic activity [21, 39]. In order to estimate the ECSA of as-prepared catalysts, the double-layer capacitance (Cdl) of each catalyst was obtained by cyclic voltammetry (CV) test (Figure S6). The Cdl of Se-NiCoP-2/CC (63.7 mF/cm2) is larger than that of NiCoP/CC (31.6 mF/cm2) and other Se-NiCoP sample, suggesting that the Se-NiCoP-2/CC has a highest electrochemical active surface area and the most exposed active sites (Figure 3(c)). Since the Se-NiCoP-x/CC (x=1, 2, 3, 4) and NiCoP/CC samples have similar morphologies, the amount of doped Se plays an important role in the incensement of ECSA.
To further obtain insight into the HER reaction kinetics, the interfacial charge transfer resistance of different catalysts in HER process was investigated by the Nyquist plots (Figure 3(d)). As the selenium content increases, the interfacial charge transfer resistance of the doped electrode decreases and then increases compared to the undoped electrode. This may be caused by the following reasons: On the one hand, doped selenium atoms with more metallic properties in NiCoP can induce lattice distortion to improve the electronic conductivity [28, 29]; On the other hand, excessive selenium doping will weaken the delocalization ability of electrons in Co and Ni atoms and increase the resistance [38]. The Se-NiCoP-2/CC exhibits a smaller diameter of semicycle, indicating that it has the lowest interfacial charge transfer resistance.
The electrochemical stability of Se-NiCoP-2/CC was evaluated by continuous CV test and chronopotentiometry test. As shown in Figure 3(e), there is no obvious deviation of current density after 1000 cycle sweeps even in the high overpotential region. Under the constant voltage (Figure 3(f)), the current density of Se-NiCoP-2/CC electrode shows no obvious decrease after the continuous hydrogen release for 20 h, which indicates the good durability of Se-NiCoP-2/CC electrode in long-term HER process. The SEM image (Figure S7(a)) and XRD pattern (Figure S7(b)) of Se-NiCoP-2/CC electrode after durability test show that the nanosheets arrays structure and phase of Se-NiCoP-2 were well retained, demonstrating the excellent stability of Se-NiCoP-2/CC electrode. The XPS spectra (Figure S8) of Se-NiCoP-2/CC electrode after durability test show that the peak positions of Co 2p, Ni 2p, P 2p and Se 3d change little, which further demonstrates that Se-NiCoP-2/CC electrode has good chemical stability. Based on the analysis above, the better HER activity of Se-NiCoP-2/CC could be attributed to the fact that Se atoms effectively modulate the electronic structure and introduce abundant defects, leading to the accelerated reaction kinetics and the increased electroactive site, which is in accordance with the reports of literature [29].
The OER performance of all samples was also measured in alkaline solution, as shown in Figure 4. The overpotential for Se-NiCoP-2/CC is 303 mV at 50 mA/cm2, which is much lower than that of NiCoP/CC (335 mV), Se-NiCoP-1/CC (310 mV), Se-NiCoP-3/CC (306 mV), Se-NiCoP-4/CC(324 mV) (Figure 4(a)). The Tafel slope of the Se-NiCoP-2/CC catalyst (107.97 mV/dec) is also smaller than those for NiCoP (165.43 mV/dec), Se-NiCoP-1/CC (118.36 mV/dec), Se-NiCoP-3/CC (112.21 mV/dec), Se-NiCoP-4/CC (146.22 mV/dec) (Figure 4(b)). These results suggest that Se doping can also improve the OER activity of NiCoP.
Finally, using Se-NiCoP-2/CC as cathode and anode, respectively, the overall water splitting performance of Se-NiCoP-2/CC in 1 mol/L KOH alkaline electrolytic cell was further studied. The Se-NiCoP-2/CC||Se-NiCoP-2/CC only needs a low voltage of 1.62 V at 50 mA/cm2 (Figure 4(c)). The catalytic activity of Se-NiCoP-2/CC is superior to the reported electrocatalysts in Table S2 (Supporting information). In addition, it can be clearly observed that obvious hydrogen and oxygen bubbles are generated on the electrodes of the cathode and anode. Furthermore, the Se-NiCoP-2/CC showed stable durability in the long-term electrochemical process (Figure 4(d)). The voltage only increased 20 mV after working for 40 h at 10 mA/cm2.
4 Conclusions
In summary, we successfully synthesized Se-NiCoP/CC hierarchical nanoarray structures by hydrothermal method and selenization/ phosphorization process with Se powder and NaH2PO2.H2O as the Se and P resources, respectively. Benefiting from the multi-level array structure, the excellent electron transport rate and abundant accessible active sites, the Se-NiCoP/CC electrode manifests outstanding HER catalytic performance in alkaline solutions. Additionally, when applied for overall water splitting, the Se-NiCoP/CC electrode also displays a superior performance and stability in alkaline solution. Consequently, the optimal Se-doped NiCoP electrode shows an excellent potential for practical applications for overall water splitting. This work may contribute to our deeper understanding of the effect of anion doping and can be applied to the reasonable design of other electrocatalysts.

Figure 4 (a) OER polarization curve; (b) Tafel slope plots; (c) Polarization curves of overall water splitting;(d) Chronoamperometry curve
Contributors
LIU Zi-xuan: Investigation, methodology, writing-original draft. WANG Xiao-long, TANG Qun-li, and XU Ya-li:Investigation, validation. HU Ai-ping and CHEN Xiao-hua: Supervision, conceptualization, writing-reviewing & editing, funding acquisition.
Conflict of interest
The authors declare no competing financial interest.
Supporting information

Figure S1 SEM images of NiCoP/CC with different resolutions

Figure S2 SEM images of Se-NiCoP-1/CC with different resolutions

Figure S3 SEM images of Se-NiCoP-3/CC with different resolutions

Figure S4 SEM images of Se-NiCoP-4/CC with different resolutions

Figure S5 EDS of (a) Se-NiCoP-1/CC, (b) Se-NiCoP-2/CC, (c) Se-NiCoP-3/CC and (d) Se-NiCoP-4/CC
Table S1 Comparison with previous reports in alkaline media

Table S2 Overpotential of different catalysts at current densities of 10 mA/cm2 and 100 mA/cm2


Figure S6 CVs curves of (a) NiCoP/CC, (b) Se-NiCoP-1/CC, (c) Se-NiCoP-2/CC, (d) Se-NiCoP-3/CC, (e) Se-NiCoP-4/CC and (f) pure carbon cloth (CC)
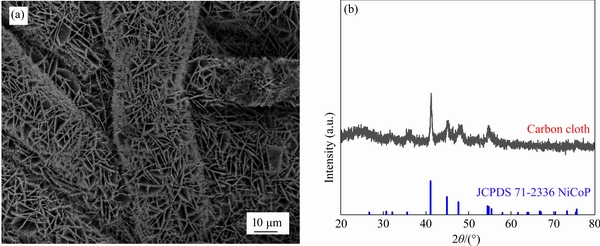
Figure S7 (a) SEM image and (b) XRD pattern of Se-NiCoP-2/CC after durability test

Figure S8 High resolution of (a) Co 2p, (b) Ni 2p, (c) P 2p and Se 2p (d) XPS spectra of Se-NiCoP-2/CC after durability test
Table S2 Comparison of cell voltages of different electrodes for overall water splitting

References
[1] LIU Bao-cang, LI Huan, CAO Bo, JIANG Jia-ning, GAO Rui, ZHANG Jun. Few layered N, P dual-doped carbon-encapsulated ultrafine MoP nanocrystal/MoP cluster hybrids on carbon cloth: An ultrahigh active and durable 3D self-supported integrated electrode for hydrogen evolution reaction in a wide pH range [J]. Advanced Functional Materials, 2018, 28(30): 1801527-1801540. DOI: 10.1002/ adfm.201801527.
[2] ZHU Yun-pei, LIU Yu-ping, REN Tie-zhen, YUAN Zhong-yong. Self-supported cobalt phosphide mesoporous nanorod arrays: A flexible and bifunctional electrode for highly active electrocatalytic water reduction and oxidation [J]. Advanced Functional Materials, 2015, 25(47): 7337-7347. DOI: 10.1002/adfm.201503666.
[3] QAZI U, JAVAID R, TAHIR N, JAMIL A, AFZAL A. Design of advanced self-supported electrode by surface modification of copper foam with transition metals for efficient hydrogen evolution reaction [J]. Int J Hydrogen Energy, 2020, 45(58): 33396-33406. DOI: https://doi.org/10.1016/j.ijhydene.2020. 09.026.
[4] WANG Yang, LI Xiao-peng, ZHANG Meng-meng, ZHOU Yuan-guang, RAO De-wei, ZHONG Cheng, ZHANG Jin-feng, HAN Xiao-peng, HU Wen-bin, ZHANG Yu-cang, ZAGHIB K, WANG Yue-sheng, DENG Yi-da. Lattice-strain engineering of homogeneous NiS0.5Se0.5 core-shell nanostructure as a highly efficient and robust electrocatalyst for overall water splitting [J]. Adv Mater, 2020, 32(40): 2000231-2000240. DOI: 10.1002/adma.202000231.
[5] ZHANG Xing, YU Xiao-lu, ZHANG Lin-jie, ZHOU Feng, LIANG Yong-ye, WANG Rui-hu. Molybdenum phosphide/ carbon nanotube hybrids as pH-universal electrocatalysts for hydrogen evolution reaction [J]. Advanced Functional Materials, 2018, 28(16): 1706523-1706530. DOI: 10.1002/ adfm.201706523.
[6] YUAN Xu, YUE Wen-bo, ZHANG Jin. Electrochemically exfoliated graphene as high-performance catalyst support to promote electrocatalytic oxidation of methanol on Pt catalysts [J]. Journal of Central South University, 2020, 27(9): 2515-2529. DOI: https://doi.org/10.1007/s11771-020-4477-9.
[7] WANG Li-ping, TIAN Jing, LI Jing-sha, ZENG Xian-guang, PENG Zhi-guang, HUANG Xiao-bing, TANG You-gen, WANG Hai-yan. Red-blood-cell-like nitrogen-doped porous carbon as an efficient metal-free catalyst for oxygen reduction reaction [J]. Journal of Central South University, 2019, 26(6): 1458-1468. DOI: https://doi.org/10.1007/s11771-019-4102-y.
[8] XIE Tian, ZHAO Hai-xia, LV Zun-hang, XIE Guang-wen, HE Yan. A highly active composite electrocatalyst Ni–Fe–P–Nb2O5/NF for overall water splitting [J]. Int J Hydrogen Energy, 2021, 46(1): 581-588. DOI: https://doi.org/10.1016/ j.ijhydene.2020.09.244.
[9] XIAO Chang-long, GADDAM R, WU Yi-lan, SUN Xiao-ming, LIANG Yan, LI Yi-bing, ZHAO Xiu-song. Improvement of the electrocatalytic performance of FeP in neutral electrolytes with Fe nanoparticles [J]. Chemical Engineering Journal, 2020: 127330. DOI: https://doi.org/ 10.1016/j.cej.2020.127330.
[10] CHEN Jia-dong, CHEN Chun-hong, CHEN Yu-zhuo, WANG Hai-yan, MAO Shan-jun, WANG Yong. Improving alkaline hydrogen evolution reaction kinetics on molybdenum carbide: Introducing Ru dopant [J]. Journal of Catalysis, 2020, 392: 313-321. DOI: https://doi.org/10.1016/j.jcat.2020.10.020.
[11] TANG Chun, GAN Lin-feng, ZHANG Rong, LU Wen-bo, JIANG Xiue, ASIRI A, SUN Xu-ping, WANG Jin, CHEN Liang. Ternary FexCo1-xP nanowire array as a robust hydrogen evolution reaction electrocatalyst with Pt-like activity: Experimental and theoretical insight [J]. Nano Lett, 2016, 16(10): 6617-6621. DOI: 10.1021/acs.nanolett.6b03332.
[12] WANG Xu-dong, XU Yang-fan, RAO Hua-shang, XU Wei-jian, CHEN Hong-yan, ZHANG Wei-xiong, KUANG Dai-bin, SU Cheng-yong. Novel porous molybdenum tungsten phosphide hybrid nanosheets on carbon cloth for efficient hydrogen evolution [J]. Energy & Environmental Science, 2016, 9(4): 1468-1475. DOI: 10.1039/c5ee03801d.
[13] YU Luo, MISHRA I, XIE Yun-long, ZHOU Hai-qing, SUN Jing-ying, ZHOU Jian-qing, NI Yi-zhou, LUO Dan, YU Fang, YU Ying, CHEN Shuo, REN Zhi-feng. Ternary Ni2(1-x)Mo2xP nanowire arrays toward efficient and stable hydrogen evolution electrocatalysis under large-current-density [J]. Nano Energy, 2018, 53: 492-500. DOI: https://doi.org/ 10.1016/j.nanoen.2018.08.025.
[14] ZHENG Xiao-zhong, CHEN Yu-zhuo, BAO Xiao-bing, MAO Shan-jun, FAN Ru-xue, WANG Yong. In situ formed bimetallic carbide Ni6Mo6C nanodots and NiMoOx nanosheet array hybrids anchored on carbon cloth: Efficient and Flexible self-supported catalysts for hydrogen evolution [J]. ACS Catalysis, 2020, 10(19): 11634-11642. https://dx.doi.org/ 10.1021/acscatal.0c03355.
[15] KIBSGAARD J, TSAI C, CHAN K, BENCK J, NoRSKOV J, ABILD-PEDERSEN F, JARAMILLO T. Designing an improved transition metal phosphide catalyst for hydrogen evolution using experimental and theoretical trends [J]. Energy & Environmental Science, 2015, 8(10): 3022-3029. DOI: 10.1039/c5ee02179k.
[16] LIN Jing-huang, YAN Yao-tian, LIU Tao, CAO Jian, ZHOU Xin, FENG Ji-cai, QI Jun-lei. Optimize the electrocatalytic performances of NiCoP for water splitting by the synergic effect of S dopant and P vacancy [J]. Inter J Hydrogen Energy, 2020, 45(32): 16161-16168. https://doi.org/10.1016/ j.ijhydene.2020.04.069.
[17] LIU Chun-lei, ZHANG Gong, YU Li, QU Jiu-hui, LIU Hui-juan. Oxygen doping to optimize atomic hydrogen binding energy on NiCoP for highly efficient hydrogen evolution [J]. Small, 2018, 14(22): 1800421-1800429. DOI: 10.1002/ smll.201800421.
[18] MA Yuan-yuan, WU Cai-xia, FENG Xiao-jia, TAN Hua-qiao, YAN Li-kai, LIU Yang, KANG Zhen-hui, WANG En-bo, LI Yang-guang. Highly efficient hydrogen evolution from seawater by a low-cost and stable CoMoP@C electrocatalyst superior to Pt/C [J]. Energy & Environmental Science, 2017, 10(3): 788-798. DOI: 10.1039/c6ee03768b.
[19] QI Yu-yang, ZHANG Long, SUN Lan, CHEN Guan-jun, LUO Qiao-mei, XIN Hong-qiang, PENG Jia-hui, LI Yan, MA Fei. Sulfur doping enhanced desorption of intermediates on NiCoP for efficient alkaline hydrogen evolution [J]. Nanoscale, 2020, 12(3): 1985-1993. DOI: 10.1039/ c9nr08583a.
[20] QU Mei-jiao, JIANG Yi-min, YANG Miao, LIU Shu, GUO Qi-fei, SHEN Wei, LI Ming, HE Rong-xing. Regulating electron density of NiFe-P nanosheets electrocatalysts by a trifle of Ru for high-efficient overall water splitting [J]. Appl Catalysis B: Environ, 2020, 263: 118324-118332. DOI: https://doi.org/10.1016/j.apcatb.2019.118324.
[21] CAO Bo, CHENG Yan, HU Ming-hao, JING Peng, MA Zhi-xue, LIU Bao-cang, GAO Rui, ZHANG Jun. Efficient and durable 3D self-supported nitrogen-doped carbon-coupled nickel/cobalt phosphide electrodes: Stoichiometric ratio regulated phase-and morphology-dependent overall water splitting performance [J]. Advanced Functional Materials, 2019, 29(44): 1906316-1906333. DOI: 10.1002/adfm. 201906316.
[22] YAN Yao-tian, LIN Jing-huang, CAO Jian, GUO Shu, ZHENG Xiao-hang, FENG Ji-cai, QI Jun-lei. Activating and optimizing the activity of NiCoP nanosheets for electrocatalytic alkaline water splitting through the V doping effect enhanced by P vacancies [J]. Journal of Materials Chemistry A, 2019, 7(42): 24486-24492. DOI: 10.1039/ c9ta09283h.
[23] FU Qiang, WU Tao, FU Gang, GAO Tang-ling, HAN Jie-cai, YAO Tai, ZHANG Yu-min, ZHONG Wen-wu, WANG Xian-jie, SONG Bo. Skutterudite-type ternary Co1–xNixP3 nanoneedle array electrocatalysts for enhanced hydrogen and oxygen evolution [J]. ACS Energy Letters, 2018, 3(7): 1744-1752. DOI: 10.1021/acsenergylett.8b00908.
[24] JIN Huan-yu, LIU Xin, CHEN Shuang-ming, VASILEFF A, LI Lai-quan, JIAO Yan, SONG Li, ZHENG Yao, QIAO Shi-zhang. Heteroatom-doped transition metal electrocatalysts for hydrogen evolution reaction [J]. ACS Energy Letters, 2019, 4(4): 805-810. DOI: 10.1021/acsenergylett.9b00348.
[25] WANG Ling, WU Hai-jun, XI Shi-bo, CHUA Sing-teng, WANG Feng-he, PENNYCOOK S, YU Zhi-gen, DU Yong-hua, XUE Jun-min. Nitrogen-doped cobalt phosphide for enhanced hydrogen evolution activity [J]. ACS Appl Mater Interfaces, 2019, 11(19): 17359-17367. DOI: 10.1021/acsami. 9b01235.
[26] ZHOU Qing-wen, SHEN Zi-han, ZHU Chao, LI Jia-chen, DING Zhi-yuan, WANG Peng, PAN Feng, ZHANG Zhi-yong, MA Hai-xia, WANG Shuang-yin, ZHANG Hui-gang. Nitrogen-doped CoP electrocatalysts for coupled hydrogen evolution and sulfur generation with low energy consumption [J]. Adv Mater, 2018, 30(27): 1800140-1800147. DOI: 10.1002/adma.201800140.
[27] YAN Liang, ZHANG Bing, ZHU Jun-lu, LIU Zhong-gang, ZHANG Hai-yan, LI Yun-yong. Callistemon-like Zn and S codoped CoP nanorod clusters as highly efficient electrocatalysts for neutral-pH overall water splitting [J]. Journal of Materials Chemistry A, 2019, 7(39): 22453-22462. DOI: 10.1039/c9ta08812a.
[28] REN Xian-pei, MA Qiang, FAN Hai-bo, PANG Liu-qing, ZHANG Yun-xia, YAO Yao, REN Xiao-dong, LIU Sheng-zhong (Frank). A Se-doped MoS2 nanosheet for improved hydrogen evolution reaction [J]. Chem Commun (Camb), 2015, 51(88): 15997-16000. DOI: 10.1039/c5cc06847a.
[29] HU Cong-ling, ZHANG Lei, ZHAO Zhi-Jian, LI Ang, CHANG Xiao-xia, GONG Jin-long. Synergism of geometric construction and electronic regulation: 3D Se-(NiCo)Sx/(OH)x nanosheets for highly efficient overall water splitting [J]. Adv Mater, 2018, 30(12): 1705538-1705545. DOI: 10.1002/adma. 201705538.
[30] YAO Na, LI Peng, ZHOU Zi-rui, ZHAO Yuan-meng, CHENG Gong-zhen, CHEN Sheng-li, LUO Wei. Synergistically tuning water and hydrogen binding abilities over Co4N by Cr doping for exceptional alkaline hydrogen evolution electrocatalysis [J]. Advanced Energy Materials, 2019, 9(41): 1902449-1902456. DOI: 10.1002/aenm.201902449.
[31] HUANG Ling, WU Hao, ZHANG Yun. One-step synthesis of CoPSe–CoSe2/CNTs as efficient electrocatalyst for oxygen evolution reaction [J]. Electrochim Acta, 2020, 331: 135362-135368. DOI: https://doi.org/10.1016/j.electacta. 2019.135362.
[32] ZHOU Guang-yao, LI Meng, LI Yan-le, DONG Hang, SUN Dong-mei, LIU Xi-en, XU Lin, TIAN Zi-qi, TANG Ya-wen. Regulating the electronic structure of CoP nanosheets by O incorporation for high-efficiency electrochemical overall water splitting [J]. Advanced Functional Materials, 2019, 30(7): 1905252-1905259. DOI: 10.1002/adfm.201905252.
[33] LUO Qiao-mei, ZHAO Yi-wei, QI Yu-yang, XIN Hong-qiang, WANG Chen, CHEN Guan-jun, SUN Jun, LIU Ming-xia, XU Ke-wei, MA Fei. Plasma-assisted nitrogen doping in Ni-Co-P hollow nanocubes for efficient hydrogen evolution electrocatalysis [J]. Nanoscale, 2020, 12(25): 13708-13718. DOI: 10.1039/d0nr01783c.
[34] HUANG Chu-qiang, YU Luo, ZHANG Wei, XIAO Qin, ZHOU Jian-qing, ZHANG Yuan-lu, AN Peng-fei, ZHANG Jing, YU Ying. N-doped Ni-Mo based sulfides for high-efficiency and stable hydrogen evolution reaction [J]. Applied Catalysis B: Environmental, 2020, 276:119137-119144. DOI: https://doi.org/10.1016/j.apcatb.2020.119137.
[35] PAN Yuan, SUN Kai-an, LIN Yan, CAO Xing, CHENG Yuan-sheng, LIU Shou-jie, ZENG Ling-you, CHEONG Weng-chon, ZHAO Di, WU Kong-lin, LIU Zhi, LIU Yun-qi, WANG Ding-sheng, PENG Qing, CHEN Chen, LI Ya-dong. Electronic structure and d-band center control engineering over M-doped CoP (M=Ni, Mn, Fe) hollow polyhedron frames for boosting hydrogen production [J]. Nano Energy, 2019, 56: 411-419. DOI: https://doi.org/10.1016/j.nanoen.2018.11.034.
[36] ZHANG Ming-yuan, HU Ai-ping, LIU Zheng, XU Ya-li, FAN Bin-bin, TANG Qun-li, ZHANG Shi-ying, DENG Wei-na, CHEN Xiao-hua. Synergistic effect of three-dimensional cobalt diselenide/carbon nanotube arrays composites for enhanced hydrogen evolution reaction [J]. Electrochimica Acta, 2018, 285: 254-261. DOI: https://doi.org/10.1016/ j.electacta.2018.07.226.
[37] WANG Jing, XU Fan, JIN Hai-yan, CHEN Yi-qing, WANG Yong. Non-noble metal-based carbon composites in hydrogen evolution reaction: Fundamentals to applications [J]. Adv Mater, 2017, 29(14): 1605838-1605872. DOI: 10.1002/adma. 201605838.
[38] CAO Er-ping, CHEN Zhi-min, WU Hao, YU Peng, WANG Ying, XIAO Fei, CHEN Shuo, DU Shi-chao, XIE Ying, WU Yi-qun, REN Zhi-yu. Boron-induced electronic-structure reformation of CoP nanoparticles drives enhanced pH-universal hydrogen evolution [J]. Angew Chem Int Ed Engl, 2020, 59(10): 4154-4160. DOI: 10.1002/ange.201915254.
[39] BAO Xiao-bing, GONG Yu-tong, ZHENG Xiao-zhong, CHEN Jia-yi, MAO Shan-jun, WANG Yong. Highly performed platinum nanosheets synthesized under in situ reaction conditions for hydrogen generation [J]. Journal of Energy Chemistry, 2020, 51: 272-279. DOI: https://doi. org/10.1016/j.jechem.2020.03.064.
[40] ZHAO Wen-xi, MA Xiao-qing, WANG Guang-zhao, LONG Xiao-jiang, LI Ya-dong, ZHANG Wan-li, ZHANG Peng. Carbon-coated CoP3 nanocomposites as anode materials for high-performance sodium-ion batteries [J]. Applied Surface Science, 2018, 445: 167-174.
[41] ZHU Chang-rong, WANG An-liang, XIAO Wen, CHAO Dong-liang, ZHANG Xiao, TIEP N H, CHEN Shi, KANG Jia-ni, WANG Xin, DING Jun, WANG John, ZHANG Hua, FAN Hong-jin. In situ grown epitaxial heterojunction exhibits high-performance electrocatalytic water splitting [J]. Adv Mater, 2018, 30(13): 1705516-1705523.
[42] LI Jia-yuan, YAN Ming, ZHOU Xue-mei, HUANG Zheng-Qing, XIA Zhao-ming, CHANG Chun-Ran, MA Yuan-yuan, QU Yong-quan. Mechanistic insights on ternary Ni2-xCoxP for hydrogen evolution and their hybrids with graphene as highly efficient and robust catalysts for overall water splitting [J]. Advanced Functional Materials, 2016, 26(37): 6785-6796.
[43] ZHANG Ying, SHAO Qi, LONG Shui, HUANG Xiao-qing. Cobalt-molybdenum nanosheet arrays as highly efficient and stable earth-abundant electrocatalysts for overall water splitting [J]. Nano Energy, 2018, 45: 448-455.
[44] HAN A-li, CHEN Huan-lin, ZHANG Han-yu, SUN Zi-jun, DU Ping-wu. Ternary metal phosphide nanosheets as a highly efficient electrocatalyst for water reduction to hydrogen over a wide pH range from 0 to 14 [J]. Journal of Materials Chemistry A, 2016, 4(26): 10195-10202.
[45] ZHANG Hao-jie, LI Xiao-peng, HaHNEL A, NAUMANN V, LIN Chao, AZIMI S, SCHWEIZER S, MAIJENBURG A W, WEHRSPOHN R B. Bifunctional heterostructure assembly of NiFe LDH nanosheets on NiCoP nanowires for highly efficient and stable overall water splitting [J]. Advanced Functional Materials, 2018, 28(14): 1706847.
[46] CHEN Peng-zuo, ZHOU Tian-pei, ZHANG Meng-xing, TONG Yun, ZHONG Cheng-an, ZHANG Nan, ZHANG Li-dong, WU Chang-zheng, XIE Yi. 3D Nitrogen-anion-decorated nickel sulfides for highly efficient overall water splitting [J]. Adv Mater, 2017, 29(30): 1701584-1701589.
[47] DENG Sheng-jue, ZHONG Yu, ZENG Yin-xiang, WANG Ya-dong, WANG Xiu-li, LU Xi-hong, XIA Xin-hui, TU Jiang-ping. Hollow TiO2@Co9S8 core-branch arrays as bifunctional electrocatalysts for efficient oxygen/hydrogen production [J]. Advanced Science, 2018, 5(3): 1700772-1700779.
[48] WANG Peng-yan, PU Zong-hua, LI Yan-hui, WU Lin, TU Zheng-kai, JIANG Min, KOU Zong-kui, AMIINU I S, MU Shi-chun. Iron-doped nickel phosphide nanosheet arrays: An efficient bifunctional electrocatalyst for water splitting [J]. ACS Appl Mater Interfaces, 2017, 9(31): 26001-26007.
[49] DU Hui-tong, XIA Lian, ZHU Shu-yun, QU Fei, QU Feng-li. Al-Doped Ni2P nanosheet array: A superior and durable electrocatalyst for alkaline hydrogen evolution [J]. Chem Commun (Camb), 2018, 54(23): 2894-2897.
[50] CHEN Peng-zuo, XU Kun, TAO Shi, ZHOU Tian-pei, TONG Yun, DING Hui, ZHANG Li-dong, CHU Wang-sheng, WU Chang-zheng, XIE Yi. Phase-transformation engineering in cobalt diselenide realizing enhanced catalytic activity for hydrogen evolution in an alkaline medium [J]. Adv Mater, 2016, 28(34): 7527-7532.
[51] XIA Chuan, LIANG Han-feng, ZHU Jia-jie, SCHWINGENSCHLOGL U, ALSHAREEF H. Active edge sites engineering in nickel cobalt selenide solid solutions for highly efficient hydrogen evolution [J]. Advanced Energy Materials, 2017, 7(9): 1602089.
[52] SIVANANTHAM A, GANESAN P, SHANMUGAM S. Hierarchical NiCo2S4 Nanowire arrays supported on Ni foam: An efficient and durable bifunctional electrocatalyst for oxygen and hydrogen evolution reactions [J]. Advanced Functional Materials, 2016, 26(26): 4661-4672.
[53] WANG Peng-cheng, LIU Xue-feng, YAN Yao-tian, CAO Jian, FENG Ji-cai, QI Jun-lei. Exploring CoP core–shell nanosheets by Fe and Zn dual cation doping as efficient electrocatalysts for overall water splitting [J]. Catalysis Science & Technology, 2020, 10(5): 1395-1400.
[54] XU Xiu-juan, DU Pu-yu, CHEN Zong-kun, HUANG Ming-hua. An electrodeposited cobalt–selenide-based film as an efficient bifunctional electrocatalyst for full water splitting [J]. Journal of Materials Chemistry A, 2016, 4(28): 10933-10939.
[55] JIA Heng-lei, JIANG Rui-bin, LU Wen-zheng, RUAN Qi-feng, WANG Jian-fang, YU J. Aerosol-spray metal phosphide microspheres with bifunctional electrocatalytic properties for water splitting [J]. Journal of Materials Chemistry A, 2018, 6(11): 4783-4792.
[56] PAN Yuan, SUN Kai-an, LIU Shou-jie, CAO Xing, WU Kong-jie, CHEONG Weng-Chon, CHEN Zheng, WANG Yu, LI Yang, LIU Yun-qi, WANG Ding-sheng, PENG Qing, CHEN Chen, LI Ya-dong. Core-shell ZIF-8@ZIF-67-derived CoP nanoparticle-embedded n-doped carbon nanotube hollow polyhedron for efficient overall water splitting [J]. J Am Chem Soc, 2018, 140(7): 2610-2618.
[57] TANG Chun, ZHANG Rong, LU Wen-bo, HE Liang-bo, JIANG Xiue, ASIRI A, SUN Xu-ping. Fe-doped CoP nanoarray: A monolithic multifunctional catalyst for highly efficient hydrogen generation [J]. Adv Mater, 2017, 29(2): 1602441-1602446.
[58] CHEN Gao-feng, MA Tian-yi, LIU Zhao-qing, LI Nan, SU Yu-zhi, DAVEY K, QIAO Shi-zhang. Efficient and stable bifunctional electrocatalysts Ni/NixMy (M = P, S) for overall water splitting [J]. Advanced Functional Materials, 2016, 26(19): 3314-3323.
(Edited by YANG Hua)
中文导读
碳布负载三维Se掺杂NiCoP纳米阵列作为碱性条件下的析氢催化剂
摘要:探索稳定高效的碱性析氢(HER)电催化剂对碱性条件下的电解水具有重要意义。通过水热反应和磷化/硒化工艺在碳布上原位生长了具有分级纳米阵列结构的Se-NiCoP/CC析氢催化剂。实验结果表明,Se掺杂可以增加NiCoP的电化学活性位点,调节NiCoP的电子结构。优化后的Se-NiCoP/CC电极在碱性电解质中表现出优异的析氢催化活性,在10 mA/cm2的电流密度下的过电位仅为79 mV。当将Se-NiCoP/CC电极同时作为碱性水电解槽的正极和负极时,在仅1.62 V的低电压下实现了50 mA/cm2的电流密度。本工作为合理设计高活性HER电催化剂提供了一种可行的方法。
关键词:Se-NiCoP 纳米棒;多级阵列结构;析氢反应;碱性条件;全水解
Foundation item: Projects(51772086, 51872087, 51971089) supported by the National Natural Science Foundation of China; Project(2018TP1037-202102) supported by Open Fund of Hunan Provincial Key Laboratory of Advanced Materials for New Energy Storage and Conversion, China; Project supported by Student National SIT Innovation Program, China; Project(2020CB1007) supported by Hunan Joint International Laboratory of Advanced Materials and Technology for Clean Energy, China
Received date: 2021-02-20; Accepted date: 2021-06-15
Corresponding author: HU Ai-ping, PhD, Professor; E-mail: hudaaipinghu@126.com; CHEN Xiao-hua, PhD, Professor; E-mail: xiaohuachen@hnu.edu.cn; ORCID: https://orcid.org/0000-0003-1054-1487
Abstract: The exploration of stable and highly efficient alkaline hydrogen evolution reaction (HER) electrocatalysts is imperative for alkaline water splitting. Herein, Se-doped NiCoP with hierarchical nanoarray structures directly grown on carbon cloth (Se-NiCoP/CC) was prepared by hydrothermal reaction and phosphorization/selenization process. The experimental results reveal that Se doping could increase the electrochemical active sites and alter the electronic structure of NiCoP. The optimized Se-NiCoP/CC electrode exhibits outstanding HER activity in alkaline electrolyte, which only needs a low overpotential of 79 mV at the current density of 10 mA/cm2. When serving as anode and cathode electrode simultaneously, the Se-NiCoP/CC electrodes achieve current density of 50 mA/cm2 at a low voltage of only 1.62 V. This work provides a feasible way to rationally design high active HER electrocatalysts.
- 3D Se-doped NiCoP nanoarrays on carbon cloth for efficient alkaline hydrogen evolution


