
Adsorption of XSD-296 resin for Cr(Ⅵ)
SHU Zeng-nian(舒增年)1, DU Rong-jun(杜荣军)2, WANG Xu(王 旭)1, Xiong Chun-hua(熊春华)3, LI Tao(李 涛)2
1. Department of Chemistry, Lishui University, Lishui 323000, China;
2. Xi’an Power Resin Factory, Xi’an 710038, China;
3. Department of Applied Chemistry, Biotechnology and Environmental Engineering, Zhejiang Gongshang University,Hangzhou 310035, China
Received 21 December 2006; accepted 19 May 2007
Abstract:
The adsorption properties of XSD-296 for Cr(Ⅵ) were studied by using chemical analysis and infrared spectrometry. Experimental results show that XSD-296 resin has a good adsorption ability for Cr(Ⅵ) at pH=2.6 in the HAc-NaAc medium. The statically saturated adsorption capacity is 235 mg/g resin. The apparent activation energy of adsorption reaction, Ea, is 16.73 kJ/mol, and the thermodynamic parameters are ?H=11.62 kJ/mol, ?G298 K=-4.13 kJ/mol. The adsorption behavior of resin for Cr(Ⅵ) is in accordance with Freundlich adsorption isotherm. Cr(Ⅵ) adsorbed on resin can be eluted by 5%NaCl-5%NaOH or 5%NH4Cl-5%NH3?H2O quantitatively. Infrared spectra and adsorption mechanism show that the functional group of resin coordinates with Cr(Ⅵ) to form co-ordination compound. The coordination molar ratio of the functional group of resin to Cr(Ⅵ) is 1:1.
Key words:
XSD-296 resin; chromium(Ⅵ); adsorption;
1 Introduction
Massive volume of wastewater containing chromium is generated during the production of electroplating, leather making, mining and dyeing etc, causing a series of pollution problem. Cr(Ⅵ) is of great toxic and can be easily absorbed by human body and accumulated inside, which would cause sickness, even cancer. Thus, chromium-contained water is considered as one of the most serious environmental pollutions. It has been listed as a major task by the State Environment Protection Administration, and it is very much meaningful for environment protection and human health to separate Cr(Ⅵ) from Cr(Ⅵ)-contained water. Currently, there are two major methods to tackle the Cr(Ⅵ)-contained water. One is the reduction system, however, with this method massive polluted earth may emerge, leading to re-pollution easily; the other is direct Cr(Ⅵ) treatment, among which the ion exchange system is of high efficiency and the re-production can be reusable, and therefore the system is popularly focused on [1-11]. In this study, the adsorption behavior and adsorption mechanism of the XSD-296 resin ([R—N(CH3)2]) for Cr(Ⅵ) were investigated.
2 Experimental
2.1 Materials and instrument
Elemental analyzer EA1110, Perkin-elmer 683 FT-IR, Sartorius PB-20 pH meter, HZ9212s temperature constant shaking machine, 722 Spectrophotometer were used.
XSD-296 resin was provided by Sian Electric Power Resin Factory; standard solution of Cr(Ⅵ) was prepared from K2Cr2O7 (analytical grade); 0.1% diphenyl carbazide solution was the developer.
2.2 Adsorption of resin and analytical method
A desired amount of treated resin was weighted and added into iodine measuring flask, and then a desired volume of the buffer solution and standard solution of Cr(Ⅵ) were added. The flask was shaken to a state of adsorption equilibrium in a shaker at constant temperature.
The amount of Cr(Ⅵ) was measured by the specteophotometric determination of diphenyl carbazide. The adsorption amount(QR) and the distribution coefficient(D) were calculated by
QR =(ρo-ρe)V/m (1)
D=QR/ρe (2)
where ρo is the initial concentration of Cr(Ⅵ) in solution (mg/mL), ρe is the equilibrium concentration of Cr(Ⅵ) in solution (mg/mL), m is the resin mass (g) and V is the total volume of solution (mL).
3 Results and discussion
3.1 Selection of medium acidity
To investigate the adsorption ability of XSD-296 resin for Cr(Ⅵ), the same adsorption tests were done at pH=0-3 in the dilute hydrochloric acid medium, pH=0-3 in the dilute sulphuric acid medium, and pH=2.6-6.5 in the HAc-NaAc medium, respectively. The results indicate that the adsorption amount is the highest at pH value of about 2 (Fig.1). At the same acidity, the adsorption amount of resin to Cr(Ⅵ) is the highest in the HAc-NaAc medium, and the lowest in the dilute sulphuric acid medium. In the three systems, there may be adsorption to Cl-, ![]() and Ac- during the adsorption process of resin to Cr(Ⅵ), and the competitive adsorption of Cl- and
and Ac- during the adsorption process of resin to Cr(Ⅵ), and the competitive adsorption of Cl- and ![]() to Cr(VI) is stronger than that of Ac-. Thus all the following experiments are performed at pH =2.6 of buffer solution HAc-NaAc.
to Cr(VI) is stronger than that of Ac-. Thus all the following experiments are performed at pH =2.6 of buffer solution HAc-NaAc.
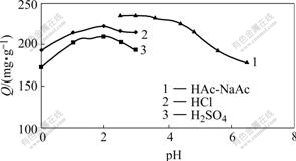
Fig.1 Effect of pH value on adsorption capacity for different systems (resin 20.0 mg, ρ0(Cr(Ⅵ))=0.20 mg/mL, T=298 K)
3.2 Determination of adsorption rate constant and adsorption activation energy
According to the experimental conditions shown in Fig.2 and the above mentioned method, a desired amount solution was taken out at intervals for the determination of remained concentrations. After the remains kept constant and volume was corrected, a series of data were obtained. The results indicate that the resin adsorption rate to Cr(Ⅵ) is very rapid. When the adsorption amount is half of that at equilibrium, the required time t1/2 is about 1.2 h, and the required time of the adsorption equilibrium is 7 h. According to BOYD method [12], the slowest process of film spreading, intraparticle spreading and chemical reaction control the exchange adsorption rate. In the light of formula -ln(1-F)=kt, a straight line is obtained by plotting -ln(1-F) versus t (correlation coefficient r=0.995 2) (Fig.3). It shows that adsorption dynamic action accords with the formula. So it can be deduced that the liquid film spreading or chemical reaction is the predominating step of the adsorption process [12]. Therefore, the adsorption rate constant can be found out from the slope of straight line, which is k298 K=1.458×10-4 s-1.

Fig.2 Adsorption dynamic curve of Cr(Ⅵ) (resin 40.0 mg, ρ0(Cr(Ⅵ))=0.24 mg/mL, T=298 K, total volume of solution 60.0 mL)

Fig.3 Determination of adsorption rate constant (resin 40.0 mg, ρ0(Cr(Ⅵ))=0.24 mg/mL, T=298 K, total volume of solution 60.0 mL)
In the same way, the adsorption rate constants were determined at different temperatures (298-313 K). They are k303 K=1.66×10-4 s-1, k308 K=1.82×10-4 s-1 and k313K= 2.05×10-4 s-1, respectively. According to the formula of Arrhenius lg k=-Ea/(2.303RT)+lg A, a straight line is made by plotting lg k versus 1/T (Fig.4). The slope of straight line is -8.739×102, the correlation coefficient is r=0.993 4. The apparent activation energy of the adsorption reaction is Ea=16.73 kJ/mol. It can be seen from the adsorption rate constants that the adsorption speed increases when the temperature is raised within the scope of experimental temperature.

Fig.4 Relationship between lg k and 1/T (resin 40.0 mg, ρ0(Cr(Ⅵ))=0.24 mg/mL, T=298 K, total volume of solution 60.0 mL)
3.3 Isotherm adsorption curve
20.0, 25.0, 30.0, 35.0, 40.0 mg of resin were weighted and put into conical flasks individually. The experimental conditions are shown in Fig.5.

Fig.5 Relationship between lg Q and lg ρe (ρ0(Cr(Ⅵ))=0.20 mg/mL, T=298 K, total volume of solution 40.0 mL)
When the adsorption equilibrium is reached, equilibrium concentration (ρe) is determined and the corresponding adsorption capacity of resin Q is calculated. The adsorption isotherm is correlated to the well-known Freundlich equation:
Q=aρe1/b (3)
or
lg Q=1/blg ρe+lg a (4)
where a and b are Freundlich constants. The straight line is obtained by plotting lg Q versus lg ρe(Fig.5), and the correlation coefficient of the straight line is 0.996 5, and Freundlich “b” value is 3.81. The fact that b is 2-10 indicates that Cr(Ⅵ) is easy to be absorbed [13].
3.4 Influence of adsorption temperature on adsorp- tion rate and determination of thermodynamic parameters
Five parts of 30.0 mg resins were weighted in iodine flask. Under the experimental condition shown in Fig.6, the distribution ratio D of the resin for Cr(Ⅵ) was determined at 283, 291, 299, 307 and 315 K. The result obviously indicates that it is favorable for the adsorption with the adsorption temperature going up. This means that the adsorption process is an endothermic one. Therefore, the adsorption reaction is a chemical adsorption. Since the adsorption processes in buffer solution, according to lgD=-?H/(2.303RT)+S/R [14], the straight line is obtained by plotting lgD vs 1/T (Fig.6). From the slope of the straight line, ?H=11.62 kJ/mol is obtained. ?S can be obtained from the intercept of the straight line, which is 52.86 J/(mol·K). In the light of ?G=?H-T?S (at 298 K), ?G298K=-4.13 kJ/mol is obtained, revealing that the adsorption reaction is a spontaneous reaction, and the impetus of XSD-296 resin adsorbing Cr(Ⅵ) makes free energy minimize and entropy increase[15]. Under condition of experimental concentration, the distribution ratio D reaches over 104 order of magnitude, which shows that when the resin adsorption to Cr(Ⅵ) reaches equilibrium, the concentration of Cr(Ⅵ) in aqueous phase is fully low, namely, the adsorption is very complete.

Fig.6 Effect of temperature on distribution ratio (resin 30.0 mg, ρ0(Cr(Ⅵ))=0.23 mg/mL, total volume of solution 50.0 mL)
3.5 Determination of complex ratio
3.5.1 Saturated capacity method
40.0 mg of resin was weighted accurately. Under the experimental condition of T=298 K, ρ0(Cr(Ⅵ))=0.24 mg/mL, the experiment was performed by using the above-mentioned method. The adsorption capacity of resin for Cr(Ⅵ) was 235 mg/g, i.e. 4.52 mmol/g. The amount of resin functional group is 4.71 mmol/g by elementary analysis. Therefore, the molar ratio of the resin functional group to Cr(Ⅵ) is 4.71?4.52, which is 1?1 approximately.
3.5.2 Equimolar method
Seven parts of different amounts of resins were accurately weighted and added into different conical flasks, and then mixed with different amounts of Cr(Ⅵ). The total amount of resin functional group and Cr(Ⅵ) was kept at 187 mmol whatever the molar ratio might be. The experiment was carried out with the same method mentioned previously. The adsorption amount (mmol) vs ρ(Cr(Ⅵ))/[ρ(Cr(Ⅵ))+ρ(R)] yields a curve shown in Fig.7 (unit of formula is mmol, R is XSD-296’s functional group). The expected adsorption amount is the biggest when the molar fraction of Cr(Ⅵ) is 0.48 shown on the abscissa. This means that the complex molar ratio of the functional group to Cr(Ⅵ) is about 1?0.92, which is 1?1 approximately. The result is consistent with that obtained from saturated capacity method.

Fig.7 Equimolar series method (T=298 K, total volume of each solution 30.0 mL)
3.6 Analysis of infrared spectra
From the above result, it can be concluded that the adsorption of Cr(Ⅵ) by XSD-296 resin (?H>0) belongs to a chemisorption. It shows that chemical bond forms between functional group of resin and Cr(Ⅵ). In order to make further approaching of the functional group of resin and Cr(Ⅵ), the infrared spectra of resin, before and after Cr(Ⅵ) is adsorbed, are compared. It is found that the characteristic adsorption peaks of resin functional group shift from 941 to 949 cm-1 (Fig.8). The reason for the shift is that resin chelates with ![]() , resulting in appearance of stretching vibration peak of the bonds Cr—O. At the same time, the characteristic adsorption peak of C—N (1 014 cm-1) is weakened, and the peaks at 858 and 811 cm-1 disappear. In addition, a new peak at 766 cm-1 is formed. It is testified obviously that nitrogen atoms of XSD-296 resin functional group act with Cr(Ⅵ), forming complex compound.
, resulting in appearance of stretching vibration peak of the bonds Cr—O. At the same time, the characteristic adsorption peak of C—N (1 014 cm-1) is weakened, and the peaks at 858 and 811 cm-1 disappear. In addition, a new peak at 766 cm-1 is formed. It is testified obviously that nitrogen atoms of XSD-296 resin functional group act with Cr(Ⅵ), forming complex compound.

Fig.8 IR spectra of XSD-296 resin: (a) Before adsorption; (b) After adsorption
3.7 Elution and recovery of resin
Five parts of 40.0 mg resin were accurately weighted and added into different conical flasks at T=298 K, and ρ0(Cr(Ⅵ))=0.20 mg/mL. The flasks were shaken until adsorption equilibriums were reached. The adsorption amount was calculated individually. The separated resin from residual aqueous phase was washed three times. After the resin was dried, different concentrations of hydrochloric acid, sodium hydroxide, ammonium muriate-ammonia water, ammonium muriate- hydrochloric acid and sodium chloride-sodium hydroxide were added into the resin, respectively. The result of elution shows that the system of 5%NaCl+ 5%NaOH or 5%NH4Cl+5%NH3·H2O is the most efficient. The elution rate is up to 100%. The adsorption capacity of the resin of elution is almost not changed and the resin can be regenerated.
4 Conclusions
1) Cr(Ⅵ) can be optimally adsorbed on XSD-296 resin in the HAc-NaAc system at pH=2.6 and the statically saturated adsorption capacity is 235 mg/g resin. Cr(Ⅵ) adsorbed on XSD-296 resin can be eluted by using 5%NaCl-5%NaOH or 5%NH4Cl-5%NH3·H2O quantitatively, and the elution rate is up to100%.
2) The adsorption behavior of XSD-296 for Cr(Ⅵ) obeys the Freundlich isotherm. The thermodynamic adsorption parameters are ?H=11.62 kJ/mol, ?G298 K= -4.13 kJ/mol. The apparent activation energy of the adsorption reaction is Ea=16.73 kJ/mol.
3) Infrared spectra and adsorption mechanism show that the functional group of resin is coordinated with Cr(Ⅵ) to form co-ordination compound. The coordination molar ratio of the functional group of resin to Cr(Ⅵ) is 1?1 approximately by saturated capacity method and equimolar method.
References
[1] Shu Zeng-nian, Xiong Chun-hua, Lin Feng. Studies on the adsorption of yhrium(Ⅲ) by macroporous phasphonic acid resin [J]. Chemical Research and Application, 1997, 9(3): 289-293. (in Chinese)
[2] Jin Yi-zhong, Xu Hao, Xie Yu-tan. Adsorption of benzene and toluene vapour on activated carbon [J]. J Chem Eng of Chinese Univ, 2004, 18(2): 258-263.
[3] Xiong Chun-hua, Wu Xiang-mei. Study on the adsorption of iminodiacetic acid resin for yttrium(Ⅲ) [J]. Chinese Journal of Inorganic Chemistry, 2003, 19(12): 1356-1360. (in Chinese)
[4] Wu Xiang-mei, Xiong Chun-hua, Shu Zeng-nian. Adsorption of silver onto thiol-resin and its mechanism [J]. Journal of Chemical Industry and Engineering, 2003, 54(10): 1466-1469. (in Chinese)
[5] Chen Yi-yong, Liang Chao, Chao Yan. Synthesis and characterization of polyacrylonitrile 2-thiosemicarbazide resin and its sorption behavior for Rh(Ⅲ), Ru(Ⅳ), Pd(Ⅱ) and Ir(Ⅳ) ions [J]. Reactive Polymers, 1998, 36: 51-58.
[6] Shu Zeng-nian, Xiong Chun-hua, Wang Xu. Adsorption behavior and mechanism of amino methylene phosphonic acid resin for Ag(Ⅰ) [J]. Trans Nonferrous Met Soc China, 2006, 16(3): 700-704.
[7] Xiong Chun-hua, Shu Zeng-nian. Adsorption behavior and mechanism of 4-amino-1,2,4-triazole resin for molybdenum(Ⅵ) [J]. Nonferrous Metals, 2000, 52(2): 61-64. (in Chinese)
[8] Xiong Chun-hua, Shu Zeng-nian, Wang Yong-Jiang. Sorption of Mo(Ⅵ) by 4-aminopyridine resin [J]. Journal of Chemical Industry and Engineering, 2005, 56(7):1267-1270. (in Chinese)
[9] SHU Zeng-nian, Wu Wei-fen, Xiong Chun-hua. Adsorption behavior and mechanism of macroporous phosphonic acid resin for Ag(Ⅰ) [J]. Acta Mineralogica Sinica, 2005, 25(2): 131-135. (in Chinese)
[10] Brykina G D, Marchak T V, Krysina L S, Belyavskaya T A. Sorption-photometric determination of copper by using AV-17 anion exchanger modified with 1-(2-thiazolyl-azo)-2-naphthol-3, 6-disulphonic acid [J]. Zh Anal Khim, 1980, 35(12): 2294-2299.
[11] Xiong Chun-hua, Shu Zeng-nian, Chen Yi-yong. Studies on the sorption of macroporous phosphonic acid resin for lanthanum [J]. Chinese Journal of Reactive Polymer, 1998, 7(2): 7-15. (in Chinese)
[12] Boyd G E, Adamson A W, Myers L S. The exchange adsorption of ions from aqueous solutions by organic zeolites Ⅱ kinetics [J]. J Am Chem Soc, 1947, 69: 2836-2848.
[13] hiro K, renichirou S. Foundation and design of adsorptions [M]. LU Zheng-li, Trans. Beijing: Chemical Industry Press, 1983: 33-36. (in Chinese)
[14] Si Gong-min, Wu Qi-hua, Zhang Le-qin. Study on the sorption behaviors and mechanism of chromium(Ⅵ) by macro-porous D252 resin [J]. Ion Exchange and Adsorption, 1990, 6(1): 1-8.
[15] Gode F, Pehlivan E. A comparative study of two chelating ion-exchange resins for the removal of chromium(Ⅲ) from aqueous solution [J]. Journal of Hazardous Materials, 2003, B100: 231-243.
__________________________
Foundation item: Project(20040501) supported by the Department of Education of Zhejiang Province, China; Project(2004465) supported by the Bureau of Science and Technology of Lishui City, China
Corresponding author: SHU Zeng-nian; Tel: +86-578-2271338; E-mail: zengnianshu@yahoo.com.cn


