
Co-intensification of gold leaching with heavy metals and hydrogen peroxide
YANG Yong-bin(杨永斌), LI Qian(李 骞), JIANG Tao(姜 涛), GUO Yu-feng(郭宇峰),
LI Guang-hui(李光辉), XU Bin(许 斌)
School of Mineral Processing and Bioengineering, Central South University, Changsha 410083, China
Received 6 July 2009; accepted 19 January 2010
Abstract:
Co-intensification was researched to accelerate gold leaching with regards to its electrochemical nature by using anodic intensifiers of heavy metal ions (Pb2+, Bi3+, Tl+, Hg2+ and Ag+) on the basis of hydrogen peroxide assistant leaching on three different types of materials which were classified as a refractory sulphide gold concentrate, an easily leachable sulphide gold concentrate, and a low grade oxide gold ore according to their leaching characteristics. The results showed that, favorable co-intensification effects on the three materials were obtained and leaching time of gold was effectively shortened to no longer than 12 h from 16 to 24 h for hydrogen peroxide assistant leaching. For the five tested heavy metal ions, Bi3+and Tl+ presented co-intensifying effect on all the three materials, and Hg2+ caused co-intensifying effect on both refractory and easily leachable sulphide gold concentrates, and Pb2+ and Ag+ only had co-intensifying effect on the easily leachable sulphide gold concentrate.
Key words:
gold leaching; co-intensification; cyanidation; heavy metals; hydrogen peroxide;
1 Introduction
Throughout the last century, a great deal of concerns have been focused upon the problem of low leaching rate of gold cyanidation and a series of intensification techniques have been worked out. Among these techniques, hydrogen peroxide assistant leaching has been proved to be an effective method to accelerate gold leaching since its appearance in 1987[1-2]. However, it seems very difficult for further progress to be made and few new techniques have been presented ever since.
It has been demonstrated that gold leaching is virtually an electrochemical process including anode dissolution of gold and cathode reduction of oxygen and other oxidants[3]. Intensification of anodic process and cathodic process can both lead to increase of gold leaching rate. When one semi-electrode is intensified to an enough extend, further intensification no longer contributes to further increase of gold leaching rate as the leaching process comes to be limited by the counter semi-electrode process. Being a cathodic intensifying technique, hydrogen peroxide assistant leaching will inevitably come to a limitation and further increase of gold leaching rate will depend on anodic intensification. Therefore, more effective intensification effect should be obtained by simultaneous promotion of both electrodes processes[4].
Some heavy metals such as Pb, Bi, Tl, Hg, and Ag were proved to be effective in intensifying anodic dissolution of gold[5-7]. On this basis, some investigations have been carried out on gold leaching with addition of Tl[4, 8], Bi[8], Pb[9], Ag[10], etc. However, few reports have been published on the practical application of these methods. The main obstacle may be attributed to the lack of overall recognition on their acting characteristics and on the adaptability of these elements in different materials with different leaching properties.
In the previous studies[11-13], the electrochemical kinetics of gold anodic dissolution intensified by these metals has been systematically investigated. On this basis, co-intensification of gold leaching has been studied based on mixed potential theory by using heavy metal ions as anodic intensifiers and hydrogen peroxide as cathodic intensifier.
In this work, co-intensification has been researched to accelerate gold leaching from different types of materials with regards to their electrochemical natures by using anodic intensifiers of heavy metals ions (Pb2+, Bi3+,Tl+, Hg2+ and Ag+) and cathodic intensifier of hydrogen peroxide.
2 Experimental
Three kinds of gold containing materials were used in this study: two concentrates with sulphides as gold-bearing minerals and one raw ore with oxides as gold-bearing minerals. The former two were labeled as sulphide gold concentrate A and sulphide gold concentrate B, respectively, and the third was named as oxide gold ore. Their chemical compositions are listed in Table 1.
From Table 1, two concentrates are high gold content materials and the oxide gold ore has only a low gold content of 3.9 g/t. In addition, the two sulphide concentrates have high S and Fe contents and noticeable Cu content, which may cause negative effects on gold leaching, while the oxide ore has little contents of these components. It was indicated by chemical phase analysis that pyrite was the main sulphide mineral of these two concentrates. Concentrate A also contained some chalcopyrite, pyrrhotite and marcasite as minor sulphide minerals, while concentrate B only contained some chalcopyrite as minor sulphide minerals; and neither pyrrhotite nor marcasite was found.
The intensifying reagents used in this study include hydrogen peroxide in the form of aqueous solution with 30% H2O2, and heavy metals in the form of analytically pure nitrate or sulphfate such as Pb(NO3)2, Bi(NO3)3, TlNO3, HgSO4, and AgNO3. Hydrogen peroxide was used as cathodic process intensifier and heavy metal salts were used as anodic process intensifiers. Besides, analytically pure sodium cyanide was used as lixiviant and chemically pure CaO was used to adjust pH value. Heavy metal salts and cyanide had been prepared in solutions before addition and CaO was added in the form of finely ground powder.
For each test, 50 g sample was first ground in a d160 mm×50 mm wet ball mill with mass fraction of 40% in grinding slurry, and then put in a 500 mL beaker. Cyanide, intensifiers, and water were added into the beaker to prepare a slurry of 20% and given concentrations of cyanide and intensifiers. The slurry was stirred with a EUROSTAR stepless speed regulation stirrer and CaO powder was added in the slurry to adjust pH to a given value in the beginning of stirring. After leaching for a given time, the slurry was filtered and the residue was dried in a electric drying oven. The residue and pregnant solution were both analyzed by atomic absorption spectrometry to determine gold content.
3 Results and discussion
3.1 Fundamental cyanide leaching characteristics
Fundamental leaching characteristics of these three kinds of gold containing materials were studied though conventional cyanide leaching in terms of gold leaching recovery as a function of leaching time. It was clearly found from Fig.1 that conventional gold leaching characteristics were quite different among three materials.
For sulphide gold concentrate A, gold leaching rate turned up very slowly. Gold leaching recovery increased slowly with increasing time especially after 36 h, and it was only 54.89% for a leaching time as long as 48 h. Therefore, sulphide gold concentrate A was a typical refractory gold concentrate which is difficult to be effectively leached by conventional cyanide leaching. In contrast, sulphide gold concentrate B exhibited a much more favourable leachability with much larger leaching rate and higher gold leaching recovery. Gold leaching recovery approached 60% within only 4 h and reached 94.23% after 24 h. For the oxide gold ore, there was also a relatively large gold leaching rate. Gold leaching recovery reached 68.8% within only 2 h and approached 80% after 8 h. However, further leaching was very difficult after 12 h and final recovery was only 84.55% after 24 h because of the low original gold content in the raw ore.
The reason for the striking difference between two kinds of concentrates might lie in their sulphide components. Concentrate A contained some amount of pyrrhotite and marcasite while concentrate B did contain either of them. As we know, pyrrhotite and marcasite were prone to be oxidized during gold cyanidation and depleted in oxygen and cyanide which were essential for gold leaching[14-15]. Herefore, gold leaching from concentrate A was seriously impeded. On the other hand, according to mixed potential theory illustrated in Fig.2, the oxidizing reaction of pyrrhotite and marcasite may act as simultaneously occurring anodic reactions which
Table 1 Chemical compositions of gold containing materials
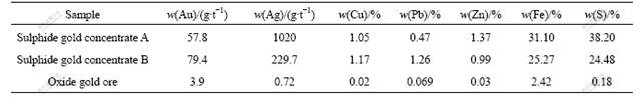
Fig.1 Gold leaching recoveries for three kinds of gold containing ores (Grinding grade <0.074 mm 99.50%, slurry density 20%, NaCN 0.2%, pH 11, and stirring speed 1 000 r/min)
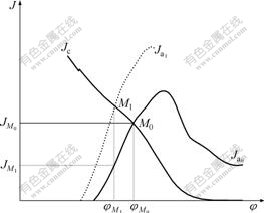
Fig.2 Diagram for illustration of decrease in current density
from easily oxidizing components (![]() , current density curve for anodic dissolution of pure gold;
, current density curve for anodic dissolution of pure gold; ![]() , current density curve
, current density curve
for anodic dissolution of imaginary easily oxidizing components; Jc current density curve for cathodic reduction of oxygen)
raise anodic curve from pure gold anodic process to total of coexisting anodic process. This will negatively shift mixed potential from M0 to M1 and result in the reduction
of gold dissolution current density from ![]() to
to ![]() .
.
On the basis of above mentioned leaching characteristics, concentrate A was classified as refractory sulphide gold concentrate; concentrate B was classified as easily leachable sulphide gold concentrate; and the oxide ore was named as low grade oxide gold ore because of its low gold content characterized leaching effect.
3.2 Co-intensification of gold leaching from refractory sulphide gold concentrate
Intensification of gold leaching from refractory sulphide gold concentrate by heavy metals was conducted by separately adding 10-5 mol/L Pb2+, Bi3+, Tl+, Hg2+, Ag+; and the results are shown in Table 2.
Table 2 Gold leaching recovery in the presence of heavy metal ions for refractory sulphide gold concentrate (%)

Grinding grade <0.074 mm 99.50%, aqueous phase-to-solid ratio 5?1, NaCN 0.2%, pH 11, stirring speed 1 000 r/min, leaching time 24 h, heavy metal concentration 10-5 mol/L
For the refractory sulphide gold concentrate, heavy metals did not show substantial promoting effect on gold leaching. Among these five kinds of metal ions, Bi3+, Tl+, Hg2+ just slightly increased gold leaching recovery and Pb2+, Ag+ did not take effect at all. This indicated that the intensifying effect of heavy metal on gold anodic dissolution could not promote the gold leaching rate for the refractory sulphide gold concentrate because easily oxidizing components depleted oxygen and cyanide needed for gold leaching and reduced mixed potential. With the negative shift of mixed potential, the difference between anodic currents with and without anodic intensifiers might decrease, which would undermine the effect of anodic intensification. Therefore, anodic intensifiers should not be suitable for gold ores containing oxygen consuming components.
In order to acquire expectant accelerating effect on gold leaching, cathodic process must be intensified in priority to diminish oxygen consuming matters and to increase anodic current. This would not only directly promote gold leaching but also help heavy metals to take effect by increasing mixed potential to the value at which the differences between the anodic curves with and without heavy metal ions become remarkable.
Hydrogen peroxide was used as cathodic intensifier for gold leaching alone and together with heavy metal ions Bi3+, Tl+, Hg2+. From Fig.3, hydrogen peroxide significantly raised gold leaching rate and gold leaching recovery, which indicated great promoting effect by cathodic intensification. Gold leaching recovery after 8 h was sharply increased from 31.88% to 60.20%. In addition, gold leaching process was further intensified by hydrogen peroxide together with heavy metal ions, exhibiting pronounced co-intensifying effect. Gold leaching recovery after 8 h was further increased to around 70%, which meant about 10% increment according to single intensification with hydrogen peroxide. This evidentially demonstrated the inducing function of cathodic intensification on effectiveness of anodic intensification and co-intensification. As for three kinds of tested metal ions, the contribution of Bi3+ to
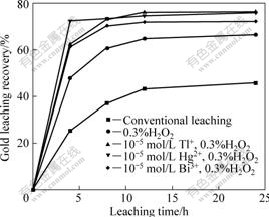
Fig.3 Effect of leaching time on gold leaching from refractory sulphide gold concentrate under co-intensification (Grinding grade <0.074 mm 99.50%, slurry density 20%, NaCN 0.2%, pH 11, stirring speed 1 000 r/min)
co-intensification was slightly less than that of Tl+ and Hg2+, and Hg2+ was more effective in the incipient 4 h of the leaching process.
It is noticed that, although the leaching process was greatly accelerated by co-intensification, gold leaching recovery was still in need of being further increased to fulfill favourable leaching. For this reason, two-step leaching was studied. According to Fig.4, the first step was arranged for 4 h, and then the seconded step was experimented. Both steps were co-intensified. From Fig 4, gold leaching recovery was effectively increased to above 90% in the second leaching step for 24 h. Therefore, two-step co-intensifying leaching was proved to be an effective way for the refractory sulphide gold concentrate.
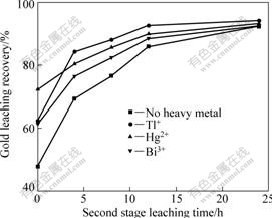
Fig.4 Effect of second step leaching time on gold leaching from refractory sulphide gold concentrate under co-intensification (First stage leaching time 4 h, slurry density 20%, NaCN 0.2%, H2O2 0.3%, pH 11, heavy metal concentration 10-5 mol/L, stirring speed 1 000 r/min)
3.3 Co-intensification of gold leaching from easily leachable sulphide gold concentrate
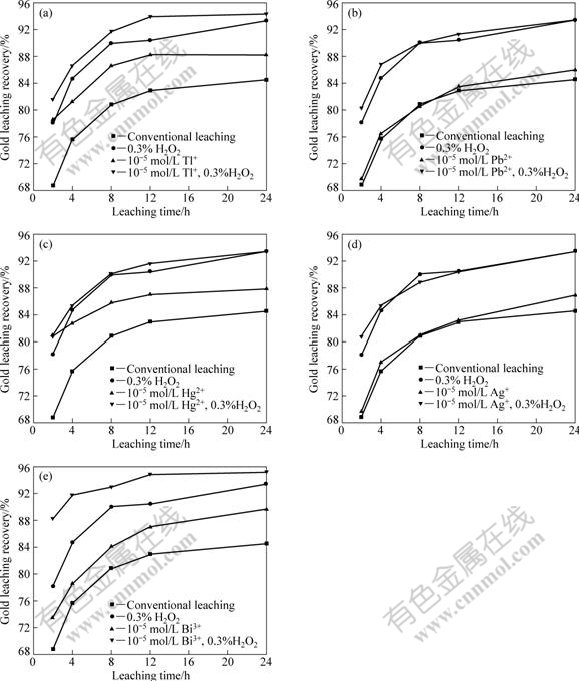
Experiments were conducted to study the effect of co-intensification on gold leaching from easily leachable sulphide gold concentrate by simultaneously using hydrogen peroxide and heavy metal ions in comparison with single intensification of semi-electrode process by singly using hydrogen peroxide or heavy metal ions. The results are shown in Fig.5. Three main aspects of information could be found from Fig.5. Firstly, gold leaching was effectively promoted by using cathodic intensifier of hydrogen peroxide. Gold leaching recovery was increased from 94.23% to more than 97% after leaching for 24 h. Secondly, anodic intensifiers of heavy metal ions presented stronger intensifying effects than hydrogen peroxide, as they not only further increased gold recovery in contrast with hydrogen peroxide but also made it possible to shorten leaching time from 24 h to 16 h. Thirdly, simultaneous use of hydrogen peroxide and heavy metal ions presented favourable co-intensifying effect which was more effective than that obtained by single intensifying with only one of the intensifiers. Gold leaching rate was further increased on the basis of intensified leaching by heavy metal ions, resulting in higher gold leaching recovery and shorter leaching time.
Although there existed some differences among effects of these five kinds of heavy metal ions mainly on leaching rate in the earlier leaching period, it is common for them that co-intensification increased gold leaching recovery to near 96% or even above 97% within 12 h and shortened leaching time to 12-16 h. In a word, for the easily leachable sulphide gold concentrate, co-intensification with anodic intensifiers of heavy metal ions and cathodic intensifiers of hydrogen peroxide not only shortened leaching time effectively but also further increased gold leaching recovery to some extend.
3.4 Co-intensification of gold leaching from low grad oxide gold concentrate
Fig.6 exhibits the co-intensifying results for the low grade oxide gold ore[11, 13]. Cathodic intensifier of hydrogen peroxide also remarkably promoted gold leaching in this case of gold leaching from oxide gold ore. Being intensified with hydrogen peroxide, gold leaching recovery was increased to 90.38% after 8 h and 93.41% after 24 h, indicating the similar magnitude of approximate 10% for each leaching time in the tested range of 0-4 h. This meant that hydrogen caused quicker and deeper leaching of gold form the oxide gold ore.
For the heavy metal ions, apparent difference existed as regard to intensifying the effect for the oxide gold ore. There were some differences from the former- mentioned gold containing materials where the heavy
Fig.5 Effect of co-intensification with different heavy metal ions on gold leaching from easily leachable sulphide gold ore (Grinding grade <0.074 mm 84.90%, aqueous phase to solid ratio 5?1, NaCN 0.2%, pH 11, stirring speed 1 000 r/min): (a) Thallium; (b) Lead; (c) Mercury; (d) Silver; (e) Bismuth
metal ions presented similar effect. There were three types of influence among these five ions in this case as follows: 1) Neither single intensifying effect nor co-intensifying effect, 2) Single intensifying effect to some extend but no noticeable co-intensifying effect, and 3) Noticeable intensifying effect whether singly using or simultaneously using cathodic intensifier of hydrogen peroxide.
Pb2+ and Ag+ belonged to the first type and Hg2+ belonged to the second type. Although Hg2+ caused some increase in gold leaching while being singly used, it was less effective than hydrogen. Moreover, it presented no noticeable co-intensifying effect while simultaneously being used with hydrogen peroxide. Bi3+ and Tl+ belonged to the third type. They presented significant intensifying effect whether being singly used or simultaneously used with hydrogen peroxide. Their single intensifying effect was not so strong as that of hydrogen peroxide. Between these two ions, it is interesting that they presented inverse comparative effect for singly intensifying and co-intensifying. While being singly used, Tl+ was more effective than Bi3+ and, while being simultaneously used with hydrogen peroxide, Bi3+ was more effective than Tl+ especially in the earlier 2 h. In cases of co-intensifying, gold leaching time was shortened to 12 h and gold leaching recovery was
Fig.6 Effect of co-intensification with different heavy metal ions on gold leaching from oxide gold ore (Grinding grade <0.074 mm 99.50%, aqueous phase-to-solid ratio 5?1, NaCN 0.2% pH 11, stirring speed 1 000 r/min): (a) Thallium; (b) Lead; (c) Mercury; (d) Silver; (e) Bismuth

increased to around 94%, which indicated an increased magnitude of nearly 10% within a half leaching time in contrast with conventional gold leaching.
4 Conclusions
1) Favourable co-intensifying effects on gold leaching were obtained for different types of gold-containing materials with different leaching characteristics and the leaching time was effectively shortened to no longer than 12 h by co-intensification of hydrogen peroxide assistant leaching. It was noted that the effects of intensification were different among the three materials.
2) For the refractory sulphide gold concentrate, heavy metal ions presented no obvious single intensifying effect on gold leaching, while Bi3+, Tl+, and Hg2+ caused co-intensifying effect and considerably increased gold leaching rate while being used in association with hydrogen peroxide.
3) For the easily leachable sulphide gold concentrate, heavy metal ions not only showed more single intensifying than hydrogen but also presented marked co-intensifying effect.
4) For the low grade oxide gold ore, Bi3+ and Tl+ had noticeable effect while being used whether for single intensification or for co-intensification.
References
[1] HUANG Kong-xuan. New advantages on gold extraction in foreign contries [J]. Hydrometallurgy, 1996(1): 9-18. (in Chinese)
[2] GUZMAN L, SGARRA M, CHIMENOS J M, FERNANDEZ M A, ESPIELL F. Gold cyanidation using hydrogen peroxide [J]. Hydrometallurgy, 1999, 52: 21-35.
[3] LIN H K, CHEN X. Electrochemical study of gold dissolution in cyanide solution [C]//SME Annual Meeting, Salt Lake City, 2000, 1-11.
[4] GUZMAN L, CHIMENOS J M, FERNANDEZ M A, SGARRA M, ESPIELL F. Gold cyanidation with potassium persulfate in the presence of a thallium(Ⅰ) salt [J]. Hydrometallurgy, 2000, 54: 185-193.
[5] CHIMENOS J M, SEGARRA M, GUZMAN L, KARAGUEORGUIEVA A, ESPIELL F. Kinetics of the reaction of gold cyanidation in the presence of a thallium(Ⅰ) salt [J]. Hydrometallurgy, 1997, 44: 269-286.
[6] JEFFREY M I, RITCHIE I M, LABROOY S R. The effect of lead on the electrochemistry of gold: Myth or magic [J]. Electrochemical Proceedings, 1996, 96(6): 284-295.
[7] TSHILOMBO A F, SANDENBERGH R F. An electrochemical study of the effect of lead and sulphide ions on the dissolution rate of gold in alkaline cyanide solutions [J]. Hydrometallurgy, 2001, 60: 55-67.
[8] SANDENBERGH R F, MILLER J D. Catalysis of the leaching of gold in cyanide solutions by lead, bismuth and thallium [J]. Minerals Engineering, 2001, 14(11): 1379-1386.
[9] DESCH?NES G, LASTRA R, BROWN J R, JIN S, MAY O, GHALI F. Effect of lead nitrate on cyanidation of gold ores: Progress on the study of the mechanisms [J]. Minerals Engineering, 2000, 13(12): 1263-1279.
[10] JEFFREY M I, RITCHIE I M. The leaching of gold in cyanide solutions in the presence of impurities Ⅱ. The effect of silver [J]. J Electrochem Soc, 2000, 147(9): 3272-3276.
[11] YANG Yong-bin. Investigation on electrochemical kinetics and application for co-investigation of gold leaching [D]. Changsha: Central South University, 2008. (in Chinese)
[12] YANG Yong-bin, LI Qian, LI Guang-hui, GUO Yu-feng, HUANG Zhu-cheng, JIANG Tao. An electrochemical investigation on intensification of gold cyanidation by heavy metal ions [C]// SCHLESINGER M E. EPD Congress 2005. Warrendale, USA: TMS (The Minerals, Metals & Materials Society), 2005.
[13] YANG Yong-bin, LI Qian, JIANG Tao, JIN yong-shi. Cyanide leaching of gold ores intensified by heavy metal ions [J]. The Chinese Journal of Nonferrous Metals, 2005, 15(8): 1283-1288. (in Chinese)
[14] SENANAYAKE G. Kinetics and reaction mechanism of gold cyanidation: Surface reaction model via Au(I)-OH-CN complexes [J]. Hydrometallurgy, 2005, 80: 1-12.
[15] LI Ding-xin, WANG Yong-lu. The extraction and refinery of precious metals [M]. Changsha: Central South University Press, 2003.
(Edited by YANG Bing)
Foundation item: Project(50725416) supported by the National Natural Science Foundation for Distinguished Young Scholars of China
Corresponding author: LI Qian; Tel: +86-731-88830547; Fax: +86-731-88830542; E-mail: csuliqian@126.com
DOI: 10.1016/S1003-6326(09)60234-X


