文章编号:1004-0609(2016)-09-1929-06
纳米CuO表面包覆对Li1.13[Ni0.5Mn0.5]0.87O2正极材料电化学性能的影响
袁 好,王先友,胡 亮,杨秀康,舒洪波
(湘潭大学 化学学院 环境友好化学与应用教育部重点实验室,
电化学能源储存与转换湖南省重点实验室,湘潭 411105)
摘 要:
为了提高球形Li1.13[Ni0.5Mn0.5]0.87O2正极材料的电化学性能,通过非均匀成核法在材料颗粒表面包覆一层纳米CuO;采用XRD、SEM、TEM和充放电测试仪对所包覆材料进行测试与表征。结果表明:适量的CuO包覆可有效地提高Li1.13[Ni0.5Mn0.5]0.87O2正极材料的电化学性能;当CuO包覆量为2%(质量分数)时,材料的电化学性能最佳。在0.1C、2.0~4.6 V充放电条件下,其首次放电容量为213.7 mA·h/g,首次库仑效率可达86.9%。此外,该材料在0.5C倍率下循环100次后其放电比容量仍为169.5 mA·h/g,容量保持率为79.3%;而未经包覆的Li1.13[Ni0.5Mn0.5]0.87O2在相同循环条件下,容量保持率仅为65.5%。
关键词:
中图分类号:TM912.9 文献标志码:A
随着便携式电子产品和清洁能源汽车的快速发展,对锂离子电池的性能提出了更高的要求[1-5]。然而,目前锂离子电池的能量密度、安全性能以及成本问题仍然制约其进一步发展。正极材料是决定锂离子电池综合性能的关键因素之一。传统商业化的正极材料,如层状LiCoO2、尖晶石Li2MnO4以及橄榄石LiFePO4,均存在放电比容量低(<200 mA·h/g)、能量密度不足等缺点,已无法满足电动汽车等领域对电池性能的高要求。因此,开发高能量密度正极材料成为锂离子电池领域的研究重点。
层状富锂正极材料,Li1+xM1-xO2(M=Ni,Co,Mn等),由于其高放电比容量(>200 mA·h/g)和低成本等优点而备受关注[6-11]。然而,由于充放电机制的特殊性和结构上的复杂性,层状富锂正极材料表现出较大首次不可逆容量损失、高电压下容量与电压衰减快和倍率性能欠佳等缺点,减缓了其实际商业化步伐。近些年来,人们通过掺杂、制备特殊形貌、不同晶型复合等方法,在一定程度上改善了富锂正极材料的电化学性能。MA等[12]通过Mo进行部分或者全部掺杂,形成Li2MoO3基富锂正极材料,有效改善电压衰退等问题;ZHANG等[13]制备了一种一次粒子沿着[010]晶面生长的二次球形富锂材料,提高了锂离子的脱出与嵌入速率,明显改善富锂正极材料的倍率等电化学性能;近年来,WANG等[14]通过水热法,成功制备出尖晶石-层状复合晶型富锂正极材料,通过尖晶石相的三维隧道结构,有效提高锂离子的扩散速率。
通过表面包覆提高材料界面稳定性和减缓正极活性物质与电解液的副反应也被认为是改善材料电化学性能的有效方法之一[15-16]。金属氧化物,如MgO[15]、TiO2[17]、Al2O3[18]等包覆通常被认为是改进锂离子电池正极材料性能的有效方法。金属氧化物包覆能够改善材料的性能,一般认为是包覆层可阻隔电解液中产生的HF等对材料表面的侵蚀,从而提高材料表面稳定性,降低界面阻抗,进而改善材料的电化学性能[19-21]。
本文作者采用非均匀成核法在富锂正极材料Li1..13[Ni0.5Mn0.5]0.87O2表面包覆一层稳定的纳米CuO膜,研究不同CuO包覆量对材料电化学性能的影响,从而获得性能优异的锂离子电池正极材料,为锂离子电池性能的改善提供可借鉴的科学依据。
1 实验
1.1 球形Li1.13[Ni0.5Mn0.5]0.87O2的制备
将预先配制好的的镍锰混合硫酸盐溶液(2.0 mol/L,Ni和Mn的摩尔比为1:1、碳酸钠溶液(2.0mol/L)和一定量的氨水并流加入到共沉淀反应釜中;严格控制反应釜内温度55 ℃、pH值7.5等反应条件,使其通过控制结晶共沉淀反应得到球形碳酸盐Ni0.5Mn0.5CO3;待碳酸盐颗粒达到要求的尺寸后,通过反复洗涤、抽滤,再在110 ℃下干燥12 h得到碳酸盐Ni0.5Mn0.5CO3前驱体;将得到的碳酸盐前躯体粉末置于坩埚中,在马弗炉中以500 ℃煅烧6 h得到氧化物;最后将氧化物与Li2CO3按一定比例混合均匀,放入马弗炉中升温至900 ℃下高温煅烧12 h,得到富锂正极材料Li1.13[Ni0.5Mn0.5]0.87O2。
1.2 CuO包覆Li1..13[Ni0.5Mn0.5]O2材料的制备
称取适量的Li1.13[Ni0.5Mn0.5]0.87O2正极材料置于无水乙醇中搅拌均匀,然后按不同包覆量(1%,2%,3%,4%)将相应的Cu(NO3)2·6H2O缓慢加入,用稀氨水调节pH值至9.4左右,持续搅拌12 h使其在正极材料颗粒的表面包覆一层Cu(OH)2,最后将上述得到的混合物进行洗涤、过滤,再在120 ℃下烘干后,放入马弗炉中在500 ℃下煅烧12 h,冷却后得到CuO包覆Li1.13[Ni0.5Mn0.5]0.87O2正极材料。
1.3 材料物理性质表征
通过日本JEOL公司生产的JSM-5600LV型扫描电子显微镜(SEM)以及JEM-2100F型透射电子显微镜(TEM)观察样品的微观形貌;采用日本理学D/max-3C型X射线衍仪(XRD)对样品进行晶体结构分析,射线源为Cu Kα,管电流20 mA,管电压36 kV,扫描速率4 (°)/min,扫描范围2θ=10°~80°;通过AA7000型原子吸收分光光度计(日本SHIMADZU公司生产)对样品进行化学元素含量的分析;采用BT9300H型激光粒度分析仪(丹东市百特仪器有限公司生产)分析样品的粒径分布。
1.4 电池的制备和电化学性能测试
将所制备的富锂正极材料与乙炔黑,石墨,粘合剂(PVDF)按质量比为80:5:5:10进行混合制浆,搅拌均匀后涂在铝箔上,在80 ℃下干燥8 h后再置于120 ℃真空干燥箱中干燥12 h得到正极片,将锂片作为负极,Celgard2400为隔膜,1 mol/L的LiPF6/(EC+DMC,体积比1:1)为电解液,在氩气气氛手套箱中组装成2025扣式电池。电池测试在新威测试充放电仪上进行,测试温度为25 ℃。此外,采用普林斯顿电化学工作站(VersaSTAT3)对样品进行电化学阻抗测试。
2 结果与讨论
图1所示为不同CuO包覆量的Li1.13[Ni0.5Mn0.5]0.87O2材料的XRD谱。从图1可以看出,包覆后的Li1.13[Ni0.5Mn0.5]0.87O2样品与包覆前的Li1.13[Ni0.5Mn0.5]0.87O2样品的XRD谱无明显变化,与标准的α-NaFeO2晶型的衍射峰基本吻合。其中,2%-CuO包覆Li1.13[Ni0.5Mn0.5]0.87O2材料的I(003)/I(104)的峰强度比值较高,说明材料中阳离子混排度小。同时该材料的(006)/(102)和(108)/(110)衍射峰分裂较明显,说明材料结晶完整,具有较好的层状结构[14]。仔细观察发现,XRD谱中并未出现CuO的特征峰,这可能由于纳米CuO仅仅包覆在Li1.13[Ni0.5Mn0.5]0.87O2材料的表面,并且CuO包覆量较少,未影响到材料的内部晶体结构。
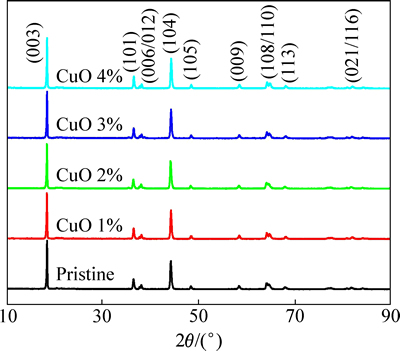
图1 不同CuO包覆量的Li1.13[Ni0.5Mn0.5]0.87O2的XRD谱
Fig. 1 XRD patterns of Li1.13[Ni0.5Mn0.5]0.87O2 with different contents of CuO coating
为了考察包覆前后材料微观形貌的变化,将未包覆和2.0% CuO包覆量的Li1.13[Ni0.5Mn0.5]0.87O2进行扫描电镜分析。图2所示为包覆前后Li1.13[Ni0.5Mn0.5]0.87O2的SEM像。从图2(a)可观察到,未包覆Li1.13[Ni0.5Mn0.5]0.87O2材料是由许多细小的纳米级一次粒子团簇而成的球形颗粒,粒径大小约为8 μm,并且一次粒子轮廓明显。从图2(b)可以看出,2.0% CuO包覆的Li1.13[Ni0.5Mn0.5]0.87O2材料的形貌较未包覆材料无明显的变化,但是包覆后颗粒表面的一次晶粒轮廓变得模糊,颗粒表面变得较为光滑。为了进一步证明CuO包覆层的存在,对包覆前后材料颗粒进行了TEM测试,结果如图3所示。通过对比可以看出,未包覆样品颗粒表面包覆后的Li1.13[Ni0.5Mn0.5]0.87O2颗粒表面存在一层明显的CuO包覆膜,其厚度约为6 nm(见图3(b))。由此可得,通过非均匀成核法成功地在Li1.13[Ni0.5Mn0.5]0.87O2材料颗粒表面包覆一层纳米CuO膜。

图2 未包覆与2.0% CuO包覆的Li1.13[Ni0.5Mn0.5]0.87O2材料的SEM像
Fig. 2 SEM images of pristine (a) and 2% CuO-coated (b) Li1.13[Ni0.5Mn0.5]0.87O2

图3 未包覆和2.0%CuO包覆的Li1.13[Ni0.5Mn0.5]0.87O2的TEM像
Fig. 3 TEM images of pristine (a) and 2% CuO-coated (b) Li1.13[Ni0.5Mn0.5]0.87O2
为了研究CuO包覆对富锂正极材料电化学性能的影响,将所得的材料组装成扣式电池进行充放电测试。图4所示为未包覆和不同CuO包覆量的Li1.13[Ni0.5Mn0.5]0.87O2正极材料在0.1C倍率下、2.0~4.6 V电压范围内的首次充放电曲线。从图4可见,未经过包覆处理的Li1.13[Ni0.5Mn0.5]0.87O2材料首次放电比容量为218.8 mA·h/g,而包覆CuO的材料其首次放电比容量均有所降低。然而,当CuO包覆量为2%时,所得到的材料的首次放电比容量仅略微衰减,仍可达213.7 mA·h/g。此外,随着包覆量的减少或者增加,材料的容量均下降的较快,CuO的包覆量为1%、3%和4%时,其首次放电比容量分别为210.8、205.3和195.4 mA·h/g。CuO包覆后的Li1.13[Ni0.5Mn0.5]0.87O2的首次放电比容量均会有不同程度的降低,可能是由于CuO是电化学惰性而影响了材料整体容量的发挥。然而,适量的CuO包覆可提高材料的首次库仑效率。当包覆量为2%时,首次库仑效率可达到最大值(87.0%),而未包覆材料的首次库仑效率仅为75.7%。
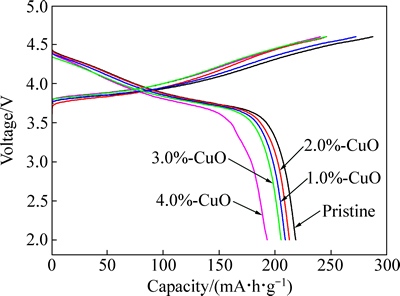
图4 未包覆和不同CuO包覆量的Li1.13[Ni0.5Mn0.5]0.87O2在2.0~4.6 V、0.1C条件下的首次充放电曲线
Fig. 4 First charge and discharge curves of pristine and CuO-coated Li1.13[Ni0.5Mn0.5]0.87O2 at 0.1C in voltage range of 2.0-4.6 V
图5所示未包覆与不同CuO包覆量的富锂正极材料Li1.13[Ni0.5Mn0.5]0.87O2在2.0~4.6 V电压范围内、0.5C倍率下的循环寿命曲线。从图5可以看出,未包覆样品在0.5C倍率下经过100 次循环后其容量保持率仅为65.2%,而CuO包覆量为1.0%、2.0%、3.0%、4.0%的样品在相同充放电条件下,其容量保持率分别为69.8%、79.3%、67.4%和66.2%。显然,当CuO包覆量为2%时,材料的循环性能最佳,明显优于未包覆和其他包覆量的材料。由此可见,适量的CuO包覆可以阻碍电解液对材料的侵蚀,减少界面副反应,进而抑制容量衰减,提高其循环性能。由于CuO本身是电化学惰性的,当包覆量过多时,造成表面惰性包覆层过厚,反而阻碍了锂离子的扩散和电子的传导,影响其循环寿命。
表1 未包覆和CuO包覆的Li1.13[Ni0.5Mn0.5]0.87O2材料的电性能比较
Table 1 The electrochemical performance of pristine and CuO-coated Li1.13[Ni0.5Mn0.5]0.87O2
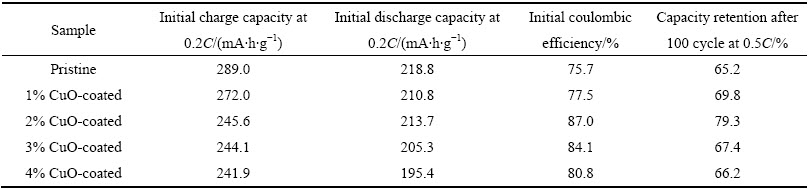

图5 未包覆和不同CuO包覆量的Li1.13[Ni0.5Mn0.5]0.87O2在0.5C、2.0~4.6 V条件下的循环寿命曲线
Fig. 5 Cycle performance curves pristine and CuO-coated Li1.13[Ni0.5Mn0.5]0.87O2 at 0.5C in voltage range of 2.0-4.6 V
倍率性能也是正极材料的另一个重要性能指标。将所制备的材料在2.0~4.6 V电压范围内,以不同的倍率进行充放电,考察其倍率性能。图6所示为未包覆和不同CuO包覆量的Li1.13[Ni0.5Mn0.5]0.87O2在0.1C、0.2C、0.5C、1C和2C倍率下的放电容量曲线。由图6可知,未经CuO包覆的材料在0.1C放电倍率下的放电比容量为218.8 mA·h/g,而在2C放电倍率下其放电比容量仅有123.6 mA·h/g。此外,经过适量CuO包覆后的材料的倍率性能有较好的改善,特别是2% CuO包覆的样品的倍率性能最佳,其在2C放电倍率下的放电比容量仍然有154.8 mA·h/g。比较其他包覆量的样品的倍率性能可知,CuO包覆的量过少或过多时,倍率性能改善的效果不明显。当包覆量过少时,CuO包覆层并不能完全覆盖在活性材料表面,因而不能最大限度的改善材料性能,而当包覆量增加到4%时,其2C放电倍率下的放电比容量仅有116.4 mA·h/g,这可能也是由于过厚的CuO包覆层反而阻碍了锂离子的扩散所致。

图6 未包覆和不同CuO包覆量的Li1.13[Ni0.5Mn0.5]0.87O2的倍率性能曲线
Fig. 6 Rate capability of pristine and CuO-coated Li1.13[Ni0.5Mn0.5]0.87O2
为了更好地解释包覆后样品电化学性能提高的原因,对未包覆和包覆后样品进行交流阻抗分析。图7所示为未包覆和CuO包覆量为2%的Li1.13[Ni0.5Mn0.5]0.87O2 在4.6 V状态下的交流阻抗谱图。从图7可见,两样品的交流阻抗谱图均是由两个半圆弧和一条斜线组成,高频部分的半弧反映活性材料在表面SEI层的阻抗,中频部分对应于电极/电解液界面的电化学过程电荷传递阻抗,而低频部分则表示锂离子在材料体相的扩散阻抗。未包覆的Li1.13[Ni0.5Mn0.5]0.87O2的SEI膜阻抗为105.3 Ω,而2% CuO包覆的Li1.13[Ni0.5Mn0.5]0.87O2的SEI膜阻抗仅74.7 Ω。显然,CuO包覆后使得活性材料在SEI膜的阻抗明显减小,从而更加有利于电子导电性的提高。此外,包覆材料也有效减小了本体材料和电解液的接触面积,减小了电解液中产生的HF等对材料的有害侵蚀,进而降低了金属离子在电解液中的溶解,从而提高了材料的循环稳定性等电化学性能。
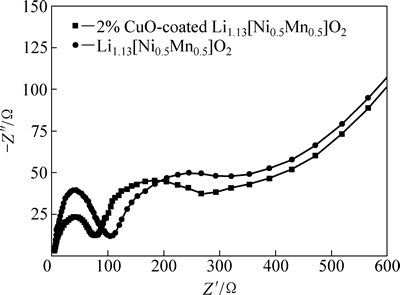
图7 未包覆和2.0%CuO包覆的Li1.13[Ni0.5Mn0.5]0.87O2的交流阻抗谱图
Fig. 7 Electrochemical impedance spectra of pristine and 2% CuO-coated Li1.13[Ni0.5Mn0.5]0.87O2
3 结论
1) 通过非均匀成核法在富锂正极材料Li1.13[Ni0.5Mn0.5]0.87O2表面包覆一层4~6 nm的CuO膜,包覆后的材料并没有影响材料的晶体结构。
2) 包覆后材料的首次放电比容量略微降低,但其具有优异的循环性能和倍率特性。其中,2%CuO包覆量的样品表现出最优的电化学性能,其在0.1C、2.0~4.6 V充放电条件下的首次放电比容量可达213.7 mA·h/g,首次库伦效率为87.0%;而未包覆材料的首次库伦效率仅为75.7%。此外,该材料在0.5C倍率下经过100次循环后容量保持率仍然为79.3%,并且在2C倍率下的放电比容量也为154.8 mA·h/g,明显优于未包覆样品的(100次循环容量保持率为65.2%,2C倍率放电比容量为123.6 mA·h/g)。
3) 交流阻抗测试表明,CuO包覆后可有效地改善材料与电解液之间的界面状况,降低电化学阻抗,从而提高材料的电化学性能。
REFERENCES
[1] TARASCON J M, ARMAND M. Issues and challenges facing rechargeable lithium batteries[J]. Nature, 2001, 414(6861): 359-367.
[2] GOODENOUGH J B, PARK K S. The Li-ion rechargeable battery: A perspective[J]. Journal of the American Chemical Society, 2013, 135(4): 1167-1176.
[3] 邵 威, 刘昌位, 郭玉忠, 吴 佳, 王剑华. 锂离子电池正极材料Li2MnO3的显微组织与电化学性能[J]. 中国有色金属学报, 2015, 25(3): 705-713.
SHAO Wei, LIU Chang-wei, GUO Yu-zhong, WU Jia, WANG Jian-hua. Microstructure and electrochemical properties of Li2MnO3 as cathode material for lithium-ion battery[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(3): 705-713.
[4] 朱伟雄, 李新海, 王志兴, 郭华军. 锂离子电池富锂材料Li[Li0.2Ni0.2Mn0.6]O2的制备及掺杂改性[J]. 中国有色金属学报, 2013, 23(4): 1047-1052.
ZHU Wei-xiong, LI Xin-hai, WANG Zhi-xing, GUO Hua-jun. Synthesis and modification of Li-rich cathode Li[Li0.2Ni0.2Mn0.6]O2 for Li-ion batteries[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(4): 1047-1052.
[5] ZHU Ji-ping, ZHANG Sheng, XIN Zhi-qiang, XU Quan-bao, SU Hui. Synthesis and electrochemical properties of LiCo0.9Ni0.05Mn0.05O2 cathode material with high rate capability for lithium ion batteries[J]. The Chinese Journal of Nonferrous Metals 2014, 24(11): 2813-2820.
[6] MARTHA S K, NANDA J, WVEITH G M, DUDNEY N J. Electrochemical and rate performance study of high-voltage lithium-rich composition: Li1.2Mn0.525Ni0.175Co0.1O2[J]. Journal of Power Sources, 2012, 199(1): 220-226.
[7] JOHNSON C S, Li N, LEFIEF C, LEFIEF C, VAUGHEY J T, THACKERAY M M. Synthesis, characterization and electrochemistry of lithium battery electrodes: xLi2MnO3· (1-x)LiMn0.333Ni0.333Co0.333O2(0≤x≤0.7)[J]. Chemistry of Materials, 2008, 20(19): 6095-6106.
[8] THACKERAY M M, KANG S H, JOHNSON C S, VAUGHEY J T, BENEDEK R, HACKNEY S A. Li2MnO3-stabilized LiMO2(M=Mn, Ni, Co) electrodes for lithium-ion batteries[J]. Journal of Materials Chemistry, 2007, 17(30): 3112-3125.
[9] 张 嘉, 朱泽华, 王海峰. 富锂锰基层状锂离子电池正极材料的研究现状[J]. 电源技术, 2015, 39(6): 1323-1326.
ZHANG Jia, ZHU Ze-hua, WANG Hai-feng. Research status of lithium-rich Mn-based layered cathode materials for Li-ion batteries[J]. Chinese Journal of Power Sources, 2015, 39(6): 1323-1326.
[10] 周罗增, 徐群杰, 汤卫平, 靳 雪, 袁小磊. 锂离子电池富锂锰基正极材料的研究进展[J]. 化学进展, 2015, 24(2): 138-144.
ZHOU Luo-zeng, XU Qun-jie, TANG Wei-ping, JIN Xue, YUAN Xiao-lei. Research Progress of Mn-Based Lithium-Rich Cathode Materials for Li-ion Batteries[J]. Journal of Electrochemistry, 2015, 21(2): 138-144.
[11] 李明明, 张英杰, 董 鹏. 锂离子电池正极材料稀土掺杂的研究进展[J]. 电源技术, 2015, 39(7): 1539-1542.
LI Ming-ming, ZHANG Ying-jie, DONG Peng. Research progress of rare earth doping cathode material for lithium ion battery[J]. Chinese Journal of Power Sources, 2015, 39(7): 1539-1542.
[12] MA Jun, ZHOU Yong-Ning, GAO Yu-rui, YU Xi-qian, KONG Qing-yu, GU Lin, WANG Zhao-xiang, YANG Xiao-Qing, CHEN Li-quan. Feasibility of using Li2MoO3 in constructing Li-Rich high energy density cathode materials[J]. Chemistry of Materials, 2014, 26: 3256-3262.
[13] ZHANG Lin-jing, LI Ning, WU Bo-rong, XU Hong-liang, WANG Lei, YANG Xiao-Qing, WU Feng. Sphere-shaped hierarchical cathode with enhanced growth of nanocrystal planes for high-rate and cycling-stable li-ion batteries[J]. Nano Letters, 2015, 15: 656-661.
[14] WANG Di, YU Rui-zhi, WANG Xian-you, GE Long, YANG Xiu-kang. Dependence of structure and temperature for lithium-rich layered-spinel microspheres cathode material of lithium ion batteries[J]. Scientific Reports, 2015, 5: 8403.
[15] HAN En-shan, LI Yan-pu, ZHU Ling-zhi, ZHAO Ling. The effect of MgO coating on Li1.17Mn0.48Ni0.23Co0.12O2 cathode material for lithium ion batteries[J]. Solid State Ionics, 2014, 255(1): 113-119.
[16] YAN Li, LIU Kai-yu, LU Mei-yu, WEI Lai, ZHONG Jian-jian, Synthesis, characterization and electrochemical performance of AlF3-coated Li1.2(Mn0.54Ni0.16Co0.08)O2 as cathode for Li-ion battery[J]. Transactions of Nonferrous Metals Society of China, 2014, 24(11): 3534-3540.
[17] ZHANG Xiao-feng, BELHAROUAK I, LI Li, LEI Yu, ELAM J W, NIE A, CHEN Xin-qi, YASSAR R S, AXELBAUM R L. Structural and electrochemical study of Al2O3 and TiO2 coated Li1.2Ni0.13Mn0.54Co0.13O2 cathode material using ALD[J]. Advanced Energy Materials, 2013, 3(10): 1299-1307.
[18] ZOU Gui-shan, YANG Xiu-kang, WANG Xian-you, GE Long, SHU Hong-bo, BAI Yan-song, WU Chun, GUO Hai-peng, HU Liang, YI Xin, JU Bo-wei, HU Hai, WANG Di, YU Rui-zhi. Improvement of electrochemical performance for Li-rich spherical Li1.3[Ni0.35Mn0.65]O2+x modified by Al2O3[J]. Journal of Solid State Electrochemistry, 2014, 18(7): 1789-1797.
[19] JU Bo-wei, WANG Xian-you, WU Chun, WEI Qi-liang, YANG Xiu-kang, SHU Hong-bo, BAI Yan-song. Excellent cycling stability of spherical spinel LiMn2O4 by Y2O3 coating for lithium-ion batteries[J]. Journal of Solid State Electrochemistry, 2014, 18(1): 115-123.
[20] LI De-cheng, YASUHIRO K, KOICHI K, HIDEYUKI N, YUICHI S. Preparation and electrochemical characteristics of Li[Nil/3Co1/3Mn1/3]O2 coated with metal oxides coating[J]. Journal of Power Sources, 2006, 160(2): 1342-1348.
[21] 朱继平, 张 胜, 辛智强, 许全保, 苏 徽. 改性LiNi1/3Col/3Mnl/3O2正极材料的合成及其电化学性能[J]. 中国有色金属学报, 2014, 24(4): 974-980.
ZHU Ji-ping, ZHANG Sheng, XIN Zhi-qiang, XU Quan-bao, SU Hui. Synthesis and electrochemical properties of modified LiNi1/3Co1/3Mn1/3O2 cathode materials[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(4): 974-980.
Effects of CuO-coating on electrochemical performances of Li1.13[Ni0.5Mn0.5]0.87O2 cathode material
YUAN Hao, WANG Xian-you, HU Liang, YANG Xiu-kang, SHU Hong-bo
(Key Laboratory of Environmentally Friendly Chemistry and Applications,
Key Laboratory of Electrochemical Energy Storage and Conversion of Hunan Province, Ministry of Education,
School of Chemistry, Xiangtan University, Xiangtan 411105, China)
Abstract: In order to improve the electrochemical performance of spherical Li1.13[Ni0.5Mn0.5]0.87O2 cathode material, CuO-coated Li1.13[Ni0.5Mn0.5]0.87O2 cathode material was synthesized by heterogeneous nucleation process. The physical and electrochemical properties of the pristine and CuO-coated materials were characterized by XRD, SEM, TEM and electrochemical measurements. The results show that the electrochemical properties of the Li1.13[Ni0.5Mn0.5]0.87O2 are significantly improved with proper amount of CuO coating. Especially, the 2% CuO-coated sample exhibits the best electrochemical properties, which delivers a high initial discharge capacity of 213.7 mA·h/g at rate of 0.1C in the voltage range of 2.0-4.6 V with coulombic efficiency of 86.9%. Additionally, the 2% CuO-coated sample shows improved cycling stability with capacity retention of 79.3% after 100 cycles at 0.5C, while only 65.5% for the pristine Li1.13[Ni0.5Mn0.5]0.87O2 under the same charge-discharge condition.
Key words: lithium-ion battery; Li-rich cathode material; copper oxide surface coating; electrochemical performance
Foundation item: Project(2012CK1006) supported by the Scientific and Technical Achievement Transformation Fund of Hunan Province, China; Project(1SCY004) supported by the Natural Science Foundation of Hunan Province, China.
Received date: 2015-10-12; Accepted date: 2016-07-11
Corresponding author: WANG Xian-you, Tel: +86-731-58293043; E-mail: wxianyou@yahoo.com
(编辑 龙怀中)
基金项目:湖南省重大科技成果转化项目(2012CK1006);湖南省教育厅科技成果产业化项目(1SCY004)
收稿日期:2015-10-12;修订日期:2016-07-11
通信作者:王先友,教授,博士;电话:0731-58293043;E-mail:wxianyou@yahoo.com
摘 要:为了提高球形Li1.13[Ni0.5Mn0.5]0.87O2正极材料的电化学性能,通过非均匀成核法在材料颗粒表面包覆一层纳米CuO;采用XRD、SEM、TEM和充放电测试仪对所包覆材料进行测试与表征。结果表明:适量的CuO包覆可有效地提高Li1.13[Ni0.5Mn0.5]0.87O2正极材料的电化学性能;当CuO包覆量为2%(质量分数)时,材料的电化学性能最佳。在0.1C、2.0~4.6 V充放电条件下,其首次放电容量为213.7 mA·h/g,首次库仑效率可达86.9%。此外,该材料在0.5C倍率下循环100次后其放电比容量仍为169.5 mA·h/g,容量保持率为79.3%;而未经包覆的Li1.13[Ni0.5Mn0.5]0.87O2在相同循环条件下,容量保持率仅为65.5%。
[3] 邵 威, 刘昌位, 郭玉忠, 吴 佳, 王剑华. 锂离子电池正极材料Li2MnO3的显微组织与电化学性能[J]. 中国有色金属学报, 2015, 25(3): 705-713.
[4] 朱伟雄, 李新海, 王志兴, 郭华军. 锂离子电池富锂材料Li[Li0.2Ni0.2Mn0.6]O2的制备及掺杂改性[J]. 中国有色金属学报, 2013, 23(4): 1047-1052.
[9] 张 嘉, 朱泽华, 王海峰. 富锂锰基层状锂离子电池正极材料的研究现状[J]. 电源技术, 2015, 39(6): 1323-1326.
[10] 周罗增, 徐群杰, 汤卫平, 靳 雪, 袁小磊. 锂离子电池富锂锰基正极材料的研究进展[J]. 化学进展, 2015, 24(2): 138-144.
[11] 李明明, 张英杰, 董 鹏. 锂离子电池正极材料稀土掺杂的研究进展[J]. 电源技术, 2015, 39(7): 1539-1542.


