文章编号:1004-0609(2010)11-2213-08
铜表面吡咯烷二硫代氨基甲酸铵自组装膜的拉曼光谱和缓蚀作用
岳忠文1, 2 , 廖强强1, 2, 王作华3, 李义久3, 陈亚琼1, 2, 葛红花1, 2
(1. 上海电力学院 上海高校电力腐蚀控制与应用电化学重点实验室, 上海 200090;
2. 上海电力学院 上海热交换系统节能工程技术研究中心, 上海 200090;
3. 同济大学 化学系,上海 200092)
摘 要:
应用表面增强拉曼光谱(SERS)和电化学方法,对铜电极表面吡咯烷二硫代氨基甲酸铵(APDTC) 的自组装单层结构、缓蚀性能和吸附行为进行研究。 SERS光谱表明:APDTC分子通过硫原子垂直吸附在铜表面形成APDTC自组装单分子膜(APDTC SAMs)。交流阻抗和极化曲线实验表明:在3%NaCl(质量分数)溶液中APDTC SAMs对铜具有很好的缓蚀作用,最高缓蚀效率可达98%; APDTC的吸附行为符合Langmuir 吸附等温式, 吸附机理是介于化学吸附和物理吸附之间的一种吸附。
关键词:
铜; 吡咯烷二硫代氨基甲酸铵; 拉曼光谱; 自组装单分子膜; 缓蚀; 吸附;
中图分类号: O646.6 文献标志码:A
Raman spectrum and inhibition effect of self-assembled monolayers of ammonium pyrrolidine dithiocarbamate on copper surface
YUE Zhong-wen1, 2, LIAO Qiang-qiang1, 2, WANG Zuo-hua3, LI Yi-jiu3, CHEN Ya-qiong1, 2, GE Hong-hua1, 2
(1. Key Laboratory of Shanghai Colleges and Universities for Electric Power Corrosion Control and
Applied Electrochemistry, Shanghai University of Electric Power, Shanghai 200090, China;
2. Shanghai Engineering Research Center of Energy-Saving in Heat Exchange Systems,
Shanghai University of Electric Power, Shanghai 200090, China;
3. Department of Chemistry, Tongji University, Shanghai 200092, China)
Abstract: The structure, corrosion inhibition and adsorption behavior of ammonium pyrrolidine dithiocarbamate (APDTC) self-assembled monolayers (SAMs) on a copper surface were investigated by surface-enhanced Raman scattering spectroscopy (SERS) and electrochemical methods. The results of SERS experiment show that APDTC SAMs is adsorbed on the copper surface by its S atoms with perpendicular orientation. The AC impedance and DC polarization results indicate that APDTC SAMs have good corrosion inhibition effects on copper in a 3% NaCl solution and its maximum inhibition efficiency can reach 98%. Adsorption of the APDTC SAMs is found to follow the Langmuir′s adsorption isotherm and the mechanism consists of a mixed adsorption between chemisorption and physisorption.
Key words: copper; ammonium pyrrolidine dithiocarbamate; Raman spectrum; self-assembled monolayer; corrosion inhibition; adsorption
自组装膜技术(SAMs)是20世纪80年代初期发展起来的较为活跃的界面化学和材料化学的前沿领域,近几年来自组装膜技术在金属的腐蚀和防护方面也得到了应用。工业上常常使用铜或铜合金等金属材料,在使用过程中不可避免要受到侵蚀性介质的腐蚀, 若在其表面制备自组装膜可有效、简便地防止腐蚀的发生。然而,对于工业上应用广泛的铁、铜、锌等金属,在其表面自组装防腐蚀性的自组装膜技术还很不成熟,亟待进一步开的发和探索[1-5]。
表面增强拉曼散射(Surface-enhanced Raman scattering, SERS) 光谱是一种高灵敏度的表面分析技术[6-8], 可测到单分子层的振动信息, 常用于研究界面吸附作用方式、分子界面取向和吸附态变化, 已被广泛应用于缓蚀作用机理的研究[9-12]。交流阻抗法是分析电极过程动力学的重要手段, 可用于观察电极单层膜的电化学过程, 阐明金属腐蚀机理和评价缓蚀剂性能[13-14]。
吡咯烷二硫代氨基甲酸铵(APDTC) 由于分子中含有配位能力很强的N、S原子,常用于原子吸收分光光度分析的螯合剂,同时也是一种有效的环境友好型金属缓蚀剂。Al-RAWAJFEH和AL-SHAMAILEH[15]研究了其在自来水中对钢管的腐蚀抑制作用, 发现缓蚀剂效率取决于溶液中Fe2+和APDTC的浓度。江红[16]用吡咯烷二硫代氨基甲酸铵代替2-巯基苯骈噻唑(2-mercaptobenzothiazole,简称MBT ), 采用线性极化法和质量损失法研究其在二级除盐水中对黄铜的缓蚀行为, 发现APDTC的缓蚀效果优于MBT的。本文作者所在课题组曾研究了APDTC自组装膜在0.5 mol/L HCl溶液中的缓蚀性能, 当APDTC自组装溶液浓度为10 mmol/L时,缓蚀效率可达95.5%[17]。
在此,本文作者利用拉曼光谱和电化学方法研究铜表面吡咯烷二硫代氨基甲酸铵自组装膜的结构及其在3%NaCl溶液中的缓蚀性能和吸附行为。
1 实验
吡咯烷二硫代氨基甲酸铵(APDTC)为淡黄色粉末, 低毒, 溶于水(20 ℃时溶解度为18.9 g/100 L水), 结构式如图1所示。APDTC为分析纯(99. 0%),上海三爱试剂有限公司生产。所用溶液均用去离子水配制。实验在三电极体系中进行, 工作电极为未组装和组装APDTC SAMs的纯铜(99.9%)电极, 辅助电极和参比电极分别为Pt电极和饱和甘汞电极(SCE)。普通拉曼光谱(Normal Raman spectrum, NRS)和SERS光谱采用Invia激光共聚焦显微Raman光谱仪(英国 Renishaw公司), SERS光谱以波长为632.8 nm的He-Ne激光器为激发光源, 谱仪的分辨率设置为3 cm-1, 选用180?背散射配制;电化学测量使用S273A型电化学工作站(美国AMETEK公司)和CHI660B型电化学工作站(上海辰华仪器公司生产); 循环伏安测量电位范围为-1.0~1.3 V, 扫描速率为20 mV/s; 极化曲线扫描速率为2 mV/s; 电化学阻抗谱测量频率范围为100 kHz~0.05 Hz, 交流激励信号峰值为5 mV; 微分电容曲线根据在各电位下测得的电化学阻抗谱拟合而得。极化曲线扫描范围为-0.25~+0.25 V (相对于开路电位),微分电容曲线扫描电位范围为-0.6~+0.6 V。本研究所示电位均相对于饱和甘汞电极(SCE)。
![]()
图1 APDTC的结构式
Fig.1 Structure of APDTC
铜电极用环氧树脂密封, 工作面积为0.78 cm2, 表面经1#~6#金相砂纸逐级打磨抛光, 用无水乙醇除油, 经去离子水冲洗干净后放入电解池, 电解液为0.1 mol/L KCl溶液, 实验前工作电极先经过氧化还原预处理, 在-0.20 V下极化处理60 s, 再于-1.1 V下极化处理60 s。 反复处理几次后立即浸入APDTC自组装液中, 控制组装时间即可在铜电极表面形成APDTC SAMs。
2 结果与讨论
图2中曲线(a)所示为纯APDTC的普通Raman光谱,曲线(b)所示为铜电极在1 mmol/L APDTC溶液中自组装8 h后形成的APDTC SAMs的SERS谱。从图2可看出,铜表面SERS谱和普通 Raman谱信号基本一致。这表明APDTC在铜表面吸附成膜后,仍然保持分子的整体结构[18]。
表1列出了铜电极表面SERS谱和普通 Raman谱中振动峰的归属。从表1中可以看出,普通Raman光谱与SERS谱有一些差别。这表明APDTC分子与铜表面发生相互作用。根SERS选择定则,只有具有垂直表面分量的振动模才能被很大程度地增强,而平行
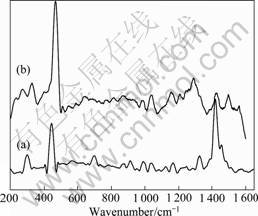
图2 纯APDTC的普通拉曼光谱和铜电极在1 mmol/L APDTC溶液中自组装8 h后的表面增强Raman光谱
Fig.2 Raman spectrum for pure APDTC (a) and surface enhanced Raman scattering spectrum of copper electrode in 1 mmol/L assembling APDTC solution for 8 h (b)
表1 APDTC分子的SERS 振动峰归属[18-21]
Table 1 SERS bands of APDTC with their assignments[18-21]
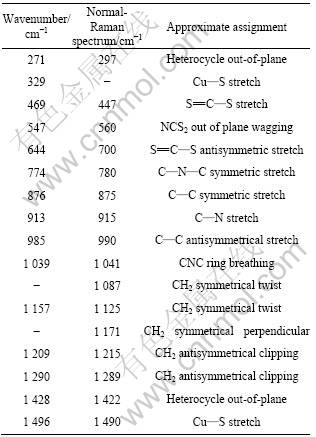
于基底表面的振动模增强非常小或接近于零;距离基底表面近的振动模增强较大,距离基底表面远的振动模增强较小[19]。普通Raman光谱的297 cm-1归属于APDTC分子中杂环面外振动[20],而SERS谱中此峰飘移至271 cm-1。这是由于APDTC分子吸附在金属表面,杂环离金属表面很近,与金属原子相互作用造成的。SERS谱在329 cm-1出现了新的吸收峰,而在普通Raman谱中并未出现, 说明APDTC吸附成膜后生成新化学键,而此峰归属于Cu—S键的伸缩振动[21]。由此可推测,APDTC分子中S原子和金属Cu成键。447 cm-1归属于APDTC分子中 S=C—S的对称伸缩振动, 在SERS谱中飘移到469 cm-1,并且强度得到了较大程度的增强, 说明APDTC分子通过硫原子垂直吸附在铜表面。664 cm-1归属于APDTC分子中 S=C—S的反对称伸缩振动,在SERS谱中飘移至700 cm-1。560 cm-1归属于N—CS2的面外摇摆, 在SERS谱中飘移至547 cm-1。其它振动峰位置在普通Raman谱和SERS谱中基本一致,无明显飘移。
图3所示为组装不同时间的APDTC SAMs的铜电极在3%NaCl溶液中的循环伏安曲线。从图3可以看出, 当电极未组装APDTC SAMs时, 在0.23 V和0.35 V两处出现明显的强阳极峰(两峰明显重叠), 分别对应CuCl的生成和CuCl2(实际是Cu2+, 因为CuCl2溶于水)的生成[22];在-0.28 V 处有很强的阴极峰。当电极组装了APDTC SAMs后, 其阳极峰完全消失,阴极峰也急剧地减弱, 说明APDTC SAMs的形成同时抑制了铜的氧化和还原。这可以用SAMs的阻挡效应来解释[2]。同时,预示着此氧化还原反应具有电化学不可逆性[23]。循环伏安图中未出现新的氧化还原峰, 说明形成的APDTC SAMs 在测量范围内具有较好的稳定性。
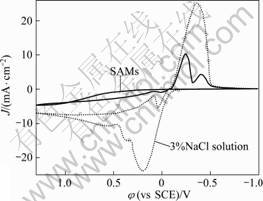
图3 自组装膜铜电极和空白电极在3%NaCl溶液中的循环伏安曲线
Fig.3 Cyclic voltammograms of copper electrodes with SAMs and naked electrodes in 3% NaCl solution
2. 3 极化曲线的测量
图4所示为在1 mmol/L APDTC 溶液中组装不同时间的铜电极在3%NaCl溶液中的极化曲线图。表2列出了用M 352腐蚀分析软件拟合得出的腐蚀电位φcorr、腐蚀电流密度Jcorr。缓蚀效率(η)可按式(1)计 算[24 ]:
![]() (1)
(1)
式中:![]() 和Jcorr 分别表示未组装和组装了SAMs的腐蚀电流密度。
和Jcorr 分别表示未组装和组装了SAMs的腐蚀电流密度。
从图4可看出, 在相同电位下, 组装了APDTC SAMs的铜电极无论是阳极电流还是阴极电流, 都有明显的降低。因此,可认为SAMs的形成增大了电极的阳极极化和阴极极化, 抑制了阳极反应过程和阴极反应过程。从表2可看出, 相对于空白电极, 组装了APDTC SAMs的铜电极在3%NaCl溶液中φcorr明显正移, Jcorr显著降低, 说明组装了APDTC SAMs的铜电
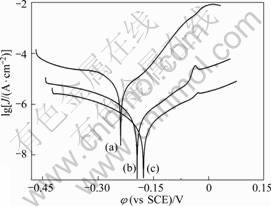
图4 在1 mmol/L APDTC溶液中组装不同时间的铜电极在3% NaCl中的极化曲线图
Fig.4 Polarization curves of copper electrodes assembled in 0.001 mol/L APDTC solution for different time and then immersed in 3% NaCl solution: (a) Before immersion; (b) 2 h; (c) 8 h
表2 组装不同时间铜电极在3% NaCl溶液中的电化学参数
Table 2 Electrochemical parameters of copper electrodes assembled for different times and then immersed in 3% NaCl solution
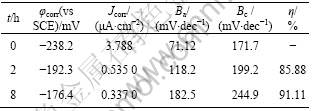
极对3% NaCl具有很好的抗腐蚀作用, 组装了8 h后缓蚀效率达91.11%。
2. 4 电化学阻抗谱的测量
图5所示为在1.0 mmol/L APDTC溶液中组装不同时间的铜电极在3% NaCl溶液中的Nyquist图。该阻抗谱图均呈一压扁的半圆形, 圆中心在实轴以下。这就是所谓的“弥散效应”。图5中的阻抗数据采用图6所示的等效电路拟合。图7所示为空白铜电极和组装SAMs的铜电极的实测数据和拟合数据的的Nyquist和Bode图。由图7可见,拟合数据和实测数据基本重合。这说明用图6所示的等效电路来拟合实测数据是比较合理的。等效电路中Rs为溶液电阻;Rct为电荷转移电阻,Rsam为膜电阻;常相角元件CPEsam和CPEdl分别代表SAMs电容和双电层电容;W 是Warburg阻抗;CPE的导纳可定义如下:
![]() (2)
(2)
式中:Q表示常相角元件;Y0表示系数;j是虚根;ω是角频率;n是指数项。当n分别为0、0.5、1.0时,CPE阻抗分别代表纯电阻、Warburg阻抗、纯电容阻 抗[22]。理想电极n值为1.0, 实际电极n值往往小于1.0,一般0.6<n<1.0,CPE近似于电容性质。自组装膜的覆盖度θ(缓蚀效率η=θ)可用式(3)表示[22, 25](Rct0表示空白电极的电荷转移电阻)。
![]() (3)
(3)
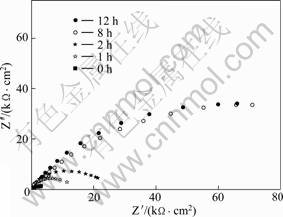
图5 在1 mmol/L APDTC溶液中组装不同时间的铜电极在3% NaCl溶液中的Nyquist图
Fig.5 Nyquist plots of copper electrodes assembled in 1 mmol/L APDTC solution for different time and then immersed in 3% NaCl solution
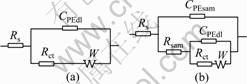
图6 空白铜电极和覆盖APDTC SAMs的铜电极的等效电路图
Fig.6 Equivalent circuit models of naked copper electrode (a) and APDTC SAMs-covered copper electrode (b)
用ZSIMPWIN软件拟合后的相关数据列于表3中。 从表3可看出溶液电阻Rs变化很小,与空白电极相比,自组装膜电极的膜电阻Rsam远大于空白电极的Rct, CPEsam和CPEdl都显著减小。Rsam越大, CPE越小, 缓蚀剂的缓蚀效果越好;而且随着组装时间的延长,Rsam逐渐增大,CPEsam和CPEdl值逐渐减小, 8 h以后基本上达到稳定值, 电极表面的覆盖度(或电极的缓蚀效率)从79.58%升高到98.21 %。因此,可认为APDTC SAMs在铜表面起到很好的抵抗NaCl的腐蚀作用。
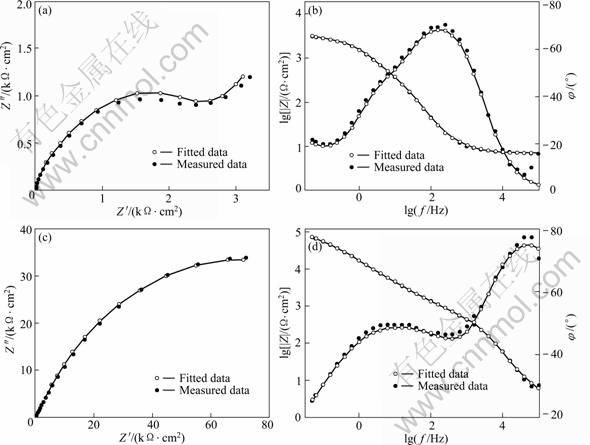
图7 实测和拟合的Nyquist和Bode图
Fig.7 Measured and fitted Nyquist and Bode plots
表3 在1 mmol/L APDTC溶液中组装不同时间的铜电极在3% NaCl溶液中的电化学参数
Table 3 Electrochemical impedance parameters for copper electrode assembled in 1 mmol/L APDTC solution for different time in and then immersed 3%NaCl solution
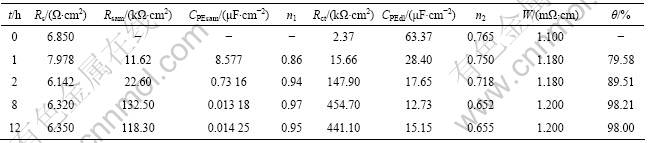
微分电容测量是研究电极表面的结构特点、来自溶液的吸附作用、发生在界面处的法拉第过程以及表面粗造度等的一种较灵敏的方法[5]。图8所示为铜电极在3% NaCl溶液中的微分电容曲线。从图8的曲线(a)可以看出, 电位从-0.6 V开始扫描, 在-0.6~-0.2 V铜电极的电容一直很小, 说明铜本身在3%NaCl溶液中具有一定的抗腐蚀能力;当电位继续正移时, 电容开始升高,在0.1 V左右出现明显的峰, 此时电极发生明显的腐蚀(对应于循环伏安图的CuCl生成), 然后电容又开始下降。这可能是因为CuCl难溶,附着在金属表面,阻止了反应进一步进行;在0.36 V左右出现第2个电容峰(对应于循环伏安图中CuCl2的生成);在0.36 V以后电容又开始下降。这是由于铜表面被氧化产生了致密的Cu2O膜,具有一定的缓蚀作用[2]。当铜电极表面组装了APDTC SAMs后,其微分电容曲线(曲线(b))所示的电容急剧减小(几乎变成直线)。这是由于有机化合物的介电常数小于水分子的介电常数,有机化合物取代吸附在铜表面的水分子而吸附在铜表面造成双电层电容减小的缘故[3, 26];同时,在曲线(b)上未见明显的电容峰, 说明在所研究的电位范围内, APDTC SAMs既无明显的脱附作用, 也未发生相变过程,具有较好的稳定性。
图8 铜电极在3% NaCl溶液中的微分电容曲线
Fig.8 Differential capacity curves for copper electrodes in 3%NaCl solution: (a) Blank; (b) With APDTC SAMs, t=8 h
2. 6 吸附行为研究
有机物分子在金属/溶液界面的吸附行为可以用式(4)表示[27 ]:
Org(sol)+xH2O(ads)![]() Org(ads)+ xH2O(sol) (4)
Org(ads)+ xH2O(sol) (4)
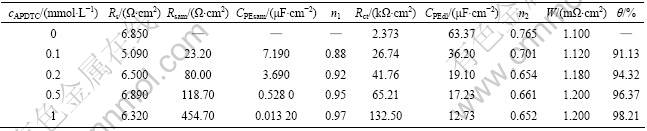
式中:Org(sol)和Org(ads)分别表示在溶液中和吸附在金属表面上的有机物分子;H2O(sol)和H2O(ads)分别表示在溶液中和吸附在金属表面上的水分子;x表示一个有机吸附分子取代水分子的比率[27-29]。为了研究铜表面的吸附行为, 测定了25 ℃时在不同浓度的APDTC溶液中, 吸附平衡后的铜电极在3%NaCl的电化学阻抗谱图, 得到图9。图谱数据用 ZSIMPWIN软件处理可得到Rct和Rsam。有机物分子表面覆盖度(θ)可以通过式(3)进行计算, 数据列于表4。将θ分别代入Temkin、Langmuir 和Freundlich吸附等温式[28]拟合, 结果表明Langmuir 吸附等温式与实验结果吻合良好。
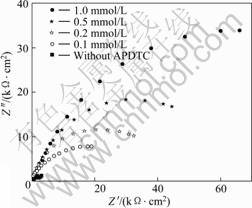
图9 在不同浓度APDTC溶液中组装的铜电极在3% NaCl溶液中的Nyquist 图
Fig.9 Nyquist plots of copper electrodes assembled in different concentrations of APDTC and then immersed in 3% NaCl solution
根据Langmuir等温式有:
![]() (5)
(5)
式中:c为缓蚀剂吸附分子浓度;K为Langmuir 吸附平衡常数。将cθ-1—c作图(见图10), 可以看出 cθ-1—c呈很好的线性关系, 线性相关系数R2为0. 999 99, 斜率为1. 010 62, 说明APDTC分子在铜表面的吸附符合Langmuir等温式模型, 每一个APDTC分子大约占据1.01个吸附点, 是单分子层吸附;同时,可得到Langmuir 吸附平衡常数K为9.895×104 L/mol。K与吸附吉布斯自由能?G0的关系如下[29]:
![]() (6)
(6)
式中:55.5为溶剂水的浓度(mol/L);R为摩尔气体常数(8.314 J/(mol·K));T 为热力学温度(K)。计算得?G0=-32.74 kJ/mol, ΔG0 <0说明APDTC分子在铜表面的吸附过程是一种自发行为。通常情况下, 当ΔG0
表4 在不同浓度APDTC溶液中组装的铜电极在3% NaCl溶液的电化学参数和覆盖度
Table 4 Electrochemical impedance parameters and surface coverage degrees of copper electrodes assembled in different concentrations of APDTC and then immersed in 3% NaCl
在-20 kJ/mol左右时, 表示带电荷的缓蚀剂分子和带电荷的金属之间的静电作用, 为物理吸附; 当ΔG0在-40 kJ/mol左右时, 表示带电荷的缓蚀剂分子通过与金属共用电子成键, 为化学吸附[20-22]。因此,可认为APDTC分子在铜表面的吸附是介于化学吸附和物理吸附之间的一种吸附。
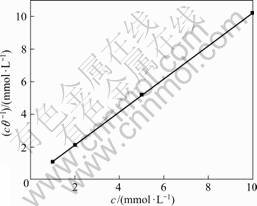
图10 根据Langmuir等温式模型的铜电极腐蚀数据拟合的吸附曲线
Fig.10 Fitted curve of corrosion data for copper electrode according to Langmuir thermodynamic kinetic model
3 结论
1) 吡咯烷二硫代氨基甲酸铵分子(APDTC) 通过硫原子垂直吸附在铜表面,形成稳定的APDTC SAMs, 并且吸附后分子整体结构保持不变。
2) 在3%NaCl溶液中自组装膜抑制铜的阳极氧化过程和阴极还原过程, 膜层电阻和电荷转移电阻明显增大, 膜层电容和固/液界面双电层电容明显降低, 表现出良好的缓蚀效果。
3) APDTC的吸附行为符合Langmuir吸附等温式, 吸附机理是介于化学吸附和物理吸附之间的一种吸附。
REFERENCES
[1] 邵海波, 王晓艳, 王建明, 王俊波, 张鉴清, 曹楚南. 碱土金属离子与EDTA对纯铝在碱性溶液中的协同缓蚀作用[J]. 物理化学学报, 2006, 22(3): 312-315.
SHAO Hai-bo, WANG Xiao-yan, WANG Jian-ming, WANG Jun-bo, ZHANG Jian-qing, CAO Chu-nan. The cooperative inhibition effects of alkaline earth metal ions and EDTA on the corrosion of pure aluminum in an alkaline solution[J]. Acta Phys-Chim Sin, 2006, 22(3): 312-315.
[2] FINKLEA H O A. Self-assembled mono layers on electrodes, in encyclopedia of analytical chemistry-electroanalytical methods[M]. Chicheser: John Wiley & Sons Ltd, 2000: 1-2.
[3] 王献群, 刘瑞泉, 朱丽琴, 宫建伟. 碱性介质中BIT, BIOHT和BIMMT对铜的缓蚀性能和吸附行为[J]. 物理化学学报, 2007, 23(1): 21-26.
WANG Xian-qun, LIU Rui-quan, ZHU Li-qin, GONG Jian-wei. Inhibition action and adsorption behaviors of BIT, BIOHT and BIMMT on Copper in alkaline medium[J]. Acta Phys-Chim Sin, 2007, 23(1): 21-26.
[4] 曹楚南, 张鉴清. 电化学阻抗谱导论[M]. 北京: 科学技术出版社, 2002: 68-69.
CAO Chu-nan, ZHANG Jian-qing. An introduction to electrochemical impedance spectroscopy[M]. Beijing: Science and Technology Press, 2002: 68-69.
[5] SCHWEIZER M, HAGENSTR?M H, KOLB D M. Potential-induced structure transitions in self-assembled monolayers: ethanethiol on Au(100)[J]. Surface Science, 2001, 490(3): L627-L636.
[6] TIAN Zhong-qun, REN B, WU De-yin. Surface-enhanced raman scattering:From noble to transition metals and from rough surfaces to ordered nanostructures[J]. J Phy Chem B, 2002, 106(37): 9463-9483.
[7] SARKA J, CHOWDHURY J, GHOSH M, DE R, TALAPATRA G B. Adsorption of 2-aminobenzothiazole on colloidal silver particles:An experimental and theoretical surface-enhanced Raman scattering study[J]. J Phys Chem B, 2005, 109(25): 12861-1267.
[8] 贾少杰, 徐抒平, 郑先亮, 赵 冰, 徐蔚青. 激光诱导沉积银膜制备光纤SERS传感器[J]. 高等学校化学学报, 2006, 27(3): 523-526.
JIA Shao-jie, XU Shu-ping, ZHENG Xian-liang, ZHAO Bing, XU Wei-qing. Preparation of SERS optical fiber sensor via laser-induced deposition of Ag film on the surface of fiber tip[J]. Chemical Journal Chinese Universities, 2006, 27(3): 523-526.
[9] CAO P G, GU R N, TIAN Z Q. Electrochemical and surface-enhanced Raman spectroscopy studies on inhibition of iron corrosion by benzotriazole[J]. Langmuir, 2002, 18(20): 7609-7615.
[10] BENTISS F, TRAISNEL M, VEZIN H, LAGRENéE M. Electrochemical study of substituted triazoles adsorption on mild steel[J]. Ind Eng Chem Res, 2000, 39(10): 3732-3736.
[11] MARCONATO J C, BULH?E L O, TEMPERINI M L. A spectroelectrochemical study of the inhibition of the electrode process on copper by 2-mercaptobenzothiazole in ethanolic solutions[J]. Electrochim Acta, 1998, 43(7): 771-780.
[12] CAO P G, YAO J L, ZHENG J W, GU R N, TIAN Z Q. Comparative study of inhibition effects of benzotriazole for metals in neutral solutions as observed with surface-enhanced raman spectroscopy[J]. Langmuir, 2002, 18(1): 100-104.
[13] 李伟华, 裴长岭, 何 乔, 张奇刚, 侯保荣. 三唑化合物在 1 mol/L 盐酸中对碳钢的缓蚀作用研究[J]. 全面腐蚀控制, 2007, 21(3): 5-8.
LI Wei-hua, PEI Chang-ling, HE Qiao, ZHANG Qi-gang, HOU Bao-rong. Study on the inhibitive effect of a new triazole derivative on corrosion of mild steel in 1 mol/L HCl solutions[J]. Total Corros Contro, 2007, 21(3): 5-8.
[14] 赵地顺, 刘会茹, 徐智策, 庞登甲, 王春芳. 植酸盐缓蚀剂及其机理研究[J]. 高等学校化学学报, 2005, 26(2): 334-336.
ZHAO Di-shun, LIU Hui-ru, XU Zhi-ce, PANG Deng-jia, WANG Chun-fang. Plant acid salt inhibitor and its inhibiting mechanism[J]. Chemical Journal of Chinese University, 2005, 26(2): 334-336.
[15] AL-RAWAJFEH A E, AL-SHAMAILEH E M. Inhibition of corrosion in steel water pipes by ammonium pyrrolidine dithiocarbamate (APDTC)[J]. Desalination, 2007, 206(1/3): 169-178.
[16] 江 红. 用APDC 替代MBT 铜缓蚀剂的试验与讨论[J]. 工业水处理, 1998, 18(3): 15-18.
JIANG Hong. The copper inhibitor substitute APDC for MBT[J]. Industial Water Treatment, 1998, 18(3): 15-18.
[17] 廖强强, 岳忠文, 朱忠伟, 王 毅, 张 羽, 周国定, 周 琪. 吡咯烷二硫代氨基甲酸铵自组装膜对铜的缓蚀作用[J]. 物理化学学报, 2009, 25: 1655-1661.
LIAO Qiang-qiang, YUE Zhong-wen, ZHU Zhong-wei, WANG Yi, ZHANG Yu, ZHOU Guo-ding, ZHOU Qi. Corrosion inhibition effect of self-assembled monolayers of ammonium pyrrolidine dithiocarbamate on copper[J]. Acta Physico-Chimica Sinica, 2009, 25: 1655-1661.
[18] HOPE G A, WALTING K, WOODS R. A SERS spectroelectrochemical investigation of the interaction of isopropyl, isobutyl and isoamyl xanthates with silver[J]. Colloids and Surfaces A: 2001, 178(1/3): 157-166.
[19] 朱纪春, 张新安, 白 莹, 莫育俊. 银表面上PVP 分子的表面增强拉曼光谱研究[J]. 河南大学学报: 自然科学版, 2004, 34(2): 20-23.
ZHU Ji-chun, ZHANG Xin-an, BAI Ying, MO Yu-jun. A study of poly( N-vinylpyrrolidone) adsorbed on silver by SERS[J]. Journal of Henan University: Natural Science, 2004, 34(2): 20-23.
[20] EL-GOGARY T M, SOLIMAN M S. Ab-initio molecular geometry and normal coordinate analysis of pyrrolidine molecule spectrochimica[J]. Spectrochimica Acta A, 2001, 57(13): 2647-2657.
[21] BARBARA P, MAURIZIO M, ADRIANO B. Surface-enhanced raman scattering investigation of the adsorption of 2-Mercaptobenzoxazole on smooth copper surfaces doped with silver colloidal nanoparticles[J]. J Phys Chem B, 2006, 110(18): 9241-9245.
[22] LI De-gang, CHEN Shen-hao, ZHAO Shi-yong, MA Hou-yi. The corrosion inhibition of the self-assembled Au, and Ag nanoparticles films on the surface of copper[J]. Colloids and Surfaces A: 2006, 273(1/3): 16-23.
[23] 邵会波, 于化忠, 程广军, 张浩力, 刘忠范. 偶氮苯硫醇衍生物自组装成膜过程考察[J]. 物理化学学报, 1998, 14(9): 846-851.
SHAO Hui-bo, YU Hua-zhong, CHENG Guang-jun, ZHANG Hao-li, LIU Zhong-fan. Study of formation process of an azobenzene self-assembled monolayer on gold surface[J]. Acta Physico-Chimica Sinica, 1998, 14(9): 846-851.
[24] 曹楚南. 腐蚀电化学原理[M]. 北京: 化学工业出版社, 2004: 232-298.
CAO Chu-nan. Principles of erosive electrochemistry[M]. Beijing: Chemical Industry Press, 2004: 232-298.
[25] GUO W J, CHEN S H, HUANG B D, MA H Y,YANG X G. Protection of self-assembled monolayers formed from triethyl phosphate and mixed self-assembled monolayers from triethyl phosphate and cetyltrimethyl ammonium bromide for copper against corrosion[J]. Electrochimca Acta, 2006, 52(1): 108-113.
[26] EL-SAYED M S, ERASMUS R M, COMINS J D. Effects of 3-amino-1, 2, 4-triazole on the inhibition of copper corrosion in acidic chloride solutions[J]. J Colloid Interface Sci, 2007, 311(1): 144-151.
[27] HNOG H G, PARK W. A study of adsorption kinetics and thermodynamics of ω-mercaptoalkyl hydroquinone self- assembled monolayer on a gold electrode[J]. Electrochimica Acta, 2005, 51(2): 579-587.
[28] BENTISS F, LEBRINI M, LAGRENéE M. Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel 2, 5-bis(n-thienyl)-1, 3, 4-thiadiazoles hydrochloric acid system[J]. Corros Sci, 2005, 47(12): 2915-2931.
[29] VILLAMIL R F V, CORIO P, RUBIM J C, AGOSTINHO S M L. Sodium dodecylsulfate-benzotriazole synergistic effect as an inhibitor of processes on copper/chloridric acid interfaces[J]. J Electroanal Chem, 2002, 535(1/2): 75-83.
基金项目:上海市重点学科建设资助项目(P1304); 上海高校电力腐蚀控制与应用电化学重点实验室开放基金资助项目(09KLSU01); 上海热交换系统节能工程技术研究中心开放基金资助项目(09SERC03); 上海市科委资助项目(08DZ2210800)
收稿日期:2009-12-13;修订日期:2010-04-22
通信作者:廖强强; 副教授, 博士; 电话: 021-65700719; E-mail: liaoqq1971@yahoo.com.cn
[11] MARCONATO J C, BULH?E L O, TEMPERINI M L.
[J]. Electrochim Acta, 1998, 43(7): 771-780.
[18] HOPE G A, WALTING K, WOODS R.
[J]. Colloids and Surfaces A: 2001, 178(1/3): 157-166.
[20] EL-GOGARY T M, SOLIMAN M S.
[26] EL-SAYED M S, ERASMUS R M, COMINS J D.


