
ARTICLE
J. Cent. South Univ. (2019) 26: 312-322
DOI: https://doi.org/10.1007/s11771-019-4003-0
Intensifying gibbsite precipitation from sodium aluminate solution by adding a mixed seed
LI Xiao-bin(李小斌), YE Pu-hong(叶普洪), ZHOU Qiu-sheng(周秋生), LIU Jing-hui(刘井辉),
PENG Zhi-hong(彭志宏), LIU Gui-hua(刘桂华), QI Tian-gui(齐天贵)
School of Metallurgy and Environment, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract:
Gibbsite precipitation from sodium aluminate solution was intensified by adding mixed industrial and self-prepared active seeds, and its mechanism was researched preliminarily. The interfacial properties of seed/aluminate solution were determined for separate industrial and active seed. Contact angles of seed/aluminate solution and the specific surface area of seeds were respectively measured by sessile drop and BET method, and the morphology and particle size of precipitates were recorded by SEM and laser diffraction. The results show that, compared with the industrial seed, the active seed has a better wettability, lower interfacial tension, and larger specific surface area, being conducive to enhancing gibbsite precipitation from sodium aluminate solution. SEM analysis of the precipitates indicates that the embedment and accumulation/agglomeration of extremely fine particles on the surface of coarse industrial seed can effectively control the content of fine particles in the precipitation product. With extra 3.1–4.6 g/L active seed, the gibbsite precipitation ratio was increased by 3.23%–3.92%. Moreover, the mass percentage of particles <45 μm in precipitation product has even a slight decrease compared with that for the traditional precipitation product or of the industrial seed itself. The result presented is favorable to developing an intensified gibbsite precipitation process for commercial alumina manufacture.
Key words:
gibbsite; seeded precipitation; sodium aluminate solution; active seed; intensification;
Cite this article as:
LI Xiao-bin, YE Pu-hong, ZHOU Qiu-sheng, LIU Jing-hui, PENG Zhi-hong, LIU Gui-hua, QI Tian-gui. Intensifying gibbsite precipitation from sodium aluminate solution by adding a mixed seed [J]. Journal of Central South University, 2019, 26(2): 312–322.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-019-4003-01 Introduction
In the commercial alumina production, there are two main research fields, i.e., efficient comprehensive utilization of red mud [1, 2], and intensification of seeded precipitation [3]. The seeded precipitation ratio can only reach 45%–52% even with seed ratio above 2 and duration 30–70 h possibly due to the high surface tension of 1.25 N/m between supersaturated sodium aluminate solution and gibbsite particles, and thus numerous intensifying seeded precipitation methods have been studied in the literature. Gibbsite precipitation was found to be affected by aluminum species and their contents in aluminate solution. LI et al [4] discovered that the precipitation ratio was increased with increasing Al(OH)4– ion content via treating sodium aluminate solution with magnetic field, and ZHANG et al [5] supported that treating sodium aluminate solution with ultrasound favored the formation of sodium aluminate ion pair and thus increased the precipitation ratio. Decreasing caustic ratio of sodium aluminate solution by solvent extraction [6] or ion membrane electrolysis [7] has been proved to be an intensifying method for the seeded precipitation. Additionally, changing the physicochemical properties of aluminate solution by adding alcohol, methanol or ammonia was reported to intensify the seeded precipitation [8–10]. Unfortunately, to date, these intensification methods are just limited in laboratory tests mainly due to their high costs, unstable effects, or application difficulties.
Noteworthy is that gibbsite precipitation preferentially occurs on the interface of gibbsite seed/aluminate solution, thus many researches have concentrated on modifying the interfacial properties of seed/aluminate solution. ZENG et al [11] thought that a slight increase of precipitation ratio by adding tetrahydrofurane was ascribed to the mild adsorption of tetrahydrofurane on active growth sites of seed surface. According to research of LI et al [12], etheric compound B35 can be adsorbed on the seed surface to distinctly reduce the interfacial tension and increase the seed hydrophilicity, increasing the precipitation ratio and gibbsite particle size. Besides organic additives, the researches by BROWN [13] and GNYRA et al [14] indicated that Ca2+ favored gibbsite agglomeration due to rendering the seed surface rougher after its adsorption on seed surfaces, whilst the precipitation ratio was not affected. To the best of our knowledge, both organic and inorganic additives were not used in the commercial alumina production to increase the precipitation ratio, largely due to their slight improvement effect, possible negative influence or remarkable cost increase.
Another important effective method for increasing seeded precipitation ratio is to add an active seed in the precipitation process [15, 16]. Compared to the industrial seed with equal seed ratio, the active seed usually has more active sites and crystal defects as a result of its remarkably high specific surface area. LIU et al [10] concluded that high seeded precipitation ratio by adding the active seed was attributed to irregular edge, poor crystalline and large specific surface area of the active seed. XIE et al [17] regarded seeded precipitation as a self-catalyzed reaction and explored the reason for different precipitation ratio when adding individual coarse seed and fine seed. Besides the studies on the active seed activity, the intensification experiments for precipitating sandy gibbsite product by adding active seed have also been investigated. YIN [18] reported that the seeded precipitation ratio was increased remarkably by adding an active seed prepared by milling coarse gibbsite particles. ZENG et al [19] also showed that an increase of precipitation ratio could be achieved by treating gibbsite seed in a boiling distilled water to remove organic contaminants adsorbed on the active seed. However, the abovementioned intensification processes were conducted under unfavorable conditions for particle agglomeration, as the solution supersaturation is not high with gibbsite precipitation proceeding. To maintain a relatively high supersaturation, ZHANG [20] systematically researched the effects of active seed preparation and feeding mode on the seeded precipitation ratio and product particle size, and his results showed that both high precipitation ratio and coarse product could not be achieved simultaneously. As an alternative method, a two- stage seeded precipitation process was proposed by LIU et al [21], in which ultrafine gibbsite particles were obtained in the first stage with high precipitation ratio of 70% by adding an active seed, and coarse boehmite product was prepared by the transformation of gibbsite to boehmite and the agglomeration of boehmite particles at elevated temperatures in the second stage. Although both high precipitation ratio and coarse borhmite product could be achieved, the process is relatively complicated and not cost effective. Under this circumstance, we proposed that it may be feasible to achieve both high precipitation ratio and coarse gibbsite product by adding a mixed seed of active seed and industrial seed. On one hand, a larger surface area of the mixed seed could accelerate gibbsite precipitation. On the other hand, attraction among gibbsite particles during the long seeded precipitation benefits gibbsite agglomeration [22, 23]. For the proposed intensified precipitation process, the effect of a self-prepared active seed on the precipitation ratio was investigated and the underlying mechanism was preliminarily analyzed by comparing interfacial properties of industrial seed/solution with those for the active seed. Moreover, the effect of the active seed on the product size was investigated.
2 Experimental
2.1 Preparation of sodium aluminate solution
Distilled water was used to prepare all solutions. All chemicals used in this work were of analytical grade and supplied by Kermel Chemical Reagent Corporation of Tianjin, China. Two sodium aluminate solutions, i.e. a plant solution and a synthetic solution, were prepared before seed precipitation. The former was simulated by dissolving industrial gibbsite into the spent Bayer liquor from Zunyi Alumina Refinery (Chalco) and by subsequent filtration 3 times, and was used for seeded precipitation experiments. The synthetic solution was prepared by dissolving aluminum trihydroxide and sodium hydroxide in distilled water, and was used for measuring the contact angle of seed/aluminate solution and surface tension of sodium aluminate solution.
2.2 Seeded precipitation experiments
Seeded precipitation experiments were carried out in a 150 mL steel bomb immersed and rotating in a preheated glycerol bath to provide an appropriate temperature and just enough agitation for the seeded precipitation. First, 60 mL sodium aluminate solution, a small amount of active seed and 102 g industrial seed were placed into the steel bomb, then the bomb was sealed and immersed in the glycerol bath. Subsequently, seeded precipitation was initiated with agitation of 40 r/min. Samples were taken from the bomb at a preset time and then centrifuged. The supernatant was used to measure the mass concentrations of caustic soda and alumina by volumetric analysis [24], and the bottom was thoroughly washed with boiling water, filtrated, and dried at 100 °C for particles size distribution (PSD) measurement and SEM observation.
2.3 Analyses
Precipitation ratio (η) was calculated by Eq. (1):
η=(1–α0/αt)×100% (1)
where α0 and αt denote the mole ratio of Na2Ok to Al2O3 in liquor at initial time and at precipitation time of t , respectively [25].
PSD curves of gibbsite particles were measured by a laser diffraction particle size analyzer (Mastersizer 2000, Malvern, UK), and before the measurement the sample was dispersed via ultrasonic of 20 W in distilled water for 3 min. Gibbsite morphology was observed by SEM (JSM-6700LV, JEOL, Japan). Phase analyses of seeds were made by the X-ray powder diffraction (XRD) analyses (Rigaku TTR-III, Japan, employing Ni-filtered Cu Kα radiation at a scanning rate of 5 (°)/min in a 2θ range from 10° to 90°). Specific surface area (SSA) of seeds was determined by the Brunauer-Emmett-Telle (BET) method in a monosorb analyzer MS-13 Quanta Chrome (Boca Raton, FL, USA).
Surface tension of sodium aluminate solution was measured by Wilhelmy plate method on an automatic surface tensiometer (QBZY-2, Shanghai Fangrui Instrument Ltd., China). The surface tensiometer was configured with a constant temperature water bath (temperature-controlled precision of ±0.1 °C). Before measurement, sodium aluminate solution was poured into the testing container of the surface tensiometer and preheated for 20 min to the preset temperature. The contact angle of seed/sodium aluminate solution or organic test liquids was conducted by sessile drop technique on a contact angle meter (CL200B, Shanghai Solon Information Technology Ltd., China). Before the measurement, 0.1 g seed powder was compressed into a thin plate (~10 MPa) by a tablet pressing machine (YP-2, Shanghai Shanyue Technology Instrument Ltd., China). The contact angle was the average value of three measurements.
Based on the contact angle of seed/organic test liquid, the surface energy of seed γSV was evaluated by the two-liquid method of OWENS et al [26] (Eqs. (2) and (3)), in which diiodomethane and glycerol were selected as the organic test liquids.
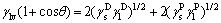 (2)
(2)
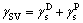 (3)
(3)
where γlv is the surface energy of test liquid;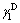 is liquid dispersion force;
is liquid dispersion force;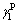 is liquid polar force;
is liquid polar force; 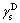 is solid dispersion force;
is solid dispersion force;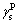 is solid polar force. At 25 °C, the values of
is solid polar force. At 25 °C, the values of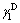 and
and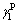 of polar test liquid of glycerol are 37.5 and 26.4 mJ/m2, respectively; and the values of
of polar test liquid of glycerol are 37.5 and 26.4 mJ/m2, respectively; and the values of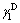 and
and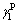 of non- polar test liquid of diiodomethane are 48.5 and 2.3 mJ/m2, respectively [27].
of non- polar test liquid of diiodomethane are 48.5 and 2.3 mJ/m2, respectively [27].
3 Results and discussion
3.1 Preparation and characterization of active seed
3.1.1 Preparation of active seed
130 mL sodium aluminate solution (Na2Ok 160 g/L, αk 1.41) was evenly added into 60 mL sodium bicarbonate solution (130 g/L) for 28 min at 40 °C with a stirring speed of 300 r/min, and the resultant slurry was subsequently stirred for another 35 min after adding the sodium aluminate solution.
3.1.2 XRD analysis
The XRD patterns of the industrial seed and self-prepared active seed (Figure 1) show that gibbsite (PDF# 76-1782) is the only detectable crystalline phase for the industrial seed, whereas both crystalline gibbsite (PDF# 76-1782) and bayerite (PDF# 15-0136) were detected for the self- prepared active seed. Moreover, the characteristic peaks of the active seed are obviously much broader than those of the industrial seed, indicating that the active seed has poorer crystallinity and may contain some amorphous phase. According to the semi-quantitative analysis [28], the percentages of gibbsite and bayerite of the active seed were calculated as 42.4 wt% and 57.6 wt%, respectively.
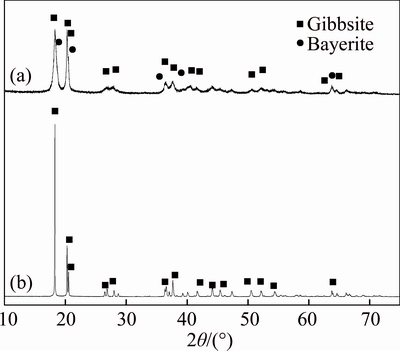
Figure 1 XRD patterns of prepared active seed (a) and industrial seed (b)
3.1.3 Morphology and PSD curves
Figures 2 and 3 respectively show the PSD curves and SEM photographs of the two seeds. As shown in Figure 2, the PSD curve of the industrial seed has a very narrow size distribution with d(0.5) of 84.16 μm, while the active seed has a very wide and bimodal particle size distribution with d(0.5) of 12.55 μm. From Figure 3, we can see that the self-prepared active seed contains numerous agglomerates consisting of small crystals. The industrial seed particles mainly take on well-round and smooth-surface with a small amount of fine particles on their surfaces.
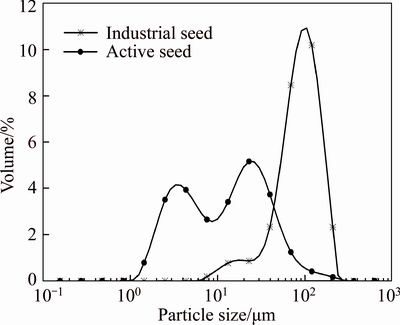
Figure 2 Particle size distribution of industrial seed and active seed

Figure 3 SEM micrographs of industrial seed and active seed:
3.1.4 Interfacial properties of seed/sodium aluminate solution
The interfacial properties of seed/sodium aluminate solution can affect gibbsite crystallization from sodium aluminate solution, therefore the interfacial properties, such as surface energy, interfacial tension, contact angle and specific surface area, for the active seed were determined and compared with those for the industrial seed.
Following two-liquid method of OWENS and WENDT, surface energy γSV values of the active seed and industrial seed were separately evaluated and the results are listed in Table 1. As shown in Table 1, the surface energy of the active seed nearly equals that of the industrial seed. However, the solvent tendency of the active seed is higher than that of the industrial seed, as the ratio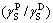 of the former is greater than that of the latter.
of the former is greater than that of the latter.
Table 1 Surface energy of active seed and industrial seed
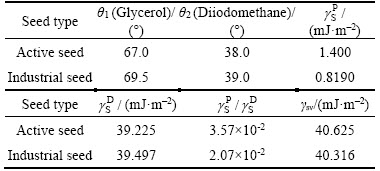
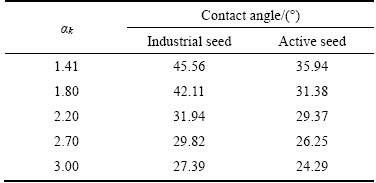
Table 2 shows the variation of surface tension of sodium aluminate solution with αk at 318 K and Na2Ok 160 g/L. It can be seen that surface tension of sodium aluminate solution first increases gradually with increasing αk from 1.41 to 2.70 and then decreases slightly with increasing αk from 2.70 to 3.00. In Table 3, the variation of contact angle of seed/sodium aluminate solution was presented under the same conditions. We can see from Table 3 that, the contact angle for the active seed/aluminate solution is much smaller than that for the industrial seed/aluminate solution at αk of 1.41–3.00, suggesting that sodium aluminate solution can better wet the active seed. As gibbsite preferentially precipitates at the interface of seed/aluminate solution, better wettability certainly favors the gibbsite precipitation.
Table 2 Variation of surface tension of sodium aluminate solution with αk (318 K, Na2Ok 160 g/L)

Table 3 Variation of contact angle of seed/sodium aluminate solution with αk for industrial seed and active seed (318 K, Na2Ok 160 g/L)
As shown in Eq. (4), at the triple phase boundary, YOUNG’s equation [29] expresses the relationship among surface energy of solid, surface tension of liquid, interfacial tension of solid/liquid, and the equilibrium contact angle of solid/liquid.
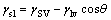 (4)
(4)
Substituting the data in Tables 1–3 into Eq. (4), the value of γsl can be calculated. The calculated values of γsl were minus, meaning that the classic YOUNG’s equation was not suitable for calculating the interfacial tension of seed /sodium aluminate solution.
ZHU et al [30, 31] proposed a method for characterizing the wetting property of limitless solid–liquid interface system:
 (5)
(5)
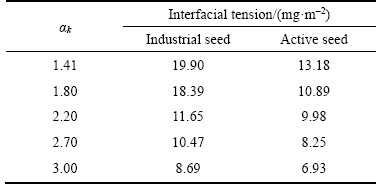
Substituting the data in Table 2 to Table 3 into Eq. (5), the variations of interfacial tension of seed/sodium aluminate solution with αk were determined. As shown in Table 4, for both the active seed and industrial seed, the interfacial tension of seed/sodium aluminate solution decreases with the increase of αk from 1.41 to 3.00, being in agreement with the result reported in our previous study [32]. Moreover, for the same sodium aluminate solution, the interfacial tension of the active seed/solution is remarkably lower than that of the industrial seed/solution, implying that gibbsite can precipitate more readily on the interface of the active seed/ aluminate solution. In addition, SSA of the industrial seed and the active seed were determined respectively as 0.03 m2/g and 16.56 m2/g. The latter is about 552 times that of the former, manifesting that adding a low concentration of the active seed may result in a high precipitation ratio.
Table 4 Variation of interfacial tension (mJ/m2) of sodium aluminate solution/seed with αk for industrial seed and active seed (318 K, Na2Ok 160 g/L)
3.2 Seeded precipitation by adding active seed alone
To explore the possibility of obtaining coarse Al(OH)3 product from sodium aluminate solution by adding the self-prepared active seed alone, seeded precipitation was performed at a preset temperature by only adding various concentrations of the active seed. The precipitation ratio and the particle size distribution of the precipitation product under different conditions are listed in Tables 5 and 6, and the corresponding SEM micrographs of the precipitates are presented in Figure 4.
Composition of the initial sodium aluminate solution was Na2Ok 160 g/L, αk 1.41; Precipitation temperature system included test 1 test 2 and test 3. Test 1: 1) isothermal reaction at 60 °C during 0–5 h;2) temperature abruptly decreased to 55 °C; 3) evenly decreased to 45 °C during 5–15 h; 4) maintained at 45 °C during 15–45 h. Test 2: isothermal reaction at 55 °C for 45 h. Test 3: Isothermal reaction at 65 °C for 45 h.
Table 5 Seeded precipitation ratio obtained by adding active seed alone

Table 6 Particle size distributions in volume fraction of precipitation product obtained by adding active seed alone
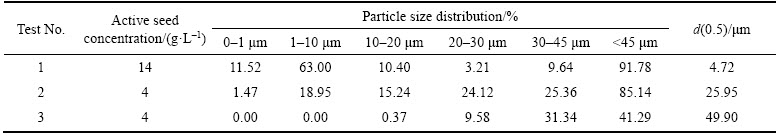

Figure 4 SEM micrographs of precipitation product obtained by adding active seed alone:
As shown in Table 5, the precipitation ratio was not obviously higher than the typical value of 45%–52% in the traditional seeded precipitation [3], when adding the active seed alone. From Table 6 and Figure 4, we can see that the precipitation product size is far below the requirement for sandy alumina production, although obvious agglomeration occurred at a low concentration of the active seed. With the precipitation ratio increasing, the particle size decreases remarkably, being in accordance with the result obtained in our previous research by adding an active seed synthesized by the similar method [15]. As for the mechanism for the refinement of precipitation product under the condition of adding active seed, WANG et al [33] and BHATTACHARYA et al [34, 16] postulated that the supersaturation in the very vicinity of active seed surface was increased by active seed dissolution into sodium aluminate solution; thereby nucleation rate of gibbsite was increased at this high supersaturation zone and fine precipitation product was obtained. In conclusion, it is hard to produce coarse gibbsite product by seeded precipitation with only adding the self- prepared active seed.
3.3 Intensification of seeded precipitation by adding mixed seed of self-prepared active seed and industrial seed
As both coarse precipitation product and high precipitation ratio couldn’t be achieved by only adding either the active seed or the industrial seed, the mixed seed consisting of the industrial seed and an extremely small amount of self-prepared active seed was adopted to enhance the gibbsite precipitation process. For the seed precipitation with the mixed seed, the initial pregnant aluminate solution was employed with mass concentration of Na2Ok 160 g/L and caustic molar ratio of αk 1.41. Industrial seed concentration was 1500 g/L, and the precipitation temperature was decreased evenly from 60 °C to 45 °C during 0–15 h and then maintained at 45 °C during 15–45 h. The effects of the active seed concentration on the precipitation ratio and particle size of precipitation product were studied, and the results are shown in Tables 7 and 8. In addition, the particle size distributions of the active seed and industrial seed are also presented in Table 9 for reference.
Table 7 illustrates that the precipitation ratio was just 51.28% for the traditional seeded process with only industrial seed of 1500 g/L (test 4), whereas the precipitation ratio was increased to 54.51%–56.38% by adding a small amount of extra self-prepared active seed. Furthermore, when the concentration of the active seed varies from 3.1 g/L to 7.7 g/L, the higher the concentration of the active seed, the higher the increment. The precipitation ratio can be increased by 5.1% when adding 7.7 g/L active seed.
Table 7 Effect of active seed concentration on precipitation ratio by adding a mixture seed of active seed and industrial seed
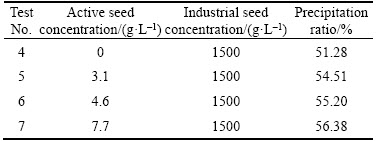
Table 8 Particle size distributions in volume fraction of precipitation products obtained by adding a mixed seed
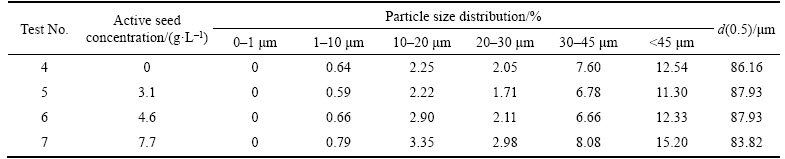
Table 9 Particle size distributions in volume fraction of self-prepared active seed and industrial seed
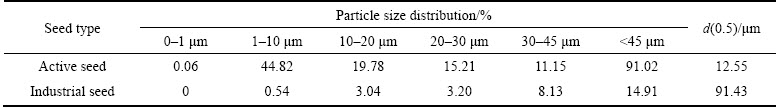
In Tables 8 and 9, with extra 3.1 or 4.6 g/L self-prepared active seed in the seeded precipitation process besides the industrial seed, the volume fraction of <45 μm particles of the precipitation product reduces slightly by comparison with that of the industrial seed and of the precipitation product by the traditional process. However, the refinement of precipitation product also occurs when the concentration of the extra self-prepared active seed reaches 7.7 g/L. So, we can draw a conclusion that both the relatively remarkable increase of precipitation ratio and coarse precipitation product can be accomplished by adding appropriate amount of extra self-prepared active seed besides the normal industrial seed during the seeded precipitation.
3.4 Preliminary mechanism analysis for intensified seeded precipitation with mixed seed
The intrinsic reasons for the acceleration of gibbsite precipitation by adding an active seed has been discussed in Section 3.1. Aiming to better understand the mechanism for the particle size variation of precipitation product by adding the mixed seed, SEM analyses of precipitation products (tests 4, 5 and 7) were conducted and the micrographs are presented in Figure 5.
Figure 5(a) shows that the precipitation product of the traditional seeded precipitation with only adding 1500 g/L industrial seed has a rather smooth surface resemble to that of the industrial seed (see Figures 3(c) and (d)). However, as shown in Figures 5(c)–(f), the morphology of the product particles obtained by the intensified process with adding the mixed seed is obviously different from that of the precipitates by the traditional process. The product particles by the intensified process were covered by numerous pellets of extremely fine particles, which may be caused by two ways. One is that the self-prepared active seeds were dispersively embedded into the surface of the industrial seed, the other is that the fine particles preferentially accumulated or agglomerated at the re-entrant corners among the faces of the industrial seed in multiply stacked structures. Moreover, the higher the active seed concentration, the more coverage the fine particles on the surface of the industrial seed. Probably, the reason why the mass percentage of particles <45 μm in the precipitation product increases with increasing extra active seed concentration (see Table 8) may be partly that, the surface area supplied by the industrial seed fails to satisfy the requirement for the agglomeration of more quantity of fine precipitate particles, as a high concentration of the active seed is prone to resulting in secondary nucleation in the gibbsite precipitation.
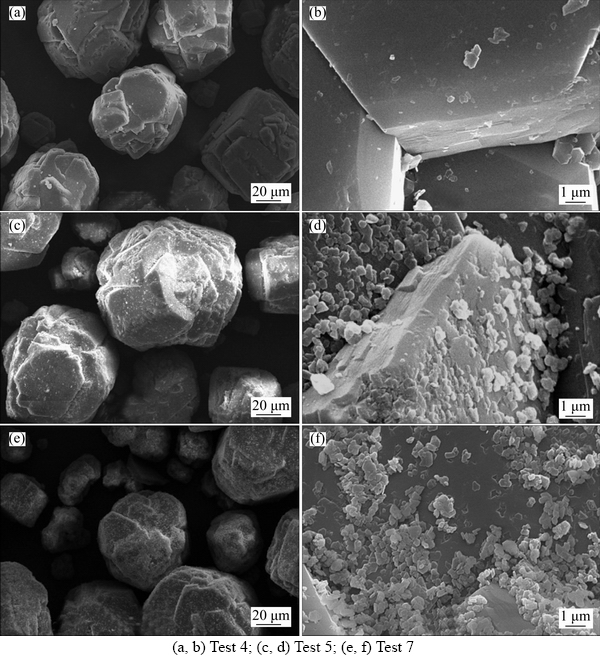
Figure 5 SEM micrographs of precipitation product by traditional process and intensified processes with mixed seed extra self-prepared active seed (g/L):
Combined the result from Figure 5 with that drawn in Section 3.1, the intensification mechanism for the seeded precipitation by adding the mixed seed of small amounts of extra self-prepared active seed and normal industrial seed has been preliminarily elucidated. The extra self-prepared active seed with favorable interfacial properties can accelerate gibbsite precipitation, while the embedment and accumulation/agglomeration of extremely fine particles on the surfaces of the industrial seed can effectively limit the content of fine particles in the precipitation product to some extent.
4 Conclusions
1) The gibbsite precipitation ratio was distinctly increased from 51.28% with only industrial seed to 54.51%–56.38% with extra 3.1–7.7 g/L self-prepared active seed. Moreover, the mass percentage of particles <45 μm in precipitation product has a small decrease with extra 3.1–4.6 g/L active seed.
2) The mechanism for the increase of gibbsite precipitation ratio by adding the extra active seed is preliminarily investigated. Compared with the industrial seed, the self-prepared active seed has more favorable interfacial properties for gibbsite precipitation, such as better wettability, lower interfacial tension, and larger specific surface area.
3) The embedment and accumulation/ agglomeration of extremely fine particles on the coarse industrial seed can effectively control the content of fine particles in the precipitation product, when adding appropriate amount of extra active seed in the intensified seeded precipitation.
References
[1] XUE Sheng-guo, YE Yu-zhen, ZHU Feng, WANG Qiong-li, JIANG Jun, WILLIAM H. Changes in distribution and microstructure of bauxite residue aggregates following amendments addition [J]. Journal of Environmental Sciences, 2019, 78: 276–286. DOI: 10.1016/j.jes.2018.10. 010.
[2] XUE Sheng-guo, WU Yu-jun, LI Yi-wei, KONG Xiang-feng, ZHU Feng, WILLIAM H, LI Xiao-fei, YE Yu-zhen. Industrial wastes applications for alkalinity regulation in bauxite residue: A comprehensive review [J]. Journal of Central South University, 2019, 26(2): 268–288. DOI: https://doi.org/1031007/s11771-019-4000-3.
[3] LI Xiao -bin, ZHAO Dong- feng , WANG Dan -qin, YAN Li. Research progress in theory and technology of gibbsite precipitation from sodium aluminate solution [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(10): 2577–2593. (in Chinese)
[4] LI Xiao-bin, WANG Dan-qin, ZHOU Qiu-sheng, LIU Gui-hua, PENG Zhi-hong. Influence of magnetic field on the seeded precipitation of gibbsite from sodium aluminate solution [J]. Minerals Engineering, 2012, 32(3): 12–18. DOI: 10.1016/j.mineng. 2012.02.015.
[5] ZHANG Bin, LI Jie, CHEN Qi-yuan, CHEN Guo-hui. Precipitation of Al(OH)3 crystals from supersaturated sodium aluminate solution irradiated with ultrasonic sound [J]. Minerals Engineering, 2009, 22(9, 10): 853–858. DOI: 10.1016/ j.mineng.2009.03.008.
[6] CHEN Nian-yi, LU Wen-cong, ZHANG Liang-miao, GU Song-qin, QI Li-juan, BAI Wan-quan. Liquid-liquid extraction modified Bayer process and its physic-chemical basis [J]. Journal of the Chinese Rare Earth Society, 2006, 24(specialissue): 78–81. (in Chinese)
[7] LI Yuan-gao, CHEN Qi-yuan, WANG Song-sen, YIN Zhou-lan, ZHANG Ping-min. Preparation of Al(OH)3 by ion membrane electrolysis and precipitation of sodium aluminate solution with seeds [J]. The Chinese Journal of Nonferrous Metals, 2008, 18(4): 974–979. DOI: 10.1016/S1003-6326(08) 60168-5. (in Chinese)
[8] WILHELMY R B. Control of form of crystal precipitation of aluminum hydroxide using co solvents and varying caustic concentration: United States, 4900537 [P]. 1990-02-13.
[9] ZHANG Ying, ZHENG Shi-li, DU Hao, XU Hong-bin, WANG Shao-na, ZHANG Yi. Improved precipitation of gibbsite from sodium aluminate solution by adding methanol [J]. Hydrometallurgy, 2009, 98(1, 2): 38–44. DOI: 10.1016/ j.hydromet.2009.03.014.
[10] LIU Gui-hua, WU Guo-yu, CHEN Wei, LI Xiao-bin, PENG Zhi-hong, ZHOU Qiu-sheng, QI Tian-gui. Increasing precipitation rate from sodium aluminate solution by adding active seed and ammonia [J]. Hydrometallurgy, 2018, 176: 253–259. DOI: 10.1016/j.hydromet.2018.02.003.
[11] ZENG Ji-shu, YIN Zhou-lan, CHEN Qi-yuan. Effect of tetracarbon additives on gibbsite precipitation from seeded sodium aluminate liquor [J]. Journal of Central South University, 2008, 15(5): 622–626. DOI: 10.1007/s11771- 008-0116-6.
[12] LI Xiao-bin, YAN Li, ZHOU Qiu-sheng, PENG Zhi-hong, LIU Gui-hua. Effect of ethers additive B35 on seeded precipitation of sodium aluminate solution [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(2): 459–464. (in Chinese)
[13] BROWN N. Effect of calcium ions on agglomeration of bayer aluminium trihydroxide [J]. Journal of Crystal Growth, 1988, 92(1): 26–32. DOI: 10.1016/0022-0248(88)90429-0.
[14] GNYRA N, BROWN N. The coarsening of Bayer alumina trihydrate by means of crystallization modifiers [J]. Powder Technology, 1975, 11(2): 101–105. DOI: 10.1016/0032- 5910(75)80035-0.
[15] TIAN Lv. Study on preparation of high whiteness aluminum trihydroxide powder from high concentration sodium aluminate solution by seeded precipitation [D]. Changsha: Central South University, 2012. (in Chinese)
[16] PRADHAN J K, GOCHHAYAT P K, BHATTACHARYA I N, DAS S C. Study on the various factors affecting the quality of precipitated non-metallurgical alumina trihydrate particles [J]. Hydrometallurgy, 2001, 60(2): 143–153. DOI: 10.1016/ S0304-386X(00)00196-1.
[17] XIE Yan-li, BI Shi-wen, YANG Yi-hong, WANG Zhi. Surface acidity of Al(OH)3 seed and its effect on precipitation of sodium aluminate liquors [J]. The Chinese Journal of Nonferrous Metals, 2000, 10(6): 896–898. (in Chinese)
[18] YIN Jian-guo. Intensifying the seeded agglomeration process of supersaturated sodium aluminate liquors [D]. Changsha: Central South University, 2007. (in Chinese)
[19] ZENG Ji-shu, YIN Zhou-lan, CHEN Qi-yuan. Intensification of precipitation of gibbsite from seeded caustic sodium aluminate liquor by seed activation and addition of crown ether [J]. Hydrometallurgy, 2007, 89(1): 107–116. DOI: 10.1016/j.hydromet.2007.07.001.
[20] ZHANG Xuan. Particle size control of products from precipitation process with seeds prepared by inducing nucleation in sodium aluminate solution [D]. Changsha: Central South University, 2011. (in Chinese)
[21] LIU Giu-hua, LI Zheng, QI Tian-gui, LI Xiao-bin, ZHOU Qiu-sheng, PENG Zhi-hong. Two-stage process for precipitating coarse boehmite from sodium aluminate solution [J]. JOM, 2017, 69(10): 1888–1893. DOI: 10.1007/s11837-017-2468-6.
[22] ADDAI-MENSAH J, RALSTON J. The influence of interfacial structuring on gibbsite interactions in synthetic Bayer liquors [J]. Journal of Colloid and Interface Science, 1999, 215(1): 124–130. DOI: 10.1006/jcis.1999.6237.
[23] ADDAI-MENSAH J, PRESTIGE C A, RALSTON J. Interparticle forces, interfacial structure development and agglomeration of gibbsite particles in synthetic Bayer liquors [J]. Minerals Engineering, 1999, 12: 655–669. DOI: 10.1016/S0892-6875(99)00050-3.
[24] WATTS H L, UTLEY D W. Volumetric analysis of sodium aluminate solutions [J]. Analytical Chemistry, 1953, 25(6): 864–867. DOI: 10.1021/ac60078a005.
[25] YANG Chong-yu. Technology of alumina production [M]. Beijing: Metallurgical Industry Press, 2011: 53. (in Chinese)
[26] OWENS D K, WENDT R C. Estimation of the surface free energy of polymers [J]. Journal of Applied Polymer Science, 1969, 13(8): 1741–1747. DOI: 10.1002/app.1969. 070130815.
[27] LUNER P E, OH E. Characterization of the surface free energy of cellulose ether films [J]. Colloids & Surfaces A: Physicochemical & Engineering Aspects, 2001, 181(1–3): 31–48. DOI: 10.1016/S0927-7757(00)00805-0.
[28] LI Xiao-bin, XU Xiang-ming, ZHOU Qiu-sheng, QI Tian-gui, LIU Gui-hua, PENG Zhi-hong, CUI Yuan-fa, LI Jian-pu. Ca3WO6 prepared by roasting tungsten-containing materials and its leaching performance [J]. International Journal of Refractory Metals & Hard Materials, 2015, 52: 151–158. DOI: 10.1016/j.ijrmhm.2015.06.003.
[29] ADAMSON A W, GAST A P. Physical chemistry of surfaces [M]. New York: Wiley, 1997: 353.
[30] ZHU Ding-yi, DAI Pin-qiang, LUO Xiao-bin, ZHANG Yuan-chao. Novel characterization of wetting properties and the calculation of liquid-solid interface tension(I) [J]. Science Technology and Engineering, 2007, 7(13): 3057–3062. (in Chinese)
[31] ZHU Ding-yi, ZHANG Yuan-chao, DAI Pin-qiang, LUO Xiao-bin. Novel characterization of wetting properties and the calculation of liquid-solid interface tension(II) [J]. Science Technology and Engineering, 2007, 7(13): 3063–3069. (in Chinese)
[32] LI Xiao-bin, YAN Li, ZHAO Dong-feng, ZHOU Qiu-sheng, LIU Gui-hua, PENG Zhi-hong, YANG Shuai-shuai, QI Tian-gui. Relationship between Al(OH)3 solubility and particle size in synthetic Bayer liquors [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(5): 1472–1479. DOI: 10.1016/S1003- 6326(13)62619-9.
[33] WANG Jian-li, CHEN Qi-yuan, LI Wang-xing, YIN Zhou-lan. Kinetics of super-fine aluminum hydroxide precipitation from sodium aluminate solutions with gel-seed [C]// Light Metals. San Francisco, California:TMS, 2009: 177–182.
[34] BHATTACHARYA I N, PRADHAN J K, GOCHHAYATP K, DAS S C. Factors controlling precipitation of finer size alumina trihydrate [J]. International Journal of Mineral Processing, 2002, 65(2): 109–124. DOI: 10.1016/S0301- 7516(01)00084-9.
(Edited by YANG Hua)
中文导读
添加混合晶种强化铝酸钠溶液的晶种分解过程
摘要:本文报道了工业晶种和自制活性晶种组成的混合晶种强化铝酸钠溶液中三水铝石结晶析出的工艺,并初步研究了其强化机理。对比研究了活性晶种/铝酸钠溶液、工业晶种/铝酸钠溶液的界面性质。分别采用坐滴法和BET法测定了晶种/铝酸钠溶液接触角及晶种比表面积;采用激光衍射法和SEM分别测定了种分产品的粒度分布及形貌。研究结果表明,相对于工业晶种,活性晶种的润湿性能更好、比表面积更大以及晶种/铝酸钠溶液界面张力更低,这些性质均利于加速铝酸钠溶液中氢氧化铝在晶种/铝酸钠溶液界面的析出。结晶产物的SEM分析表明,细小颗粒会嵌入、堆积及附聚到粗粒度工业晶种表面,从而可有效地控制结晶产物中的细颗粒含量。额外加入3.1~4.6 g/L活性晶种,种分分解率可提高3.23%~3.92%,且种分产物中 <45 μm颗粒含量比工业晶种及传统种分产物的均略有减少。研究结果有利于为氧化铝工业生产开发一种新的铝酸钠溶液晶种分解过程强化工艺。
关键词:三水铝石;晶种分解;铝酸钠溶液;活性晶种;强化
Foundation item: Project(51604309) supported by the National Natural Science Foundation of China; Project(2015BAB04B01) supported by the National Key Technology R & D Program of China
Received date: 2018-11-05; Accepted date: 2018-12-15
Corresponding author: ZHOU Qiu-sheng, PhD, Professor; Tel: +86-731-88830453; E-mail: qszhou@csu.edu.cn; ORCID: 0000-0003- 0159-0041
Abstract: Gibbsite precipitation from sodium aluminate solution was intensified by adding mixed industrial and self-prepared active seeds, and its mechanism was researched preliminarily. The interfacial properties of seed/aluminate solution were determined for separate industrial and active seed. Contact angles of seed/aluminate solution and the specific surface area of seeds were respectively measured by sessile drop and BET method, and the morphology and particle size of precipitates were recorded by SEM and laser diffraction. The results show that, compared with the industrial seed, the active seed has a better wettability, lower interfacial tension, and larger specific surface area, being conducive to enhancing gibbsite precipitation from sodium aluminate solution. SEM analysis of the precipitates indicates that the embedment and accumulation/agglomeration of extremely fine particles on the surface of coarse industrial seed can effectively control the content of fine particles in the precipitation product. With extra 3.1–4.6 g/L active seed, the gibbsite precipitation ratio was increased by 3.23%–3.92%. Moreover, the mass percentage of particles <45 μm in precipitation product has even a slight decrease compared with that for the traditional precipitation product or of the industrial seed itself. The result presented is favorable to developing an intensified gibbsite precipitation process for commercial alumina manufacture.


