Preparation and characterization of Ni nanopowders prepared by anodic arc plasma
WEI Zhi-qiang(魏智强)1, QIAO Hong-xia(乔宏霞)2,DAI Jian-feng(戴剑锋)1, FENG Wang-jun(冯旺军)1,
WANG Qing(王 青)1, LI Wei-xue(李维学)1, YAN Peng-xun(闫鹏勋)3
(1. School of Sciences, Lanzhou University of Technology, Lanzhou 730050, China;
2. College of Civil Engineering, Lanzhou University of Technology, Lanzhou 730050, China;
3. School of Physical Science and Technology, Lanzhou University,Lanzhou 730000, China)
Abstract:
Ni nanopowders were successfully prepared in large quantities by anodic arc discharged plasma method with homemade experimental apparatus in inert gas. The particle size, microstructure and morphology of the particles were characterized via X-ray diffractometry(XRD), transmission electron microscopy(TEM) and the corresponding selected area electron diffractometry(SAED). The specific surface area and pore parameters were investigated by nitrogen sorption isotherms at 77K with Brunauer-Emmett-Teller(BET) equation and Barrett-Joyner-Halenda (BJH) method. The chemical compositions were determined by X-ray energy dispersive spectrometry (XEDS) and element analysis. The experimental results indicate that this method is convenient and effective, and the nanopowders with uniform size, higher purity, weakly agglomerated and spherical chain shape are gotten. The crystal structure of the samples is FCC structure as the bulk materials, the particle size distribution ranges from 20 to 70nm, and the average particle size is about 46nm obtained by TEM and confirmed by XRD and BET results. The specific surface area is 14.23m2/g, specific pore volume is 0.09cm3/g and average pore diameter is 23nm.
Key words:
anodic arc plasma; Ni nanopowders; particle size; structure; composition CLC number: TG146.15;
Document code: A
1 INTRODUCTION
In recent years, a great deal of attention has been paid to metal nanoparticles because of their novel physical and chemical properties[1, 2]. The nickel nanopowders as a new type of material have many applications in the electronic industry, chemical engineering, national defense, high technology, etc[3-5]. Due to the fact that the properties and application aspects of the nanopowders depend strongly on the particle size and morphology. The preparation methods and characterization of these powders have been main topics in the field of metal materials. Many techniques have been developed to prepare nano-metal powders, such as vapor decomposition, sputtering, high energy ball milling, chemical vapor condensation, sol-gel processing, gas-phase chemical reaction, laser high temperature burning method, micro-emulsion and hydrothermal synthesis[6-9]. However, some limitations still exist in the above-mentioned methods. For example, such powders prepared by these methods usually cannot be isolated; the majority of the procedures cannot be fulfilled promptly and generally cannot be adaptable to industrial production; in addition, commercial exploitation of nanopowders is currently limited by high synthesis cost.
From a practical viewpoint, it is vital to develop a way to manufacture high quality nanopowders in large quantities at low cost. For this reason, an efficient preparation method has been developed in our laboratory to prepare high quality nanopowders[10], which hopefully will meet all these conditions. Some metals have been prepared and ample evidences are available to show that this technique is a very promising method and has some advantages as follows: 1) this method is convenient, inexpensive and effective; 2) the nanopowders with uniform size, are weakly agglomerated, and have a narrow size distribution and low impurity contamination; 3) the properties(particle size, morphology and other characteristics) can be easily improved by varying the technological parameters[11]; and 4) this is a continuous production method and is suitable for bulk production in factory.
In this paper, we report a convenient approach to prepare Ni nanopowders by anodic arc discharging plasma method in inert atmosphere. The particle size, mircostructure, morphology, specific surface area, pore parameters, and chemical composition of the samples by this process were characterized via X-ray diffractometry(XRD), transmission electron microscopy(TEM) and the corresponding selected area electron diffractometry(SAED), Brunauer-Emmett-Teller(BET) surface area analyzer, X-ray energy dispersive spectrometry (XEDS) and element analysis instrument, and the results were discussed.
2 EXPERIMENTAL
The schematic diagram of the experimental installation designed for obtaining metal nanopowders is presented in Fig.1. The experimental apparatus mainly consists of the stainless steel vacuum chamber, the gas supply device, the DC power supply, the plasma generator with high frequency initiator, the vacuum pump, the water-cooled collection cylinder, the fixed water-cooled copper anode crucible and plumb moveable tungsten cathode in a vacuum chamber.

Fig.1 Schematic diagram of experimental installation
In the process of preparation, the vacuum chamber was pumped to 10-3Pa and then was backfilled with inert argon(purity 99.99%) to near 103Pa. The electric arc in the argon environment was automatically ignited between the wolfram electrode and the nozzle(well cooled) by high frequency initiator. Under argon pressure and electric discharge current, the ionized gases were driven through the nozzle outlet and formed the plasma jet[12]. The bulk metal Ni was heated and melted by the high temperature of the plasma. The melt detached from the metal surface when the plasma jet kinetic energy exceeded the melt superficial energy, and then evaporated into atom soot. The metal atoms diffused around and collided with inert gas atoms to decrease the germ forming energy, and then the nano Ni drops were formed by collision among the metal atoms[13, 14]. The as-formed drops were stable when their potential energy was the minimum as the particle was spherical. The drops deposited before their deposition in enclosure, and the formed particles finally solidified on the inner walls of the cooled collection cylinder[15, 16]. The loose nanopowders can be obtained after a period of passivation and stabilization with working gas.
To investigate the structure of Ni nanopowders, the as-obtained powders were analyzed by Japan Rigaku D/max-2400 X-ray diffractometer using monochromatic high-intensity CuKα radiation(λ=1.54056, 40kV, 100mA). The average grain size of the powder was estimated from X-ray line broadening measurements according to the Scherrer formula.
The particle size and morphology of the sample were examined by transmission electron microscopy(TEM) and the corresponding selected area electron diffraction(SAED) with a Japan JEOL JEM-1200EX microscope with an accelerating voltage of 80kV.
The specific surface area and pore parameters were measured by nitrogen sorption isotherms at 77K. The data were conducted automatically on a micromeritics ASAP-2010 porosity analyzer(Micromerities Corp, USA). From the sorption data, the specific surface area of Ni nanopowders was evaluated by using the Brunauer-Emmett-Teller(BET) equation, and the average pore diameter and cumulative pore volume of pores were estimated by Barrett-Joyner-Halenda(BJH) method.
The main constituent elements and their relative content were examined by X-ray energy dispersive spectrometer(XEDS) that was attached to the scanning electron miscroscope(SEM, JEOL Ltd., Tokyo, Japan). Impurities, such as nitrogen, hydrogen, oxygen and carbon were determined by using element analysis instrument (Elemental Vario EL, Germany).
3 RESULTS AND DISCUSSION
3.1 TEM Results
Fig.2(a) shows representative TEM image of Ni nanoparticles. It can be seen from the image that the particles distribute homogeneous, and a few large agglomerates of particles are seen. Because of their extremely small dimensions and high surface energy, most of the particles appear to be fairly uniform in size, with smooth surface and spherical in chain or random shape. The spherical chainlike morphology is the result of magnetic force and surface tension collaboration between the ultra-fine particles. Fig.2(b) shows the corresponding selected-area electron diffraction(SAED) pattern. It can be indexed to be the FCC structure. Random particles orientation and small particles cause the widening of diffraction rings that is made up of many diffraction spots, which indicates that the nanoparticles are of polycrystalline structure. Fig.3 shows the particle size distribution of Ni nanopowders. It can be seen that the particle sizes range from 20 to 70nm, and the average diameter is about 47nm by calculation, showing a relatively narrow size distribution.
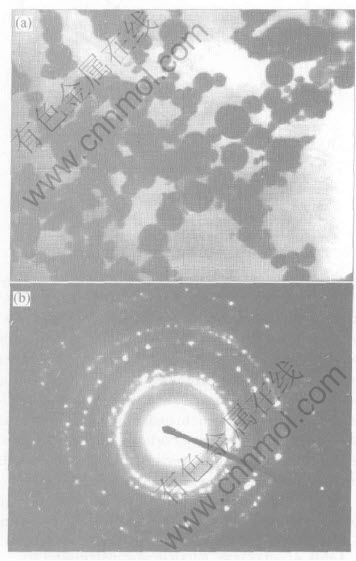
Fig.2 TEM micrograph(a) and selected area electron diffraction pattern(b) of Ni nanopowders
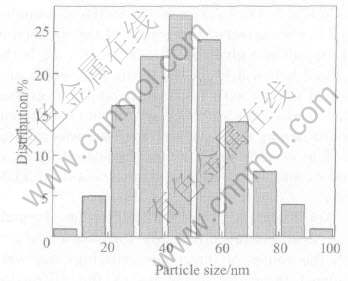
Fig.3 Particle size distribution of Ni nanopowders
3.2 XRD results
Although TEM may give us direct information of the microstructures of the nanopowders, statistically the XRD could provide us more reliable knowledge. Fig.4 shows the X-ray diffraction(XRD) pattern for the samples. The diffraction peaks are broad, suggesting that the sample consists of very small particles. On the other hand, all the peaks with 2θ values of 44.52°, 51.88°, 76.40°, 92.96°and 98.48°correspond to the (111), (200), (220), (311), (222) planes of the bulk metallic Ni, respectively. The XRD spectrum does not reveal any other phase except the characteristic peaks of nickel, which can be assigned to Ni face centered cubic(FCC) phase. This result shows that the physical phases of the nickel nanoparticles synthesized in this work have high purity.

Fig.4 XRD pattern of Ni nanopowders
The average grain size of Ni nanopowders was estimated from X-ray pattern broadening measurements. The calculation was done using the (111) diffraction peak in the XRD spectra according to the Scherrer formula: ![]() , where d is the crystallite size; K=0.89 is approximately the Scherrer constant related to the shape and index(hkl) of the crystals; λ is the wavelength of the X-ray(CuKα, 1.4954); θ is the diffraction angle; and B is the corrected half-width of the diffraction peak(in radians) given by B2=B2m-B2s. Bm is the measured half-width and Bs is the half-width of a standard sample with a known crystal size greater than 100nm. The effect of geometric(instrumental) broadening on the reflection peaks was calibrated. The average grain size is calculated to be around 42nm, which agrees with the result of TEM image.
, where d is the crystallite size; K=0.89 is approximately the Scherrer constant related to the shape and index(hkl) of the crystals; λ is the wavelength of the X-ray(CuKα, 1.4954); θ is the diffraction angle; and B is the corrected half-width of the diffraction peak(in radians) given by B2=B2m-B2s. Bm is the measured half-width and Bs is the half-width of a standard sample with a known crystal size greater than 100nm. The effect of geometric(instrumental) broadening on the reflection peaks was calibrated. The average grain size is calculated to be around 42nm, which agrees with the result of TEM image.
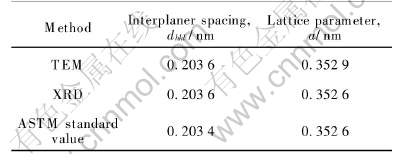
According to electron diffraction formula Rdhkl=L and X-ray diffraction formula λ=2dhkl·cosθ, the values of interplaner spacings dhkl were calculated from the diameters of the diffraction rings, as well as obtained from XRD analysis. For FCC structure, dhkl=![]() , and the lattice parameter (a) of (111) Miller plane can be calculated respectively. Compared with standard ASTM data (a= 0.3526nm) in Table 1, it can be seen that the lattice parameter is in good agreement with each other.
, and the lattice parameter (a) of (111) Miller plane can be calculated respectively. Compared with standard ASTM data (a= 0.3526nm) in Table 1, it can be seen that the lattice parameter is in good agreement with each other.
Table 1 Comparison of interplaner spacings(dhkl) and lattice parameter (a) with standard ASTM data
3.3 BET results
Fig.5 shows the typical nitrogen sorption isotherms of Ni nanopowders. It shows that the sample presents typical IV adsorption. In the low-pressure region(p/p0〈0.8), the adsorption-desorption isotherms are relative flat. Adsorption and desorption completely are over-lapped because adsorption mostly occurs in the micropores. In the high relative pressure region(p/p0>0.8), the isotherms increase rapidly, and form a lag loop owing to capillary agglomeration phenomenon.
The surface area analysis was carried out on the as-prepared powders by BET. Fig.6 shows BET plots of Ni nanopowders. The specific surface area is 14.23m2/g, which is calculated with the multi-point BET-equation. Assuming that the particles have solid, spherical shape with smooth surface and same size, the surface area can be related to the average equivalent particle size by the equation: DBET=6000/(ρ·Sw), where D is the average diameter of a spherical particle, nm; Sw represents the measured surface area of the powder, m2/g; and ρ is the theoretical density, g/cm3. The calculated average equivalent particle size is 46nm.
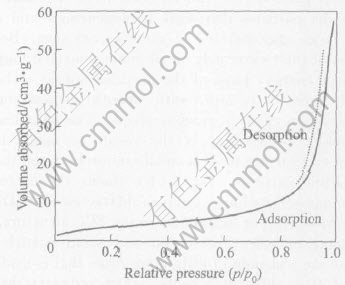
Fig.5 N2 adsorption-desorption isotherms of Ni nanopowders

Fig.6 BET plots of Ni nanopowders
The average particle size values of the as-prepared powders, calculated from the Scherrer formula, obtained based on TEM data and estimated from BET values are listed in Table 2. We notice that the particle size obtained from the BET and the TEM methods, agree very well with the result given by X-ray pattern broadening measurement. The results of TEM observations and BET methods further confirm and verify the relevant results obtained by XRD as mentioned above.
Fig.7 shows the typical BJH pore size distri-bution curves of Ni nanopowders. The experimen- tal fact indicates that the micropore with a size smaller than 40nm is observed, and the average pore diameter estimated from the peak position is about 23nm with narrow pore size distribution. Moreover, such micro-pores have not been observed within particles by TEM (Fig.2). Therefore, these particles are actually grain clusters, i.e. small polycrystals. By assuming full saturation of the pores at the relative pressure of 0.95, the cumulative pore volume of pores is approximately 0.09cm3/g.
Table 2 Average particle size calculated by various methods

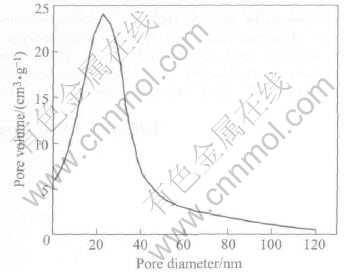
Fig.7 BJH pore size distribution curves of Ni nanopowders
3.4 Chemical composition analysis
There are four elements (nickel, nitrogen, silicon and oxygen) in the powder from the X-ray energy dispersive spectrometry(XEDS) result shown in Fig.8. It is obvious that the silicon peak is caused by the glass substrate on which Ni nanopowders are mounted for XEDS analysis. Table 3 lists XEDS quantitative microanalysis result that indicates a predominance of nickel(98.30%, mass fraction) as the main nanopowders constituents. Impurities, such as nitrogen, hydrogen, oxygen
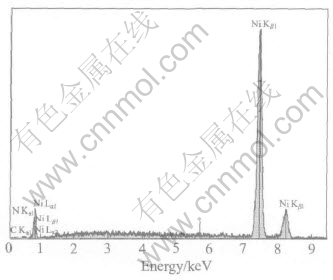
Fig.8 X-ray energy dispersive spectrometry of Ni nanopowders
and carbon were determined by element analyze instrument. Table 4 lists C, H, N and O contents of the samples. It shows that C, H, N and O contents are very low. We can judge that it is conceivable for N2, CO2 and O2 to exist in the surface of the particle as it is exposed in air. It has been proved that the peaks of the impurities in the product by this method are very weak, that is to say, the samples are highly pure.
Table 3 X-ray energy dispersive spectrometry quantitative microanalysis of Ni nanopowders
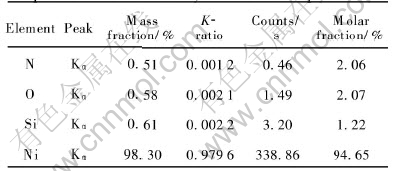
Table 4 Chemical composition analyses of Ni nanopowders(mass fraction, %)

4 CONCLUSIONS
Ni nanopowders were successfully prepared by anodic arc discharged plasma with homemade experimental apparatus in inert gas. This is a convenient and effective method that can produce the nanopowders with desirable characteristics, including uniform size, higher purity, weak agglomeration, narrow size distribution and spherical chain shape. The crystal of the samples has FCC structure as same as the bulk materials. The particle size distribution of the powders ranges from 20 to 70nm, and the average particle size is about 47nm obtained by TEM and confirmed by XRD and BET results. The specific surface area is 14.23m2/g, specific pore volume is 0.09cm3/g and average pore diameter is 23nm.
REFERENCES
[1]ZHANG Wen-wei, CAO Qing-qi. Structural, morphological, and magnetic study of nanocrystalline cobalt-nickel-copper particles [J]. Journal of Colloid and Interface Science, 2003, 257: 237-243.
[2]CUI Z L, DONG L F, HAO C C. Microstructure and magnetic property of nano-Fe particles prepared by hydrogen arc plasma [J]. Mater Sci Eng, 2000, 286A: 205-207.
[3]Valiev R Z. Structure and mechanical properties of ultrafine-grained metals [J]. Mater Sci Eng, 1997, 234-236A: 50-66.
[4]LIU P, WANG Y M. Study on twin stacking faults in ultrafine nickel [J]. Materials and Design, 2000, 21: 155-157.
[5]Cranqvist C G, Buhrman R A. Ultrafine metal particle [J]. J Appl Phys, 1976, 47: 2200-2219.
[6]CHEN Y J, CAO M S, TIAN Q. A novel preparation and surface decorated approach for α-Fe nanoparticles by chemical vapor-liquid reaction at low temperature [J]. Materials Letters, 2004, 58: 1481-1484.
[7]CHEN De-hong, CHEN Dai-rong. Hydrothermal synthesis and characterization of octahedral nickel ferrite particles [J]. Powder Technology, 2003, 133: 247-250.
[8]ZHENG H G, LANG J H, ZENG J H. Preparation of nickel nanopowders in ethanol-watersystem (EWS) [J]. Materials Research Bulletin, 2001, 36: 947-952.
[9]CHEN B J, SUN X W, XU C X. Growth and characterization of zinc oxide nano/micro-fibers by thermal chemical reactions and vapor transport deposition in air [J]. Physica E, 2004, 21: 103-107.
[10]WEI Zhi-qiang, WEN Xian-lun,YAN Peng-xun. Study of preparing Ni nanopowders by anodic arc plasma[J ].The Chinese Journal of Nonferrous Metals, 2003, 13(5): 1136-1140.( in Chinese)
[11]WEI Zhi-qiang, YAN Peng-xun.The effects of technology parameters of anodic arc discharged plasma on the preparation of Ni nanopowders [J]. Rare Metal Materials and Engineering, 2004, 33(3): 305-308. (in Chinese)
[12]Loan B. Nanoparticle production by plasma [J]. Mater Sci Eng, 1999, 68B: 5-9.
[13]Scott J H J, Majetich S A. Morphology, structure, and growth of nanoparticles produced in a carbon arc [J]. Phys Rev B, 1995, 52: 12564-12571.
[14]Kwan A J W. Arc-discharge ion sources for heavy ion fusion [J]. Nuclear Instruments and Methods in Physics Research, 2001, 464A: 569-575.
[15]Maeda S, Jwabuchi S. Differential scanning calorimeter of the coalescence growth of fine offine smoke particles [J]. Jpn J Appl Phys, 1984, 23(7): 830-834.
[16]Kaito C. Coalescence growth mechanism of smoke particles [J]. Jpn J Appl Phys, 1985, 24(3): 261-264.
Foundation item: Project(GS012-A52-047) supported by the Bureau of Science & Technology of Gansu Province, China
Received date: 2004-06-15; Accepted date: 2004-10-08
Correspondence: WEI Zhi-qiang, PhD; Tel: +86-931-2975732; E-mail: zqwei7411@163.com


