J. Cent. South Univ. (2016) 23: 2483-2491
DOI: 10.1007/s11771-016-3307-6
Effects of Mg content on pore structure and electrochemical corrosion behaviors of porous Al-Mg alloys
HE Wen-yuan(何文远)1, 2, XIAO Yi-feng(肖逸锋)1, 2, 3, WU Liang(吴靓)1, 2, XU Yan-fei(许艳飞)1, 2,
QIAN Jin-wen(钱锦文)1, 2, HE Yue-hui(贺跃辉)3, 4, ZHENG Xue-jun(郑学军)1, 2, 3
1. School of Mechanical Engineering, Xiangtan University, Xiangtan 411105, China;
2. Key Laboratory of Welding Robot and Application Technology of Hunan Province,
Xiangtan University, Xiangtan 411105, China;
3. Engineering Research Center of Complex Trajectory Processing Technology and Equipment of
Ministry of Education, Xiangtan University, Xiangtan 411105, China;
4. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract:
Porous Al-Mg alloys with different nominal compositions were successfully fabricated via elemental powder reactive synthesis, and the phase composition, pore structure, and corrosion resistance were characterized with X-ray diffractometer, scanning electron microscope and electrochemical analyzer. The volume expansion ratio, open porosity and corrosion resistance in 3.5% (mass fraction) NaCl aqueous solution of the alloys increase at first and then decrease with the increase of Mg content. The maxima of volume expansion ratio and open porosity are 18.3% and 28.1% for the porous Al-56%Mg (mass fraction) alloy, while there is the best corrosion resistance for the porous Al-37.5% Mg (mass fraction) alloy. The pore formation mechanism can be explained by Kirkendall effect, and the corrosion resistance can be mainly affected by the phase composition for the porous Al-Mg alloys. They would be of the potential application for filtration in the chloride environment.
Key words:
porous Al-Mg alloys; reactive synthesis; pore structure; electrochemical corrosion behaviors;
1 Introduction
In the seawater desalination industry, it is necessary for the seawater pretreatment to intercept seaweed, sediment, suspended solids and microbes, etc, and filtration occupies an important position as a core part in seawater pretreatment process [1-2]. Currently, the quartz sand filter has been widely used in this filtration process, but there are some drawbacks. For example, the high water-head loss increases the energy consumption, and the low filtration velocity affects the efficiency of pretreatment [3-4]. On the other hand, the porous materials have received great attention as the preferred filters in seawater pretreatment [5-6]. The porous ceramics are of good corrosion resistance, but they are limited by the brittleness and poor weldability [7]. The porous metals are of good mechanical properties, while they will be also defeated due to their poor corrosion resistance [8]. Compared with porous ceramics and porous metals, porous intermetallics have the potential to be used as the novel filters, because they are of good mechanical property and environmental resistance, together [9]. Therefore, a novel porous intermetallic to bear chloride corrosion will be anticipated.
Al-Mg alloys have been widely used in vehicle industry for the good combined properties, such as high specific strength (strength to mass ratio) and good welding property [10-11], while in recent years they are becoming increasingly popular for the good corrosion resistance in chloride environment as a surface protective layer of pure Mg or Mg alloys [12-13]. Considering the good combined properties and corrosion resistance, Al-Mg alloys are anticipated to be used as a kind of porous materials, because they may solve the aforementioned seawater pretreatment problem effectively. However, there is rare report on preparing porous Al-Mg alloys, and it is not clear how the Mg content affects the pore structure and corrosion resistance, which may restrict the good applications of porous Al-Mg alloys for seawater pretreatment.
In order to understand the effects of Mg content on pore structure and corrosion resistance, the porous Al-Mg alloys with Mg contents from 20% to 80% were prepared by elemental powder reactive synthesis free of any pore former. X-ray diffraction (XRD) and scanning electron microscopy (SEM) with an energy dispersive spectrometer (EDS) system were used to characterize the phase constitution and pore structure. Open circuit potential (OCP), electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization were applied to elucidate the corrosion resistance. The pore formation mechanism and effect factors of corrosion resistance were also discussed for the porous Al-Mg alloys.
2 Experimental
The raw materials were 99.9% purified Al and Mg powders with the average particle size of 100-150 mm, and the porous Al-Mg alloys with seven nominal compositions at the range of Al-20%Mg to Al-80%Mg were fabricated via elemental powder reactive synthesis. The Al and Mg powders were blended in a cylinder mixer for 10 h, and no lubricant was added in the mixture in order to maintain a sound metal-metal contact to enhance the atomic interdiffusion. Then, the mixture was cold-pressed into compact discs with a dimension of Φ30 mm×3 mm under 50 MPa for 90 s. The discs were sintered in a vacuum furnace under 1×10-3 Pa at the temperature of 435 °C for 4 h. The heating rate was less than 5 °C/min, in order that the discs can preserve the original shape and avoid the possible self-propagation high-temperature synthesis procedure. The porous Al and Mg discs were also prepared with the above synthesis procedure to compare corrosion resistance with the porous Al-Mg alloys.
Before and after the sintering, the disc dimensions were measured by vernier caliper to determine the volume expansion ratio. The sintered discs with seven nominal compositions were characterized by XRD (Ultima IV, Rigaku, Japan) to identify the crystalline phases. The open porosity was measured by the Archimedes method [14]. The pore morphology and material composition were characterized by SEM (JSM-6490LV, JEOL, Japan) with EDS (Inca 250, Oxford, UK) system. The corrosion tests, such as OCP, EIS and potentiodynamic polarization in 3.5% NaCl aqueous solution were performed by the electrochemical analyzer (CorrTest CS350, CorrTest Instrument Co., China) at room temperature, and the corrosion resistances were evaluated for the porous pure Al, pure Mg and porous Al-Mg with seven nominal compositions. In the corrosion tests, the discs served as the working electrodes were carefully washed by ultrasonic cleaning in absolute ethyl alcohol for 30 min. Then, they were positioned at a glass corrosion cell kit, leaving a circular (1.0±0.02) cm2 metal surface in contact with the electrolyte. In OCP tests, the discs were monitored for 8 h after they were immersed into 3.5% NaCl solution. The EIS tests were immediately performed after the OCP test in the same solution. In EIS tests, the Nyquist and Bode plots were recorded at OCP in the frequency ranging from 105 Hz to 10-2 Hz, with the sinusoidal signal perturbation of 5 mV (vs SCE). Immediately after the EIS tests, the potentiodynamic tests were conducted by stepping the potential with a scan rate of 1 mV/s from -200 mV (vs SCE) to 1 V (vs SCE) relative to OCP. All corrosion tests were repeated three times for reproducibility.
3 Results and discussion
3.1 Effect of Mg content on pore structure
3.1.1 Effect of Mg content on volume expansion
Since the pore structure can be controlled by the change of volume expansion during the sintering procedure [15], it is necessary to research the effect of Mg content on volume expansion. The axial, radial and volume expansion ratios are given in Fig. 1 for the porous Al-Mg alloys with different Mg contents. Obviously, the axial, radial and volume expansion ratios increase at first and then decrease with the increase of the Mg content. As for the porous Al-56%Mg alloy, there are the maxima of 9.4%, 4.0% and 18.3% for the axial, radial and volume expansion ratios, respectively. Generally, the volume expansion can be generated by the phase transformation and the formation of pores in the synthetic process [16]. To understand the volume expansion of porous Al-Mg alloys, the authors consider two possible reactions between Al and Mg [17] that may lead to the volume change in the sintering process:
12Al+17Mg→Al12Mg17, ΔV=-1.258% (1)
2Al12Mg17+27Al→17Al3Mg2, ΔV=+0.949% (2)
where ΔV is calculated based on the densities of the pre-reaction materials and the reacted products, and the negative value of ΔV means the volume contraction. As can be seen from Eqs. (1) and (2), both the volume changes of -1.258% and +0.949% of the two reactions are too small to be comparable to the measured value of 18.3%. As a consequence, the volume expansion is not caused by phase transformation but mainly by formation of pores during the sintering process. The original shape of the compact discs was still preserved under such huge expansion ratios, indicating that the compact discs with controllable shape can be in fact obtained.
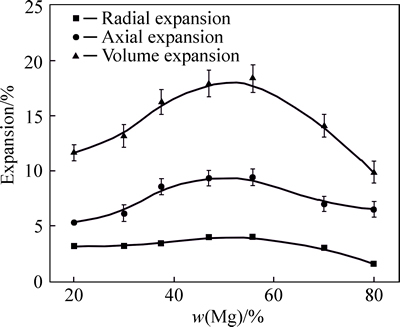
Fig. 1 Effect of Mg content on volume expansion of porous Al-Mg alloys
3.1.2 Effect of Mg content on phase composition
The phase composition should be identified to understand the forming process of the pores [18], and XRD diffraction patterns of porous Al-Mg alloys sintered at 435 °C are given in Fig. 2 for the different Mg contents. Here, four different phases, such as α(Al), β(Al3Mg2), γ(Al12Mg17) and δ(Mg) phases, are detected. When the content of Mg is 20%, the main phase is α(Al) solid solution with a little amount of β(Al3Mg2) phase. As the Mg content increases from 20% to 30%, the main phase is β(Al3Mg2) with the α(Al) phase peaks becoming weaker. As for the Mg content of 37.5%, the α(Al) phase disappears meanwhile the β(Al3Mg2) phase is only detected. With the continuous increase of Mg content, the diffraction peak of γ(Al12Mg17) phase appears. As the content of Mg up to 56%, only very little β(Al3Mg2) phase is detected and the main phase is γ(Al12Mg17). The δ(Mg) diffraction peak becomes stronger and the β(Al3Mg2) diffraction peak disappears with increasing the amount of Mg. Both of the γ(Al12Mg17) and δ(Mg) phases are detected in the porous Al-70%Mg and Al-80%Mg alloys. The results illustrate that the phase changes along with the varied Mg content, and the single β(Al3Mg2) phase and the nearly pure γ(Al12Mg17) phase can be separately achieved for the cases of porous Al-37.5%Mg alloy and Al-56%Mg alloy. According to the Al-Mg binary phase diagram [19], pure single γ(Al12Mg17) phase can be obtained in porous Al-56%Mg alloy, but γ(Al12Mg17) phase with little β(Al3Mg2) phase is obtained. The possible reason is the narrow composition range of γ(Al12Mg17) phase at room temperature.
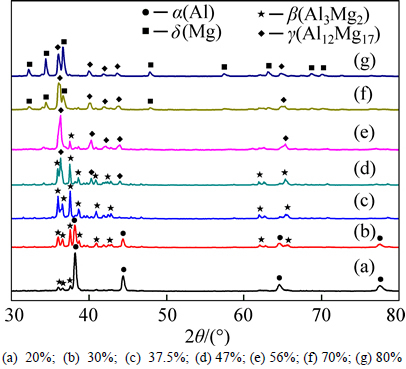
Fig. 2 XRD patterns of porous Al-Mg alloys with different Mg contents sintered at 435 °C:
3.1.3 Effect of Mg content on open porosity
Since the open porosity is critically important for the application of the porous alloys, it is important to find out the variation of the open porosity as a function of Mg content. Figure 3 shows the variation of open porosity in green, sintered discs and open porosity formed during sintering process as a function of the Mg content. The open porosity in the green discs decreases almost linearly with the increase of the Mg content, because the Mg powders can be easily distorted to fill in the clearance pores in the discs during the cold pressing. The open porosity in the final sintered discs exhibits the similar variation of volume expansion with different Mg contents in the sintering process, and this indicates that the pores have great dependence upon the Mg content. It can be further proven that the volume expansion of porous Al-Mg alloys is mainly caused by the formation of pores during the reactive synthesis process with the verification in Fig. 1. The maximum open porosity is 28.1% for the porous Al-56%Mg alloy. The results imply that the open porosity of porous Al-Mg alloys can be controlled through controlling the Mg content during the sintering procedure.
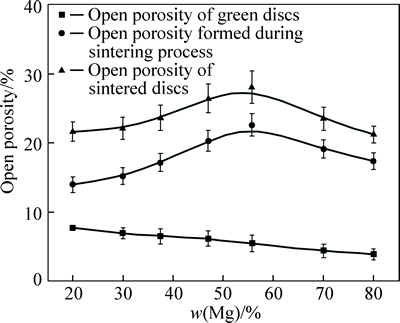
Fig. 3 Effect of Mg content on open porosity of porous Al-Mg alloys
3.1.4 Effect of Mg content on pore morphology
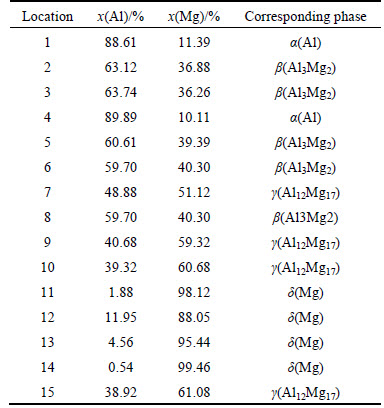
SEM in the back scattered electron (BSE) imaging mode was employed to evaluate the pore morphology, and the SEM micrographs for porous Al-Mg alloys with different Mg contents are given in Figs. 4(a)-(g). In these figures, some isotropic and large interconnected pores can be observed for the porous Al-Mg alloys, and the general pore size increases at first and then decreases with the increase of the Mg content, which means that the variation of porosity is consistent with the results shown in Fig. 3. The different contrast represents different chemical composition regions in the porous Al-Mg alloys, and they can be respectively seen from the inserted high-magnification images in Figs. 4(a)-(g). The material composition and the corresponding phase of the marked points in Figs. 4(a)-(g) are determined by the EDS analysis, and the results are summarized in Table 1. It can be seen that the phase determined by the EDS analysis is in good agreement with the XRD results shown in Fig. 2. Obviously, the Mg content of the sintered porous Al-Mg alloys not only dramatically affects the volume expansion and open porosity, but also has a great influence on the pore morphology.
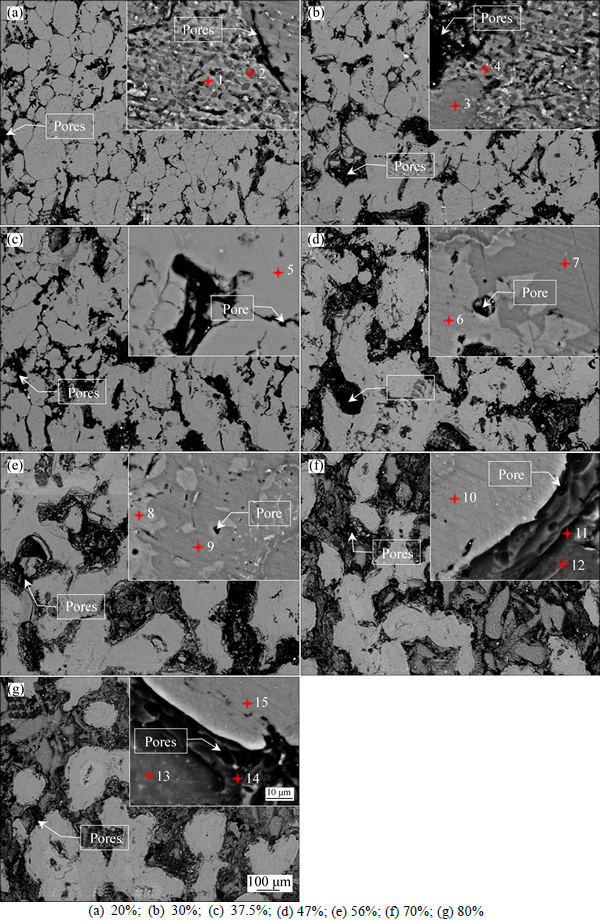
Fig. 4 SEM micrographs (BSE mode) of porous Al-Mg alloys with different Mg contents (Insets are magnification images of discs):
Table 1 EDS analysis results corresponding to marked positions in Fig. 4
3.2 Pore formation mechanism of porous Al-Mg alloys
A possible reaction process is proposed to explain the pore structure variation with Mg content. Since there is the low porosity for the green disc formed by pressing Al and Mg particles as shown in Fig. 3, the large number of pores in Fig. 4 should be formed during the sintering process. There are two possible phase transformation of Eqs. (1) and (2) in the sintering process for the porous Al-Mg alloys [17]. As can be seen from Eqs. (1) and (2), there are little volume constriction in the two phase transition process, which means that the pore formation due to the phase transformation is ignored.
For the Mg content larger than 56%, there will be only one phase transformation of Eq. (1) for porous Al-Mg alloys in the sintering process. During the phase transition process of Eq. (1), since the intrinsic diffusion rate of Al is much faster than that of Mg in the Al-Mg binary system [20], the net movement of Al element will be balanced by the opposite net vacancy flux to lead to the excessive vacancies. To reduce the Gibbs free energy of the system, the supersaturated vacancies are condensed into Kirkendall pores [21]. That is to say, with the faster diffusion of Al element, Kirkendall pores form at the site of Al elements as a result of aggregation and collapse of vacancies. Finally, the Kirkendall pores substitute the original site of the Al element with their dimension consistency until the completion of the diffusion reaction. Therefore, the pores formed in this phase transition process were generated from the exhaustion of the Al powders in the discs. For this reason, the porosity increases with increasing the Al content as the Mg content is higher than 56%.
For the Mg content less than 56%, all the two phase transformations of Eqs. (1) and (2) occur in the diffusion reaction process. With the decrease of Mg content, only part of Al powders can react with Mg because of the surplus Al powders, and the Kirkendall pores generated in the phase transition process of Eq. (1) are reduced accordingly. On the other hand, few Kirkendall pores could be formed in the phase transition process of Eq. (2), because there are no significant distinction between the diffusion rates of Al and Mg in γ(Al12Mg17) phase. It is confirmed in the literatures on Al-Mg diffusion couple [22-23]. Therefore, the porosity decreases with the decrease of the Mg content, when it is below 56%.
As a consequence, the maximum open porosity of porous Al-Mg alloys can be obtained at the composition of Al-56%Mg. The pore formation in porous Al-Mg alloys is mainly attributed to the inter-particle pores formed during the pressing procedure and the Kirkendall pores formed during the solid-state sintering process.
3.3 Effect of Mg content on electrochemical corrosion behaviors
3.3.1 Effect of Mg content on OCP
The open circuit potentials were recorded for the porous pure Al, pure Mg and Al-Mg alloys exposed to 3.5% NaCl aqueous solution, and the OCP versus time curves are given in Fig. 5. The variation trend of the OCP with time is similar for all the porous Al-Mg alloys. The open circuit potentials of all the discs shift toward more noble direction to achieve relatively stable values after immersion for 8 h, and these shifts are indications of the passivation of electrode surface. The highest OCP of the porous Al-Mg alloys in relatively stable situation exhibits for porous Al-37.5%Mg alloy, indicating that it is of the optimum corrosion resistance.
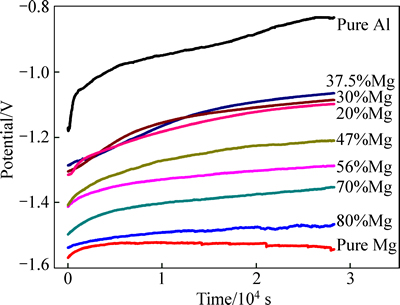
Fig. 5 OCP versus time curves of porous pure Al, pure Mg and Al-Mg alloys with different Mg contents measured in 3.5% NaCl aqueous solution
3.3.2 Effect of Mg content on EIS
The EIS results of the porous pure Al, pure Mg and Al-Mg alloys are presented in Fig. 6. Figure 6(a) shows the Nyquist plots and they reveal a capacitive arc at high and intermediate frequencies. The diameter of the arc with metal dissolution is dependent on the charge- transfer resistance, i.e. corrosion resistance in the corrosion process [24-25]. As shown in Fig. 6(a), the diameters of capacitive arcs of the porous Al-Mg alloys increase at first and then decrease with the increase of the Mg content. Except the porous pure Al, the porous Al-37.5%Mg alloy has a maximum diameter of capacitive arcs and corresponds to the best corrosion resistance. At low frequencies, the appearance of tails in Nyquist plots of porous Al-70%Mg, Al-80%Mg and pure Mg is attributed to the relaxation of adsorbed species, such as Mg(OH)ads+ or Mg(OH)2. This means that the inductive loop can be ascribed to the pit formation [26].
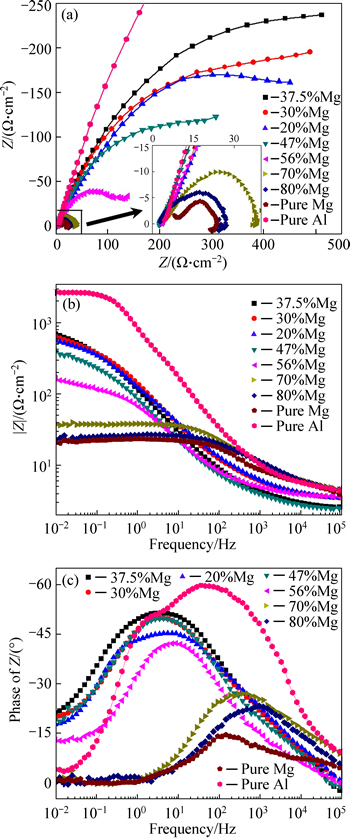
Fig. 6 Nyquist plots (a) and Bode plots (Frequency-|Z| (b) and frequency-phase plot (c)) of porous pure Al, pure Mg and Al-Mg alloys with different Mg contents in 3.5% NaCl aqueous solution
Figures 6(b) and (c) show the Bode plots which describe the modulus of impedance (|Z|) and phase angle (θ) versus frequency, respectively. The Bode plots depict three distinct regions for all porous Al-Mg, pure Al and pure Mg discs. At the high frequency region (of about 104 Hz), |Z| versus frequency is constant and associated with a phase angle which is close to 0°in Bode-phase plots, indicating that the impedance is dominated by the electrolyte resistance in this frequency range (resistive region). At intermediate frequency region, the Bode magnitude displays a linear slope of about -1, which indicates a capacitive behavior [27-28]. An interpretation concerning the nature of the double electric layer formation can be made between 100 Hz and 103 Hz [28]. At low frequency region between 10-2 Hz and 10-1 Hz, |Z| versus frequency represents the polarization resistance of the alloy discs, and the maximum value of |Z| with frequency is attained by porous pure Al, followed by porous Al-37.5%Mg, Al-30%Mg, Al-20%Mg, Al-47%Mg, Al-56%Mg, Al-70%Mg and Al-80%Mg. The lowest |Z| is associated with the porous pure Mg. These results are in good agreement with the aforementioned discussion based on the Nyquist plots shown in Fig. 6(a).
3.3.3 Effect of Mg content on potentiodynamic polarization results
The potentiodynamic polarization curves are presented in Fig. 7 for porous pure Al, pure Mg and Al-Mg alloys. Generally, the cathodic polarization curves are assumed to represent the cathodic hydrogen evolution through water reduction, while the anodic ones represent the dissolution of Al-Mg intermetallic. It is noted that all the cathodic parts of the curves clearly reveal the existence of a similar current slope, while it varies for the anodic ones. No defined passive region can be observed in the anodic region of porous pure Mg, Al-80%Mg and Al-70%Mg alloys. Porous Al-20%Mg, Al-30%Mg and Al-37.5%Mg alloys exhibit more obvious passive region than porous Al-56%Mg and Al-47%Mg alloys.
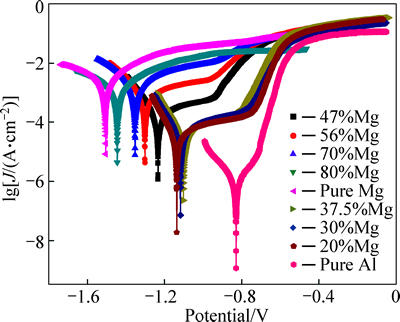
Fig. 7 Potentiodynamic polarization curves of porous pure Al, pure Mg and Al-Mg alloys with different Mg contents measured in 3.5% NaCl aqueous solution
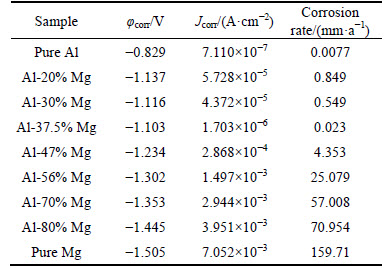
Three kinds of corrosion parameters can be determined from the potentiodynamic polarization curves and they are summarized in Table 2. It is obvious that the free corrosion potential (φcorr) can be read out directly from Fig. 7, and the porous Al-37.5%Mg alloy has a nobler φcorr than other porous Al-Mg alloys. In fact, the value of φcorr represents a thermodynamic characteristic of a given metal-electrolyte system, but not the kinetics of material corrosion [29]. The corrosion current density (Jcorr) value can more accurately reflect the corrosion rate than φcorr. Therefore, the increased Jcorr values of porous Al-Mg alloys correspond to the significantly higher anodic kinetics and lower cathode kinetics. The Jcorr values were determined by extrapolating the linear Tafel segments of the anode and cathode polarization curves, and the Jcorr values of porous Al-Mg alloys decrease at first and then increase with the increase of the Mg content. Except the porous pure Al, the corrosion rate (CR) calculated by Jcorr [30] reveals the lowest corrosion rate of the porous Al-37.5%Mg alloy. These results indicate that the Al-37.5%Mg alloy has the best corrosion resistance in all the porous Al-Mg alloys.
Table 2 Corrosion parameters obtained from potentiodynamic polarization curves
3.4 Effect factors of corrosion resistance
The corrosion resistance of porous Al-Mg alloys can be affected by the two factors: the pore structure and the phase composition. To understand the variation of corrosion resistance with the Mg content, it is necessary to study the relationships among the pore structure, the phase composition and the corrosion resistance, respectively.
In order to present the relationships among the pore structure, the phase composition and the corrosion resistance more intuitively, the variations of open porosity, phase composition and electrochemical parameters with Mg content are plotted together in Fig. 8 for the porous Al-Mg alloys. It is shown that the variations of open porosity and electrochemical parameters with the Mg content are inconsistent. In fact, with the change of open porosity, the pore size, pore shape and the smoothness of pores’ inner walls change correspondingly for the porous Al-Mg alloys [31]. It is difficult to provide a complete characterization of the pore structure by solely measuring the open porosity. For the discs with a lower open porosity, the majority of the pores are the isolated small pores (see Figs. 4(a), (b), (f) and (g)), which is unlikely to have trapped appreciable volumes of solution. With the open porosity increases, the interconnectivity of the pores is markedly improved and the pore size increases remarkably (Figs. 4(c), (d) and (e)). The liquid can flow freely in the large and interconnected pores. Moreover, the real surface area of the porous Al-Mg alloys generally increases with increasing the open porosity. Therefore, the pore structure of the porous Al-Mg alloy with higher open porosity would be more prone to induce localized corrosion, and the corrosion resistance of the porous Al-Mg alloys would be worse with the open porosity increasing, but that didn’t happen. This indicates that the pore structure is not a crucial factor of influencing the corrosion resistance of porous Al-Mg alloys. On the other hand, recent researches [32-33] show that the α(Al) phase is of the best corrosion resistance in the four typical phases of the Al-Mg alloys, followed by β(Al3Mg2) phase, γ(Al12Mg17) phase and δ(Mg) phase. Considering the phase transformation behaviors with different Mg contents, the corrosion resistance of the porous Al-Mg alloys should get worse with increasing the Mg content. It is in good agreement with the corrosion resistance of porous Al-Mg alloys in Fig. 8 as the Mg content is higher than 37.5%. Nevertheless, when the Mg content increases from 20% to 37.5%, the corrosion resistance of the porous Al-Mg alloys gets better instead and the porous Al-Mg alloy with single β(Al3Mg2) phase shows the best corrosion resistance. This may be because the galvanic corrosion [34] occurs between the two phases α(Al) and β(Al3Mg2) of the porous Al-Mg alloys. Furthermore, as presented in Table 2, the φcorr of porous Al-37.5%Mg and Al-56%Mg alloy are -1103 mV (vs SCE) and -1302 mV (vs SCE), respectively, which are nearly consistent with the φcorr of β(Al3Mg2) (-1150 mV (vs SCE)) and γ(Al12Mg17) phase (-1350 mV (vs SCE)) reported by LUNDER et al [32] and BIRBILIS and BUCHHEIT [33]. Thus, the phase composition plays an important role in improving the corrosion resistance of porous Al-Mg alloys. As a consequence, the key factor in determining the corrosion resistance of porous Al-Mg alloys is not the pore structure but the phase composition.
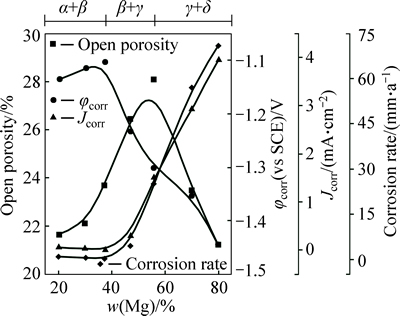
Fig. 8 Evolutions of open porosity, phase composition and extracted electrochemical parameters from polarization curves with different Mg contents
4 Conclusions
Porous Al-Mg alloys with nominal compositions ranging from Al-20%Mg to Al-80%Mg were successfully fabricated by elemental powder reactive synthesis using Al and Mg powders free of any pore-forming agents. The sintered porous Al-Mg alloys exhibit four typical phases: α(Al), β(Al3Mg2), γ(Al12Mg17) and δ(Mg), and the volume expansion ratio, open porosity and corrosion resistance increase at first and then decrease with the increase of the Mg content. The porous Al-56%Mg alloy is of the maximum volume expansion ratio and the open porosity value of 18.3% and 28.1%, respectively, while the porous Al-37.5%Mg alloy exhibits the best corrosion resistance. The evolution of the pore structure for porous Al-Mg alloys with different Mg contents is in accordance with the pore formation mechanism caused by the Kirkendall effect, and the variation of the corrosion resistance is mainly due to the change of the phase composition. One of the advantages of these porous Al-Mg alloys lies in the fact that they can be fabricated by a simple and cost effective way with controllable pore structure parameters in large scale. The promising pore structure and good corrosion resistance of these porous Al-Mg alloys are sufficient for the filtration application in the chloride environment.
References
[1] CHENG Jun, CHENG Si, CAO Xiao-fan. Application of PFSC in seawater desalination retreatment [J]. Advanced Materials Research, 2014, 945: 3510-3513.
[2] CHENG Jun, ZHANG Jing, YU Xiang. Application of new complex flocculant in seawater desalination pretreatment [J]. Applied Mechanics and Materials, 2014, 556: 57-59.
[3] WANG Xin-min, ZHAO Bin, ZHANG Qin-li, XU Dong-sheng. Cemented backfilling technology with unclassified tailings based on vertical sand silo [J]. Journal of Central South University of Technology,2008, 15: 801-807.
[4] VOUTCHKOV N. Considerations for selection of seawater filtration pretreatment system [J]. Desalination, 2010, 261(3): 354-364.
[5] XU Pei, DREWES J E, HEIL D. Beneficial use of co-produced water through membrane treatment: Technical-economic assessment [J]. Desalination, 2008, 225(1): 139-155.
[6] CHERYAN M. Ultrafiltration and microfiltration handbook [M]. Lancaster, USA: CRC Press, 1998: 3.
[7] HSIEH H P. Inorganic membranes for separation and reaction [M]. New York, USA: Elsevier, 1996: 263.
[8] LEFEBVRE L P, BANHART J, DUNAND D. Porous metals and metallic foams: Current status and recent developments [J]. Advanced Engineering Materials, 2008, 10(9): 775-787.
[9] GAO Hai-yan, HE Yue-hui, ZOU Jin, XU Nan-ping, LIU C T. Tortuosity factor for porous FeAl intermetallics fabricated by reactive synthesis [J].Transactions of Nonferrous Metals Society of China, 2012, 22(9): 2179-2183.
[10] HIRSCH J, AL-SAMMAN T. Superior light metals by texture engineering: Optimized aluminum and magnesium alloys for automotive applications [J]. Acta Materialia, 2013, 61(3): 818-843.
[11] KULEKCI M K. Magnesium and its alloys applications in automotive industry [J]. The International Journal of Advanced Manufacturing Technology, 2008, 39(9/10): 851-865.
[12] HE Mei-feng, LIU Lei, WU Ya-ting, ZHONG Cheng, HU Wen-bin. Influence of microstructure on corrosion properties of multilayer Mg-Al intermetallic compound coating [J]. Corrosion Science, 2011, 53(4): 1312-1321.
[13] WU Chao-yun, ZHANG Jin. State-of-art on corrosion and protection of magnesium alloys based on patent literatures [J]. Transactions of Nonferrous Metals Society of China,2011, 21(4): 892-902.
[14] HERNANDEZ A, CALVO J I, PRADANOS P, TEJERINA F. Pore size distributions in microporous membranes. A critical analysis of the bubble point extended method [J]. Journal of Membrane Science, 1996, 112(1): 1-12.
[15] JIANG Yao, HE Yue-hui, XU Nan-ping, ZOU Jin, HUANG Bai-yun, LIU C T. Effects of the Al content on pore structures of porous Ti-Al alloys [J]. Intermetallics, 2008, 16(10): 327-332.
[16] GAO Hai-yan, HE Yue-hui, SHEN Pei-zhi, ZOU Jin, XU Nan-ping, JIANG Yao, HUANG Bai-yun, LIU C T. Porous FeAl intermetallics fabricated by elemental powder reactive synthesis [J]. Intermetallics, 2009, 17(12): 1041-1046.
[17] JIE J C, WANG H W, ZOU C M, WEI Z J, LI T J. Precipitation in Al-Mg solid solution prepared by solidification under high pressure [J]. Materials Characterization, 2014, 87: 19-26.
[18] DONG Hong-xing, HE Yue-hui, JIANG Yao, WU Liang, ZOU Jin, XU Nan-ping, HUANG Bai-yun, LIU C T. Effect of Al content on porous Ni–Al alloys [J]. Materials Science and Engineering A, 2011, 528(13): 4849-4855.
[19] MASSALSKI T B, OKAMOTO H, SUBRAMANIAN P, KACPRZAK L. Binary alloys phase diagrams [M]. Vol II. Materials Park, Ohio, ASM International, 2001: 867.
[20] FUNAMIZU Y, WATANABE K. Interdiffusion in the Al-Mg system [J]. Transactions of the Japan Institute of Metals, 1972, 13(4): 278-283.
[21] SPRINGER H, KOSTKA A, DOS SANTOS J F, RAABE D. Influence of intermetallic phases and Kirkendall-porosity on the mechanical properties of joints between steel and aluminium alloys [J]. Materials Science and Engineering A, 2011, 528(13): 4630- 4642.
[22] BRENNAN S, BERMUDEZ K, KULKARNI N S, SOHN Y. Interdiffusion in the Mg-Al system and intrinsic diffusion in β-Mg2Al3 [J]. Metallurgical and Materials Transactions A, 2012, 43(11): 4043-4052.
[23] KULKARNI K N, LUO A A. Interdiffusion and phase growth kinetics in magnesium-aluminum binary system [J]. Journal of Phase Equilibria and Diffusion, 2013, 34(2): 104-115.
[24] OSORIO W R, FREITAS E S, GARCIA A. EIS and potentiodynamic polarization studies on immiscible monotectic Al-In alloys [J]. Electrochimica Acta, 2013, 102: 436-445.
[25] LIU Jian-hua, ZHAN Zhong-wei, LI Song-mei, YU Mei. Corrosion resistance of waterborne epoxy coating pigmented by nano-sized aluminium powder on steel [J]. Journal of Central South University, 2012, 19: 46-54.
[26] BRETT C M A, DIAS L, TRINDADE B, FISCHER R, MIES S. Characterisation by EIS of ternary Mg alloys synthesised by mechanical alloying [J]. Electrochimica Acta, 2006, 51(8): 1752- 1760.
[27] LIAO Cui-jiao, HE Yue-hui, YANG Jun-sheng, NAN Bo, LIU Xin-li. Effect of carburization on electrochemical corrosion behaviours of TiAl alloy [J]. Materials Science and Engineering B, 2013, 178(7): 449-456.
[28] MANSFELD F, KENDIG M W. Evaluation of anodized aluminum surfaces with electrochemical impedance spectroscopy [J]. Journal of the Electrochemical Society, 1988, 135(4): 828-833.
[29] MORENO J M C, VASILESCU E, DROB P, OSICEANU P, VASILESCU C, DROB S L, POPA M. Surface analysis and electrochemical behavior of Ti-20Zr alloy in simulated physiological fluids [J]. Materials Science and Engineering B, 2013, 178(18): 1195-1204.
[30] HANDBOOK A S M. Corrosion [M]. Vol. 13. Materials Park, OH, ASM International, 1987: 893-902.
[31] SUN X T, KANG Z X, ZHANG X L, JIANG H J, GUAN R F, ZHANG X P. A comparative study on the corrosion behavior of porous and dense NiTi shape memory alloys in NaCl solution [J]. Electrochimica Acta, 2011, 56(18): 6389-6396.
[32] LUNDER O, LEIN J E, AUNE T K, NISANCIOGLU K. The role of Mg17Al12 phase in the corrosion of Mg alloy AZ91 [J]. Corrosion, 1989, 45(9): 741-748.
[33] BIRBILIS N, BUCHHEIT R G. Electrochemical characteristics of intermetallic phases in aluminum alloys an experimental survey and discussion [J]. Journal of the Electrochemical Society, 2005, 152(4): B140-B151.
[34] MANSFELD F, KENKEL J V. Galvanic corrosion of Al alloys-III. The effect of area ratio [J]. Corrosion Science, 1975, 15(4): 239-250.
(Edited by YANG Bing)
Foundation item: Project(IRT_14R48) supported by the Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China; Projects(51271158, 51272158, 51401175, 51504213) supported by the National Natural Science Foundation of China; Project([2009]17) supported by the Changjiang Scholar Incentive Program, China; Project(CX2015B224) supported by the Hunan Provincial Innovation Foundation for Postgraduate, China; Project(2015WK3021) supported by the Hunan Provincial Key Research Program, ChinaReceived date: 2016-01-04; Accepted date: 2016-05-11
Corresponding author: XIAO Yi-feng, Professor, PhD; Tel: +86-731-58292214; E-mail: sanyxyf@163.com; ZHENG Xue-jun, Professor, PhD; Tel: +86- 731-58293210; E-mail:zhengxuejun@xtu.edu.cn
Abstract: Porous Al-Mg alloys with different nominal compositions were successfully fabricated via elemental powder reactive synthesis, and the phase composition, pore structure, and corrosion resistance were characterized with X-ray diffractometer, scanning electron microscope and electrochemical analyzer. The volume expansion ratio, open porosity and corrosion resistance in 3.5% (mass fraction) NaCl aqueous solution of the alloys increase at first and then decrease with the increase of Mg content. The maxima of volume expansion ratio and open porosity are 18.3% and 28.1% for the porous Al-56%Mg (mass fraction) alloy, while there is the best corrosion resistance for the porous Al-37.5% Mg (mass fraction) alloy. The pore formation mechanism can be explained by Kirkendall effect, and the corrosion resistance can be mainly affected by the phase composition for the porous Al-Mg alloys. They would be of the potential application for filtration in the chloride environment.


