J. Cent. South Univ. Technol. (2007)05-0623-06
DOI: 10.1007/s11771-007-0119-8 ![]()
Preparation of jarosite by Acidithiobacillus ferrooxidans oxidation
LIU Jian-she(柳建设), LI Bang-mei(李邦梅), ZHONG De-yi(钟得意),
XIA Le-xian(夏乐先), QIU Guan-zhou(邱冠周)
(School of Mineral Processing and Bioengineering, Central South University, Changsha 410083, China)
Abstract:
The formation of jarosite in the presence of Acidithiobacillus ferrooxidans (A. ferrooxidans) was researched to ascertain the conditions of producing minimum precipitation. The effects of salt concentration and pH on the characteristics of jarosite formed in K2SO4/(NH4)2SO4-FeSO4 inorganic salt solution and 9K medium were studied by using the measurements of scanning electron microscope, X-ray diffraction, Fourierism transform infrared analysis, thermogravity/differential thermogravity analysis and particle size analysis to evaluate the product. The results indicate that the formation of jarosite begins when A. ferrooxidans reaches logarithmic growth phase in 9K medium, and a higher pH value is beneficial to the formation of jarosite. The jarosite formed in 9K medium has smaller and more concentrative particle size and smoother surface than that formed in inorganic salt solution.
Key words:
jarosite; A. ferrooxidans; inorganic salt solution; 9K medium; characteristic ;
1 IntroductionJarosite [KFe3(SO4)2(OH)6] is a member of the isostructural jarosite-alunite group of minerals that are based upon the formula Mn(Fe3+)6(SO4)4(OH)12, where M may be K, NH4, Na, Ag or Pb, and n=2 for monovalent cation and n=1 for the divalent cation[1]. Jarosite occurs commonly in the oxidized portions of sulphide ore deposits, fluvial environments contaminated by acid rock or acid mine drainage (ARD, AMD), wastes produced from the metallurgical extractive industry, acid sulphate soils and clay seams and beds. Jarosite is of considerable interest in geological, environmental, and metallurgical fields in recent years, because it sorbs and co-precipitates many kinds of potentially toxic elements such as As and Pd[2]. In our country, the study on jarosite is mainly focused on its use of enriching heavy metal irons in metallurgy industry. Using small additions of Portland cement or lime, the compress strength and water resistance of jarosite materials can be increased due to that the amorphous gel and hydrate form in the crystal structure. These materials can be used in general civil engineering applications as bases or sub-bases of roads, airfields and dams, replacing natural crushed stones, gravel, sand, and so on, and in the production of bricks, tiles and other products[3-5]. The jarosite produced by bacteria is likely to possess more uniform and perfect crystals than the natural ones due to the effect of the extracellular polymers of microbes, and therefore has a more promising application in the material field.
In order to review the influences of jarosite on the growth of A. ferrooxidans and the oxidation of FeSO4, in this work the amount and characteristics of jarosite formed under different conditions, i.e. pH, temperature and concentrations of inorganic salts were investigated.
2 Materials and methodsA. ferrooxidans YTW, isolated from an acid mine drainage in Hubei Province, was used in this work. Bacteria were cultured in 9K medium developed by SILVERMAN et al[6]. All the cultures in our experiments were carried out in 500 mL flasks with shaking at 180 r/min and 30 ℃. When Fe2+ was exhausted, the precipi- tation was collected by filtering the cultures. The precipitates were washed five times with 5 mmol/L H2SO4 to eliminate the attached bacteria and other impurities and dried at 65 ℃ for 10 h[7].
X-ray diffraction (XRD) patterns of the precipitations were recorded by D/Max 2500 X-ray diffractometer(Rigaku Cor, Japan) using Cu Kα at 45 kV, 250 mA and 2θ=5?-8? scanning range, 8(?)/min scanning rate. SEM images were obtained by KYKY2800 scanning electron microscope (China). TG/DTA analysis was performed in a WCT-2A differential thermal balance (China) from 30 to 900 ℃ at 10 ℃/min. The particle sizes of precipitations were tested with Mastersizer 2000 Ver.3.01(Malvern Co., UK). Bacterial population was counted by the blood platelet counter technique and observed with OLYMPUS CX31 microscope(Olympus Co., Japan). The pH value was adjusted with vitriol and monitored by a pH meter (PHS-3C, China). The concentration of ferrous iron was determined with the potassium dichromate titration method.
3 Results and discussion
3.1 Formation process of precipitation in 9K mediumAt first, formation process of precipitation with A.ferrooxidans at pH 2.00 in 9K medium was tested. The initial bacterial population was about 1.3×106/mL. The changes of concentration of Fe3+ and bacterial population, and the changes of precipitation mass and pH values are shown in Figs.1 and 2, respectively.
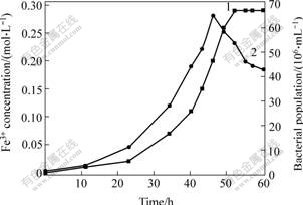
Fig.1 Production of Fe3+ and bacterial population vs. time at pH 2.00
1—Bacterial population; 2—Production of Fe3+
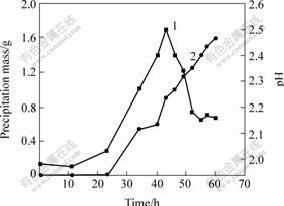
Fig.2 Precipitation mass and pH vs cultivation time
1—Precipitation mass; 2—pH
It can be seen that the increase of precipitation has the same trend with the produce rate of Fe3+ and the increase of bacterial population. The precipitation is gained at the logarithmic growth phase of A. ferrooxidans. Appropriate Fe3+ concentration and pH
value are the formation qualifications of precipitation. And from the results we can obtain more bacteria with less precipitation when growth for 40-46 h.
3.2.1 K2SO4/(NH4)2SO4-FeSO4 inorganic salts solutions
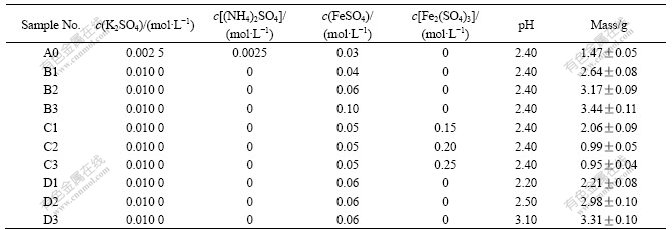
A. ferrooxidans. strain YTW cells were obtained from the late logarithmic growth phase and filtered to remove deposition, centrifuged at 10 000 r/min for 10 min, washed twice with sterilized water (adjusted pH to 2.0 by H2SO4) and suspended in different inorganic solutions. The initial bacterial population in solution was about 1×108/mL.
The comparative experiments results are shown in Table 1. From groups B and C, it can be seen that in K2SO4-FeSO4 solution with bacteria in suspension the amount of the initial Fe2+ advances the produce of precipitation while Fe3+ restrains it. And the reason perhaps is the initial Fe3+ restrains the oxidation of Fe2+. In group D, the rise of pH causes the increase of the precipitation at pH 2.20-3.10. The precipitation formed in group A was compared to that in 9K medium.
Table 1 Precipitation of jarosite in different salt solutions
A. ferrooxidans strain YTW inoculants were also obtained from the late logarithmic growth phase and filtered to remove deposition, washed twice with sterilized water (adjusted pH to 2.0 by H2SO4) and cultured in 9K medium under different conditions (Table 2). The initial bacterial population was about 1×107/ mL.
Table 2 Masses of precipitation produced in different 9K media
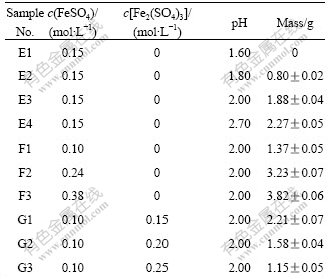
From group E in Table 2, it can be seen that in 9K media the rise of pH also causes the increase of the precipitation at pH value of 1.60-2.70, and there is no visible precipitation when the pH is 1.60. And groups F and G show the same trend with groups B and C in Table 1, that the initial Fe2+ advances the produce of precipitation while Fe3+ restrains it.
3.3 Structure and composition of precipitateThe composition and structure of these typical precipitate B2 and F2 were analyzed by X-ray diffraction, scanning electron microscopy, Fourier transform infrared spectroscopy, thermal analysis and particle size analysis.
3.3.1 Scanning electrical microscope observation
It is observed that there are many differences among the surface appearances of these three samples shown in Figs.3 and 4. The surface of F2 is obviously smoother than that of B2, although they are all comprised of densely hexagonal lozenges aggregates. The most possible reason is that the growth of A.ferrooxidans promotes the oxidation of Fe2+ to Fe3+ and the supply rate of Fe3+ influences the crystal modality of jarosite[8].
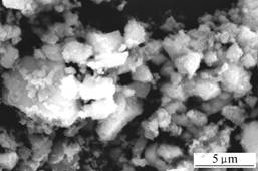
Fig.3 SEM image of sample B2
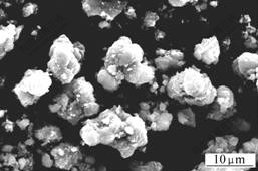
Fig.4 SEM image of sample F2
The particle size of F2 is about 10 μm, congregated by several units. This is due to the cellular polysaccharides of A.ferrooxidans enhancing the aggregation of jarosite.
3.3.2 X-ray diffraction analysis
The XRD patterns of samples are shown in Figs.5 and 6. The unit cell parameters were calculated by least square refinement based on the location and intensity of the peaks (Table 3).
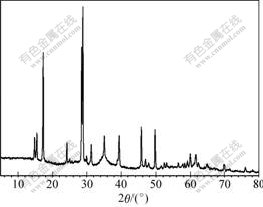
Fig.5 XRD pattern of sample B2
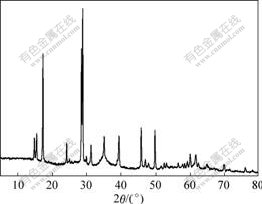
Fig.6 XRD pattern of sample F2
Table 3 Unit cell parameters of jarosite in experiment
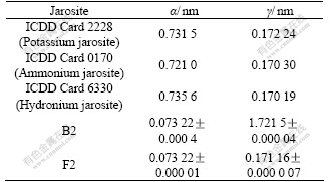
XRD patterns indicate that the precipitations of B2 and F2 are potassium jarosite, and no other crystalline phases in these samples are present at detectable levels.
The unit parameters of jarosite in our experiment and ICDD Card 2228 (potassium jarosite), ICDD Card 0170(ammonium jarosite) and ICDD Card 6330 (hydronium jarosite) are shown in Table 3.
The unit cell parameters “α” and “γ” of these three samples are all intermediate among those reported for potassium jarosite, ammonium jarosite and hydronium jarosite. That also indicates that a part of K+ is possibly substituted by H3O+ in the process of formation. Numerous investigators have demonstrated that substitutions occur among jarosite in the univalent cation site, especially between K+, Na+, NH4+ and H3O+[9-10]. So, the concentration of other ions must be strictly controlled to avoid impurities in the crystal.
3.3.3 Fourier transform infrared (FTIR) spectrometry
The FTIR spectra of the samples are shown in Figs.7 and 8, in which the main vibration bands are marked.
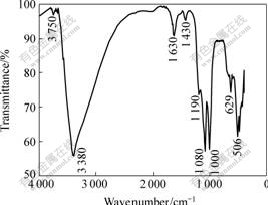
Fig.7 FTIR spectrum of sample B2
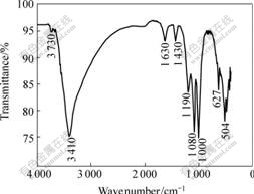
Fig.8 FTIR spectrum of sample F2
The FTIR spectra of these three jarosite samples formed in different solutions are basically in accordance with that of standard jarosite reported by POWERS et al[11]. The intense absorption observed in the region from 3 380 to 341 cm-1 can be attributed to O—H stretching. The bands observed at 1 630 cm-1 are attributed to H—O—H deformation. Three absorption bands at 1 190 and 1 080 cm-1 are due to the υ3 (doublet) vibrations of SO42-[12]. Several absorptions were also observed in the region from 400 to 1 000 cm-1. The absorption at 627 and 629 cm-1 can be attributed to the υ4 vibration mode of SO42-, which could be distinguishable by IR due to the decrease in symmetry of the sulfate species in the jarosite structure. Several researchers suggested that the band observed near 1 008 cm-1 is due to O—H deformations rather than υ1 (SO42-). Note that the vibrations υ1 (SO42-) and υ2 (SO42-) could not be seen here, most probably due to the overlapping with other nearby intense absorptions[11-13]. The bands observed near 504 cm-1 are attributed to vibrations of FeO6 coordination octahedra, the band near 1 430 cm-1 is the adsorption peak of NH4+, which suggests that A. ferrooxidan could fix nitrogen by itself [14].
The thermal decomposition of the samples B2 and F2 are shown in Figs.9 and 10, separately.
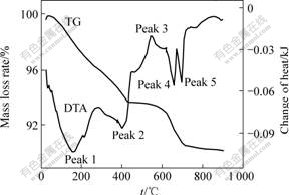
Fig.9 TG/DTA curves of sample B2

Fig.10 TG/DTA curves of sample F2
Although the main peaks of TG/DTA curves of samples B2 and F2 are almost the same, there are some differences, the curves of B2 is more complex.
The first endothermic peak (labeled peak 1 in Figs.9 and 10.) was observed at 160 ℃. It is attributed to the loss of H2O molecules referred to as “adsorbed” water. The loss from 30 to 260 ℃ of B2 and F2 are 3.55% and 3.54%, respectively.
The peak 2 was observed at 400 ℃(labeled peak 2 in Figs.9 and 10). By the analysis of XRD of the decomposition of potassium jarosite in this temperature range, some investigators found KFe(SO4)2 and hematite (Fe2O3). So, the reaction can be summarized as follows:
KFe3(SO4)2(OH)6→KFe(SO4)2+Fe2O3+H2O (1)
There is an exothermic peak near 550 ℃(labeled peak 3 in Figs.9 and 10), possibly caused by the phase transformation of hematite[15].
FTIR analysis shows the presence of SO2 (produced by decomposition of SO3) in the gas phase from 550 to 700 ℃[16-18]. So, SO3 loss accounts for the endothermic peaks at these temperatures. The losses of B2 and F2 in this temperature range are 2.78% and 2.72%, respectively. The reaction can be conferred as:
KFe(SO4)2 →Fe2O3+K2SO4+SO3(g) (2)
The particle size of samples B2 and F2 is shown in Fig.11.
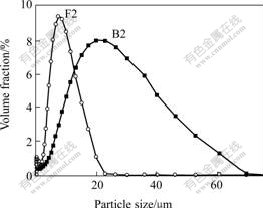
Fig.11 Particle size distribution of samples B2 and F2
The d0.5 size of B2 and F2 is 19.38 and 7.42 μm, respectively, and the d0.9 size is 41.95 and 14.43 μm, respectively. The particle size of F2 is smaller and more concentrative than that of B2. The result is consistent with SEM that of, and also proves that the jarosite formed in 9K medium is more excellent in material characteristics than in inorganic salt solution.
4 Conclusions
1) The precipitates formed in both 9K media and K2SO4/(NH4)2SO4-FeSO4 inorganic salt solutions are potassium jarosite or the mixtures of potassium jarosite and ammonium jarosite. The unit parameters of the precipitations are intermediate among the reported ones of potassium jarosite, ammonium jarosite and hydronium jarosite, and a part of K+ are substituted by H3O+.
2) The stretching,deformation and vibration bands of O—H, H—O—H and υ3, υ4 of SO42- and FeO6 coordination octahedral, accord with that reported in literatures, the adsorption peak of NH4+ possibly suggests the nitrogen fixation of A.ferrooxidan.
3) In 9K medium the precipitation is gained when A.ferrooxidans reaches logarithmic growth phase. There is no visible precipitation when pH is more below than some degree. The initial Fe2+ advances the produce of precipitation while Fe3+ restrains it in both 9K media and K2SO4/(NH4)2SO4-FeSO4 inorganic salt solutions. The higher the pH value, the more the precipitation formed.
4) The jarosite decomposes into KFe(SO4)2 and Fe2O3 and let “adsorbed” water out at 30-500 ℃. KFe(SO4)2 decomposes into Fe2O3 and K2SO4, and emits SO3 gas at 550- 700 ℃. The phase transformation of hematite near 550 ℃ performs an exothermic peak in the curves.
5) The jarosite formed in 9K medium has a smaller and more homogeneous size and smoother surface than that formed in salt solution, and is likely more excellent in material characteristics.
References
[1] FROST R L, RACHAEL-ANNE W. A Raman spectroscopic study of selected natural jarosites[J]. Sectrochimica Acta Part A, 2006, 63(1): 1-8.
[2] SMITH A M L, HUDSON-EDWARDS K A. Dissolution of jarosite [KFe3(SO4)2(OH)6] at pH 2 and 8: Insights from batch experiments and computational modeling[J]. Geochimica et Cosmochimica Acta, 2006, 70(3): 608-621.
[3] VS_EVOLOD A M, HAROLDO A P, PATRICIO R I. Potential application of acid jarosite wastes as the main component of construction materials[J]. Construction and Building Materials, 2005, 19(1): 141-146.
[4] ASOKAN P, SAXENA M, SHYAM R A. Hazardous jarosite use in developing non-hazardous product for engineering application[J]. Journal of Hazardous Materials, 2006, 137(3): 1589–1599.
[5] TSAKIRIDISA P E, AGATZINI-LEONARDOUA S, OUSTADAKISA P, et al. Examination of the jarosite–alunite precipitate addition in the raw meal for the production of Portland cement clinker[J]. Cement and Concrete Research, 2005, 35(11): 2066-2073.
[6] SILVERMAN M P, LUNDGREN D G. Studies on the chemoautotrophic iron bacterium Thiobacillus ferrooxidans. I: An improved medium and a havesting procedure for securing high xellun yields[J]. J Bacterial, 1959, 77(5): 642-647.
[7] WANG Hong-mei, BIGHAM J M. Formation of schwertmannite and its transformation to jarosite in the presence of acidophilic iron-oxidizing microorganisms[J]. Materials Engineering, 2006, 26(10): 588-592.
[8] SASAKI K, KONNO H. Morphology of jarosite-compounds precipitated from biologically and chemically oxidized Fe ions[J]. The Canadian Mineralogist, 2000, 38(1): 45-56.
[9] KUBISZ J. Studies on synthetic alkali-hydronium jarosites. I: Synthesis of jarosite and natrojarosite[J]. Mineralogia Polonicam 1970, 1(1): 47–57.
[10] RIPMEESTER J A, RATCLIFFE C I, DUTRIZAC J E, et al. Hydronium in the alunite-jarosite group[J]. The Canadian Mineralogist, 1986, 24: 773–784.
[11] POWERS D A, ROSSMAN G R, SCHUGAR H J, et al. Magnetic behavior and infrared spectra of jarosite, basic iron sulfate and their chromate analogs[J]. J Sol Stat Chem, 1975, 13(1/2): 1–13.
[12] ARKHIPENKO D K, DEVIATKINA E T, PALCHIK H A. Kristallokhimicheskiye osobennosti sinteticheskikh yarozitov [J]. Trudy Instituta Geologii I Geofiziki, 1987, 653: 31–70. (in Russian)
[13] DROUET C, NAVROTSKY A. Synthesis, characterization, and thermochemistry of K-Na-H3O jarosites[J]. Geochimica et Cosmochimica Acta, 2003, 67(11): 2063-2076.
[14] V?CTOR P, MERCEDES M P. Nitrogen fixation in acidophile iron-oxidizing bacteria: The nif regulon of leptospirillum ferrooxidans[J]. Research in Microbiology, 2004, 155(9): 703–709.
[15] YAN Xin. Preparation by solid phase reaction and characterization of iron oxide nanometer particle[J]. Journal of Yancheng Institute of Technology: Natural Science, 2002, 15(4): 24-26. (in Chinese)
[16] ASOKAN P, MOHINI S. Jarosite characteristics and its utilization potentials[J]. Science of the Total Environment, 2006, 359(1/3): 232-243.
[17] QIU Guan-zhou, LIU Jian-she, WANG Dian-zuo, et al. Iron behaviour in growth of thiobacillus ferrooxidans[J]. Journal of Central South University of Technology: Natural Science, 1998, 26(1): 226-228.(in Chinsese)
[18] YANG Yu, PENG Hong, QIU Guan-zhou, et al. Stochastic simulation of growth curves of Acidithiobacillus ferrooxidant[J]. Journal of Central South University of Technology, 2006, 13(5): 473-476.
Foundation item: Projects(50321402; 50374075) supported by the National Natural Science Foundation of China; project(2004CB619204) supported by the
National Key Fundamental Research and Development Program of China
Received date: 2007-01-25; Accepted date: 2007-03-28
Corresponding author: LIU Jian-she, Professor; Tel: +86-731-8830546; E-mail: ljscsu@263.net
(Edited by YANG Hua)
Abstract: The formation of jarosite in the presence of Acidithiobacillus ferrooxidans (A. ferrooxidans) was researched to ascertain the conditions of producing minimum precipitation. The effects of salt concentration and pH on the characteristics of jarosite formed in K2SO4/(NH4)2SO4-FeSO4 inorganic salt solution and 9K medium were studied by using the measurements of scanning electron microscope, X-ray diffraction, Fourierism transform infrared analysis, thermogravity/differential thermogravity analysis and particle size analysis to evaluate the product. The results indicate that the formation of jarosite begins when A. ferrooxidans reaches logarithmic growth phase in 9K medium, and a higher pH value is beneficial to the formation of jarosite. The jarosite formed in 9K medium has smaller and more concentrative particle size and smoother surface than that formed in inorganic salt solution.


