Electrochemical flotation of ethyl xanthate-pyrrhotite system
ZHANG Qin(张 芹), HU Yue-hua(胡岳华), GU Guo-hua(顾帼华), NIE Zhen-yuan(聂珍缓)
(School of Resources Processing and Bio-engineering, Central South University,Changsha 410083, China)
Abstract:
The flotation behavior of pyrrhotite was investigated by using ethyl xanthate as a collector. The results show that pyrrhotite has good floatability from pH 2 to 11, and poor flotability when pH>12. The reagents adjusting potential are ammonium persulfate ((NH4)2S2O8) and sodium dithionite (Na2S2O4). The flotation of pyrrhotite is dependent on pulp potential at certain pH value. The potential-pH range for pyrrhotite flotation was established. Cyclic voltammetry and FTIR spectroscopy analysis show that the major adsorption product of ethyl xanthate on pyrrhotite is dixanthogen. The intensity of FTIR signals of dixanthogen adsorbed on pyrrhotite and the anode current of a pyrrhotite electrode and flotation response of pyrrhotite are correlated with pulp potentials.
Key words:
ethyl xanthate; pyrrhotite; electrochemical flotation; FTIR spectroscopy; cyclic voltammetry CLC number: TD952;
Document code: A
1 INTRODUCTION
Pyrrhotite(Fe1-xS, 0〈x〈0.125) is an iron sulfide mineral widely encountered in base-metal sulfide ores. Because of its changeable stoichiometries and crystal structure (i.e. x is different), pyrrhotite exhibits a variety of flotation characteristics which depend upon the conditions of oxidation, pH and pulp potentials. Woods and his co-workers[1-4] studied the reaction and the production of the surface of pyrrhotite by XPS, linear potential sweep voltammetry and chemical analysis. Their results showed that the pyrrhotite surface(as fracture faces) oxidize immediately when exposed to ambient air to form an overlayer of iron(Ⅲ) hydroxide (or hydrated oxide) covering an iron-deficient sulfide lattice, the metal content of which decreases with increasing exposure(i.e. oxidation) time. This air oxidation mechanism is similar to that found in XPS studies of galena(PbS)[5], pyrite(FeS)[6] and chalcopyrite(CuFeS2)[7]. Heyes and Trahar[8]reported that at acidic pH values, good flotation can be achieved without collector addition. Cheng et al[9] reported the cathodic decomposition behavior of pyrrhotite in deoxygenated solution, showing that pyrrhotite did not exhibit significant collectorless flotation, and when ethyl xanthate was used as collector, the floatability of pyrrhotite decreased with cathodic treatments, the more negative the potentials applied and the longer the time used for polarization, the lower the recoveries were. The relation of pulp potential and flotation recovery was offered. The electrochemical study of the surface reactions that occur during the flotation of nickeliferous pyrrhotite in the recovery of nickel and the platinum groupmetals was made by Buswell and Nicol[10]. Their results showed that the formation of dixanthogen on pyrrhotite surface was thermodynamically favourable in plant flotation slurries. Leppinen and Yoon[11] studied the electrochemical reactions involving ethyl xanthate and chalcocite, chalcopyrite, pyrite and galena in situ using an electrochemical ATR cell. They investigated the reaction productions of minerals surface, and found that the IR signal intensity which is a measure of xanthate adsorption and flotation recovery response of minerals is correlated with pule potentials. In this paper, the flotation behavior of pyrrhotite is investigated by using ethyl xanthate as a collector. The interaction mechanism for the pyrrhotite and the ethyl xanthate is studied using cyclic voltammetric measurement. Reaction productions on the pyrrhotite surfaces are examined by FTIR reflection spectra analysis.
2 EXPERIMENTAL
2.1 Materials
The pyrrhotite of FeS1.13 used in this investigation was from Dachang No.100 ore body, Guangxi Province. Mineral lumps were crushed and pyrrhotite grains of several millimeters in diameter were handpicked. The picked minerals were ground in a ceramic ball mill and sieved to 〈0.1mm and stored under nitrogen. This sample contained 93.86% pure pyrrhotite. The stock solution for flotation was made of distilled water and reagents[12], and is shown in Table 1 with the corresponding pH values.
Table 1 Stock solution for flotation
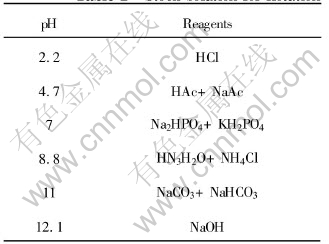
The reagents adjusting potential were ammonium persulfate ((NH4)2S2O8) and sodium dithionite (Na2S2O4). All reagents for tests were analytical grade except the frother butyl ether alcohol was industry grade. The dosage of the frother was 10mg/L.
2.2 Flotation tests
The flotation tests were carried out in a micro flotation cell with 25mL of effective volume. The amount of sample used for each experiment was 2.2g, which was ultrasonically washed for 5min to remove any possible oxides on the mineral surface. The washing solution was decanted and the fresh stock solution of given pH was added before flotation. The pulp potential in each experiment was adjusted by the reagents and measured before frother addition. The electrodes to measure pulp potential were a saturated calomel electrode (SCE, 0.245V vs SHE) and a platinum electrode with geometric surface area 0.28cm2. The SCE was checked periodically to ensure accurate measurement. Before each experiment, the platinum electrode was cleaned. The flotation time was 4min. The flotation recovery (R) was calculated by
![]()
where m1 and m2 are the floated and un-floated fractions, respectively.
2.3 Cyclic voltammetry measurement
Cyclic voltammetry was used to characterize redox reaction that occurs at the pyrrhotite surface. Cyclic voltammetry was done by using EG&GPARC electrochemical measurement system which was made in USA. The setup consisted of Model 273 multiple-function potentiostat, M270 measurement software and PC computer. The pyrrhotite electrode was prepared from pure crystals pyrrhotite which had been picked out from this mineral sample. The pyrrhotite electrode was polished with 600 grit silicon carbide paper and then rinsed with distilled water before experiment. The sweep rate was fixed at 0.01V/s.
2.4 FTIR reflection spectra measurement
The FTIR reflection spectra were obtained using the infrared spectrophotometer (type NEXUS 470). The experimental procedure with powdered samples was as follows. The pyrrhotite sample of 0.7g was suspended in 25mL of buffer solution at the corresponding pH and desired reagent concentrations. After the addition of each reagent, the suspension was stirred for 15min and then settled for 15min. The solution was filtered. The treated sample was first dried in a vacuum and then used for FTIR reflection spectra measurement.
3 RESULTS AND DISCUSSION
3.1 Potential-pH area of pyrrhotite flotation
The flotation recovery of pyrrhotite as a function of pH and pulp potential is given in Fig.1. The results show that the pyrrhotite exhibits good flotability from pH 2 to pH 11 when using ethyl xanthate as a collector. Only when pH>12, the flotation recovery begins to decline. But the pulp potential decreases with the increasing pH.
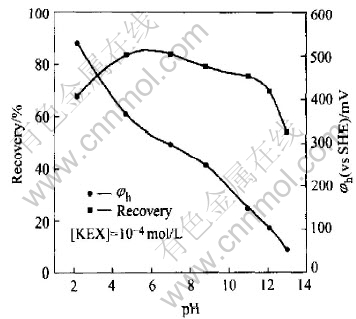
Fig.1 Flotation recovery of pyrrhotite as function of pH and pulp potential
The dependence of pyrrhotite flotation recovery on pulp potential is presented in Fig.2 at pH 4.7, 8.8, 12.1 and 12.7, which were fixed by different buffer solutions, respectively. It may be seen that pyrrhotite can only be floated in a very narrow range of the pulp potential at a given pH condition. If the upper and lower potential limits for flotation are defined at 50% of flotation recovery, a relationship between pyrrhotite flotation upper (φh(u)) and lower (φh(l)) potential limit potential can be established and is presented in Fig.3. It can be seen that the flotation of pyrrhotite may occur only in a certain range of pulp potential φh(l)〈φh〈φh(u) at different pH values. In the neutral and weak acidic solution, there is a wider range of pulp potential for the flotation. The flotation potential range becomes more narrow with the increasing pH value. For example, at pH 8.8, the optimal potential range for flotation of pyrrhotite is about 150-320mV. The maximum flotation occurs at potential of 250mV. When pH is 12.7, the flotation of pyrrhotite can almost not be above 50%, no matter how potential changes.
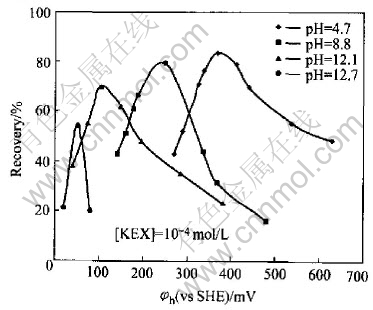
Fig.2 Relationship between pyrrhotite flotation recovery and pulp potential
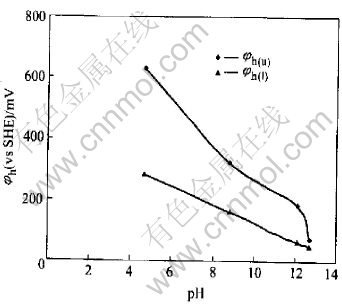
Fig.3 Relationship between pulp pH and pyrrhotite flotation potential upper and lower limit
3.2 Anodic oxidation of pyrrhotite surface in presence of ethyl xanthate
The anodic scan sections of cyclic voltammetry for pyrrhotite electrode in pH 7, 8.8, 11, 12.1, 12.7 buffer solutions with ethyl xanthate are respectively presented in Fig.4. The cyclic voltammetry curve at pH 8.8 is also presented in Fig.4. It can be seen from Fig.4 that the anodic current peak emerges at about 0.1V. As pH value increasing, the peak moves to the left. This peak may correspond to the formation of dixanthogen. The oxidation of ethyl xanthate forming dixanthogen is as follows:
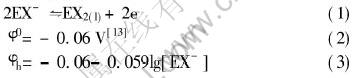
When the concentration of ethyl xanthate is 5×10-3mol/L, φh=0.08V, which reasonably well agrees with the results in Fig.4.
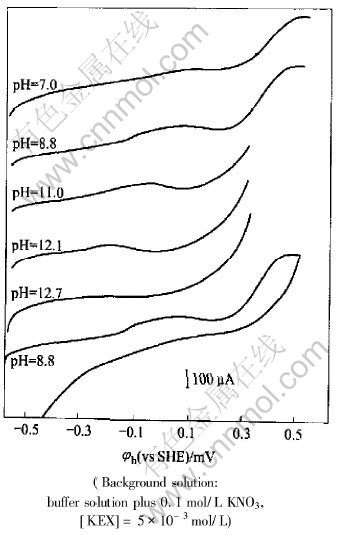
Fig.4 Anodic scan sections of cyclic voltammetry for pyrrhotite electrode in different pH buffer solutions at potential scan rate of 10mV/s
When the hydrophobic entity diethyl dixanthogen is formed, the flotation of pyrrhotite can be realized.
It can be also seen from Fig.4 that anodic peak moves toward left. When pH value is 12.7, the anodic peak disappears, the anodic current increases rapidly. It is indicated that only the oxidation of pyrrhotite self takes place on the surface of pyrrhotite, and no dixanthogen is formed at pH=12.7. So the flotation of pyrrhotite can not be carried out. This phenomenon may correspond to the following reactions:
FeS1.13+7.52H2O=Fe(OH)3+1.13SO2-4+ 12.04H++9.78e(4)
2FeS1.13+9.39H2O=2Fe(OH)3+ 1.13S2O2-3+12.78H++10.52e(5)
3.3 FTIR reflection spectra analysis of ethyl xanthate adsorption on pyrrhotite surface
The FTIR reflection spectra of KEX solid, KEX in solution, diethyl dixanthogen(EX2) and iron (Ⅲ) ethyl xanthate (Fe(EX)3) are shown in Fig.5. Only the 950-1350cm-1 region is shown, where most of the important vibrations of xanthate functional groups are observed. From Fig.5, it can be seen that the characteristic absorption bands of ethyl xanthat are as follows: the stretching vibration band of the C—O—C at 1100-1172cm-1, C-S at 1049cm-1 and 1008cm-1. When diethyl dixanthogen is formed, the stretching vibration
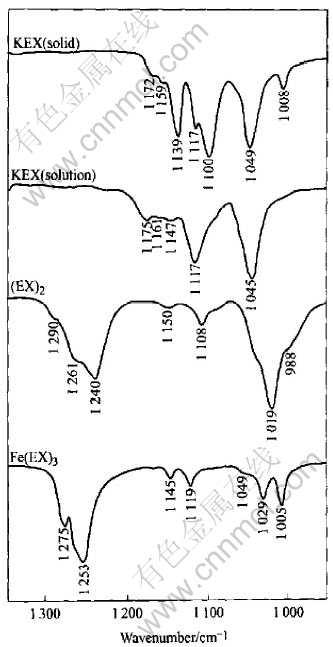
Fig.5 FTIR spectra of KEX (solid),KEX (solution), EX2 and Fe(EX)3[14]
band of C-S moves to lower wavenumber and shifts to 1019cm-1 and 998cm-1, and that of C—O—C moves to higher wavenumber and shifts to 1240-1290cm-1. When iron(Ⅲ) ethyl xanthate is formed, the stretching vibration band of C-S moves to lower wavenumber and shifts to 1029cm-1 and 1005cm-1.
The FTIR reflection spectra of ethyl xanthate adsorption on pyrrhotite are shown in Fig.6. From Fig.6, it can be seen that the characteristic absorption bands of diethyl dixanthogen at 1023cm-1, 1242cm-1, and 1263cm-1 appear on the surface of pyrrhotite, indicating that the dominate hydrophobic species on pyrrhotite surface are dixanthogen. The effect of pulp potential on the adsorption of ethyl xanthate on pyrrhotite was examined and the results are presented in Fig.7. At pH 8.8 the ethyl xanthate adsorption on pyrrhotite is mainly of dixanthogen independent of potential in the range of 140-250mV due to the occurrence of almost same dixanthogen characteristic band. However, the intensity of IR signals is changed at various potential values. It is demonstrated that IR signals of the characteristic dixanthogen peaks and hence dixanthogen adsorption on pyrrhotite decrease with decreasing potential from 250mV to 140mV. It explained well the results of flotation in Fig.2 and Fig.3. At pH 8.8, the flotation recovery and dixanthogen adsorption on pyrrhotite are decreased with the decrease of pulp potential from 250mV to 140mV. The effect of pH on FTIR signal intensity of xanthate adsorption on pyrrhotite and pyrrhotite flotation recovery is plotted in Fig.8. It can be seen from Fig.8 that IR signal intensity corresponds well to flotation recovery. At weak acidic pulp solution, the IR signal intensity is the strongest.
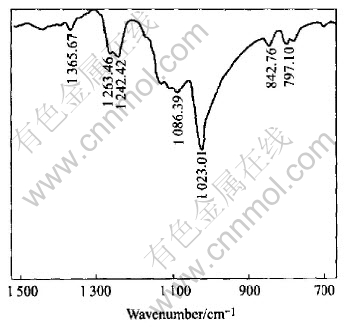
Fig.6 FTIR spectra of ethyl xanthate adsorbed on pyrrhotite
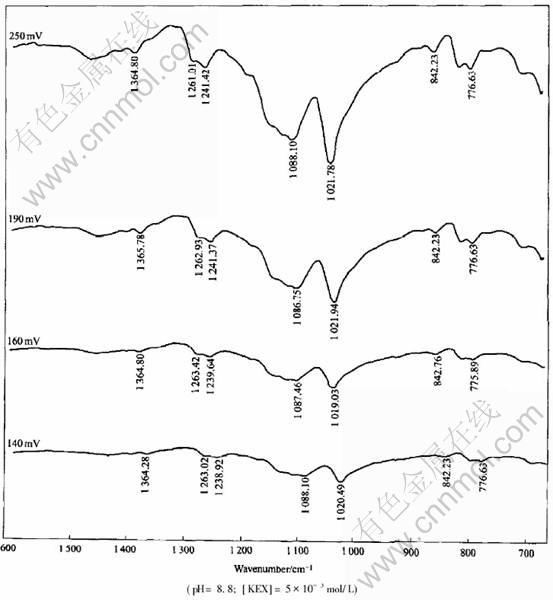
Fig.7 FTIR spectra of ethyl xanthate adsorbed on pyrrhotite at different pulp potential
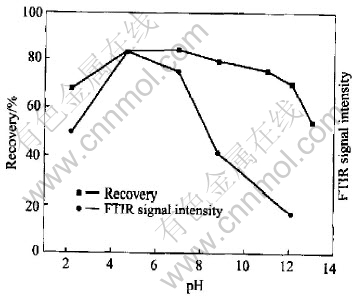
Fig.8 Effect of pH on FTIR signal intensity of xanthate adsorption on pyrrhotite and pyrrhotite flotation recovery
4 CONCLUSIONS
1) Pyrrhotite has good floatability from pH 2 to pH 11, and poor flotability pH>12.
2) The flotation of pyrrhotite depends on pulp pH and pulp potential. In a suitable range of pulp potential at certain pH, the flotation of pyrrhotite can occur.
3) Through potential scan of cyclic voltammograms for pyrrhotite electrode and analysis of FTIR reflection spectra measuring, the major production of ethyl xanthate reaction with pyrrhotite is diethyl dixanthogen, which attributes to the flotation of pyrrhotite.
REFERENCES
[1]Buckley A N, Woods R. X-ray photoelectron spectroscopy of oxidized pyrrhofite surfaces—Ⅰ Exposure to air [J]. Appl Surf Sci,1985, 22/23: 280-287.
[2]Buckley A N, Woods R. X-ray photoelectron spectrpscopy of oxidized pyrrhotite surfaces—Ⅱ Exposure to aqueousolutions [J]. Appl Surf Sci, 1985, 20: 472-480.
[3]Hamilton I C, Woods R. An investigation of surface oxidation of pyrite and pyrrhotite by linear potential sweep voltammetry [J]. J Electroanal Chem, 1981, 118: 327-343.
[4]Steger H F. Oxidation of sulfide minerals—(Ⅶ)Effect of temperature and relative humidity on the oxidation in of pyrrhotite [J]. Chem Geol, 1982, 35: 281-295.
[5]Buckley A N, Woods R. An X-ray photoelectron spectroscopic study of the oxidation of galena [J]. Appl Surf Sci, 1984, 17: 401-414.
[6]Buckley A N, Woods R. The surface oxidation of pyrite [J]. Appl Surf Sci, 1987, 27: 437-452.
[7]Buckley A N, Woods R. An X-ray photoelectron spectroscopic study of the oxidation of chalcopyrite [J]. Aust J Chem, 1984, 37: 2403-2413.
[8]Heyes G W, Trahar W J. The flotation of pyrite and pyrrhotite in the absence of conventional collectors[A]. Proceeding-The Electrochemical Society[C]. 1984, 10: 219-232.
[9]Cheng X , Iwasaki I, Smith K A. An electrochemical study on cathodic decomposition behavior of pyrrhotite in deoxygenated solutions [J]. Mineral & Metallurgical Processing, 1994, 1(3): 160-167.
[10]Buswell A M, Nicol M J. Some aspects of applied electrochemistry of the flotation of pyrrhotite [J]. Journal of Applied Electrochemistry, 2002, 32(12): 1321-1329.
[11]Leppinen J O, Bssilio C I, Yoon R H. In-situ FTIR study of ethyl xanthate adsorption on sulfide under conditions of controlled potential [J]. International Journal of Mineral Processing, 1989, 26: 259-274.
[12]ZOU Tong-hui. Analytical Chemical Handbook (Ⅱ) [M]. Beijing: Chemical Industry Press, 1997. 335-349. ( in Chinese)
[13]WANG Dian-zuo, HU Yue-hua. Solution Chemistry of Flotation [M]. Changsha: Hunan Science and Technology Press, 1988. 298. (in Chinese)
[14]Leppinen J O. FTIR and flotation investigation of the adsorption of ethyl xanthate on activation and non-activated sulfide mineral [J]. Int J Miner Process, 1990, 30: 245-263.
Foundation item: Project (50234010) supported by the National Natural Science Foundation of China; Project (2001CCA03100) supported by Pre-Research of the National Basic Research Program of China
Received date: 2004-04-27; Accepted date: 2004-07-09
Correspondence: ZHANG Qin, PhD candidate; Tel: +86-731-8830482; E-mail: zq81219074@163.com


