Trans. Nonferrous Met. Soc. China 25(2015) 4183-4191
Solvent extraction of V(V) and Cr(III) from acidic leach liquors of ilmenite using Aliquat 336
A. A. NAYL1,2, H. F. ALY2
1. Chemistry Department, College of Science, Aljouf University, Skaka 2014, Saudi Arabia;
2. Hot Laboratories Centre, Atomic Energy Authority, Cairo 13759, Egypt
Received 1 December 2014; accepted 8 February 2015
Abstract:
Extraction of V(V) and Cr(III) from acidic sulfate leach liquors of ilmenite using 0.4 mol/L Aliquat 336 chloride in kerosene was carried out. Different parameters affecting the extraction process such as equilibrium time, sulfate concentration, Aliquat 336 concentration, equilibrium pH and the extraction temperature were investigated. Extraction of V(V) and Cr(III) by Aliquat 336 involved anion exchange mechanism, and the extracted species are [(VO2SO4)R4N]org at low equilibrium pH for V(V) and [R4N-Cr(OH)4]org at high equilibrium pH for Cr(III). Calculated thermodynamic parameters show that the extraction process is endothermic reaction for V(V) and exothermic for Cr(III). Also, calculated values of DGex and DSex indicate that the extraction reactions of V(V) and Cr(III) proceed as non-spontaneous reaction is more random. V(V) and Cr(III) were stripped, precipitated, separated and calcined at 500 °C for 2.0 h to produce the corresponding oxide in pure form after rinsing and drying.
Key words:
extraction; acidic leach liquor; Aliquat 336; V(V); Cr(III);
1 Introduction
Vanadium and chromium are regarded as strategic metals and their compounds are widely used in many industries. Vanadium and chromium are widely used in different metallurgical industries. In the last decades, due to their characteristic properties, considerable attention has been attracted to different oxides to design some novel and strategical materials that can be used in electro-, radio-, and microwave equipments, nuclear industries and in the leather processing, etc [1-4]. The presence of such metals or their compounds in large quantities is one of the major environmental problems [5] that many countries are facing. Therefore, the recovery, removal and extraction of such substances are very necessary to bring down the concentration of such hazardous metal ions below the discharge standards.
In the last decades, large quantities of ilmenite ores are used in production processes of titanium and other metals. The commercial techniques used for the manufacture of such metals from ilmenite are sulfate and chloride routes [6-8]. Therefore, many metallic impurities such as Fe, Al, Mg, Cr, V, also are leached with titanium. Therefore, this waste is regarded as secondary resources for these metals.
Solvent extraction is a common method used to extract, separate and concentrate vanadium and chromium from leach liquors. It is one of the most important techniques used for recovery and separation of vanadium and chromium from different leach liquors [9-15]. Many extractants are tested for the extraction of vanadium and chromium from various acidic aqueous solutions using different types of extractants such as HDEHP [16-22], chelated extractant LIX63 [23], Aliquat 336 [12,24,25], Alamine 336 [26] and CYANEX 272 [27].
Some novel techniques have been studied for the solvent extraction of Cr(III) and Cr(VI) using Aliquat 336 (trialkylmethylammonium chloride) as the extractant [28-31].
As reported by previous studies, Aliquat 336 was used as a good extractant for metals and phase transfer catalyst. Also, it shows good characteristics in the extraction of Cr(III, IV) ions from their aqueous solutions in anionic forms as Cr(OH)4- and CrO42-, respectively [5].
In the extraction process of Cr(III), both cationic and anionic species of Cr(III) were extracted mainly from acidic or neutral media. Namely, cationic complexes of Cr(III) were extracted by acidic extractants [32-34] while anionic complexes of Cr(III) were extracted with Aliquat 336 [35]. Many studies have proven that, the quaternary ammonium compounds are effectively extractants for Cr(III) from the alkaline model solutions. In these studies, the effects of different parameters affecting the process of Cr(III) extraction were examined [31,36,37]. Very limited research works are done for extraction of Cr(III) and V(V) ions from acidic leach liquors of ilmenite.
In the present work, the extraction of V(V) and Cr(III) from ilmenite leach sulfate solutions by 0.4 mol/L Aliquat 336 in kerosene was studied as a function of equilibrium time, sulfate concentration, Aliquat 336 concentration, equilibrium pH, temperature, and equilibrium mechanism. In addition, different isotherm parameters were studied and calculated for the extraction system. The organic solutions were stripped using various strippers and pure forms of V(V) and Cr(III) were obtained.
2 Experimental
2.1 Materials
All the chemical reagents used were of analytical grade and deionized water was used throughout the experiments. Sulfuric acid, sodium sulfate and sodium hydroxide were obtained from BDH Company for chemicals while kerosene (non-aromatic) was supplied by Misr Petroleum Ltd Company, Egypt.
The ilmenite used in the present study was a natural placer mineral obtained from Abu Ghalaga region in the Eastern Desert in Egypt [38]. The bulk chemical compositions of the ilmenite ore sample (0.18% V2O5 and 0.38% Cr2O3), ilmenite paste (0.17% V2O5 and 0.37% Cr2O3) and the final obtained oxides in this study were determined using energy dispersive X-ray fluorescence (EDX) spectrometer Model (Oxford) attached with SEM Model JEOL-JSM-5600 (Table 1) [38].
Aliquat 336 chloride (R4N–Cl), (Aldrich, USA) diluted by kerosene was used in the preparation of the organic phase. Generally, Aliquat 336 is a water insoluble quaternary ammonium salt composed of a large organic cation associated with a chloride ion as shown below:

Since the ammonium structure has a permanent positive charge, Aliquat 336 forms salts with different anions over a wider pH range than primary, secondary and tertiary amines. This was reflected on its use in many applications in recovery of several metals from solutions [39].
2.2 Leaching procedure
All the experiments were conducted batch wise and the leaching experiments were performed in 500 mL conical flasks. After adding a measured amount of the ilmenite sample (0.18% V 2O5 and 0.38% Cr2O3) and potassium hydroxide solution, the mixture of the reactants was heated under stirring speed of 375 r/min. As reported in our previous work [38], the decomposition conditions by KOH were generally fixed at 70% KOH solution, 150 °C, 3.0 h mixing time, a liquid to solid mass ratio of 5:1. At the end of each decomposition experiment, the slurry was filtered, washed with distilled water and the residue paste was taken for acid leaching investigations under specific stirring speed of 375 r/min. The leached metal ions were calculated by dissolving the paste sample (contain 0.17% V2O5 and 0.37% Cr2O3) in 6.0 mol/L H2SO4 in acid/ilmenite paste mass ratio of 9:1 and reaction time of 2.5 h [38]. The obtained solutions were then analyzed for iron, chromium, vanadium, titanium and other metals to calculate leaching efficiency by inductively coupled plasma optical emission spectrometry (ICP-OES) using the system ULTIMA-2 ICP, Jobin Yvon, France and by different chemical reagents.
2.3 Extraction procedure
The extraction procedure was carried out by shaking same volume (5.0 mL) of the leach liquor solution and organic solution (Aliquat 336) phases in stoppered glass tubes for 30 min using a thermostated shaking water bath adjusted at 25 °C. The initial pH of leach liquor was adjusted by H2SO4 or NaOH to the desired value. The data obtained showed that, 20 min was a sufficient time to reach the extraction equilibrium for both metals, except otherwise cited. After few test experiments, about 5.0% (volume fraction) iso-decanol was chosen and used as phase modifier irrespective of the concentration of the solvent used during the extraction.
Table 1 Bulk chemical compositions of ilmenite ore and ilmenite paste used in study [38] (mass fraction, %)
To determine the concentrations of V(V) and Cr(III), known volumes were taken from the aqueous phase before and after extraction assay. The distribution ratio (D) was calculated using the following expression:
D=[(co-c)/c]XVaq/Vorg (1)
where Vaq and Vorg refer to the volumes of the aqueous and the organic phases, respectively, and co is the original metal concentration in the aqueous phase before extraction and c is the metal concentration in the aqueous phase after extraction.
The extraction rate (E) was calculated using the following equation (with organic/aqueous O/A volume ratio equal to unity):
E=100D/(D+1)×100% (2)
The separation feasibility of V(V) from Cr(III) was evaluated and calculated in terms of the separation factor (S(V/Cr)) between V(V) and Cr(III) and defined by the following equation:
S(V/Cr)=DV/DCr (3)
All the extraction and stripping experiments were carried out at ambient temperature of (25±1) °C. In all experiments, the phase ratio was kept at unity except otherwise stated.
3 Results and discussion
As reported in our previous work [38], the recovery rates of iron, titanium, vanadium, chromium, magnesium and aluminum, from leaching process of alkaline paste of ilmenite by 6.0 mol/L H2SO4 were 91%, 89%, 86%, 68%, 83%, 85%, respectively. The effect of various parameters on the extraction process was optimized and described below for the extraction of V(V) and Cr(III) from the leach solution containing 2.1×10-3 mol/L V, 5.41×10-3 mol/L Cr, 0.627 mol/L Ti, 0.39 mol/L Fe, 2.73×10-2 mol/L Mg, 1.74×10-2 mol/L Al and 6.4×10-4 mol/L Ln.
In acidic sulfate solutions, SOLE [40] suggested that almost dominant titanium species were TiOSO4, TiO(SO4)22- and TiO2+. For the pH of 0.5-2.5, most dominant iron species were 71% FeSO4+ and 15% FeHSO42+, respectively [41]. In addition, when pH is less than 4.8, predominated species of aluminum is Al3+ [42]. Accordingly, in this work, Aliquat 336, which acts as anion exchanger, cannot extract these species.
3.1 Extraction process
The selective recovery, extraction and separation of V(V) and Cr(III) from leach sulfuric acid liquor solutions were carried out by using 0.4 mol/L Aliquat 336 in kerosene as they form anionic species in these acidic solutions.
3.1.1 Effect of equilibrium time
The effects of equilibrium time on the extraction of V(V) and Cr(III) from leach liquor of sulfuric acid media with 0.3 mol/L Na2SO4 solution were studied by 0.4 mol/L Aliquat 336 in kerosene at a 1:1 phase ratio for various equilibrium periods from 1.0 to 20 min at 25 °C and pH 2.0 (Fig. 1). The data obtained show that, the extraction rate increases with the increase in contact time to reach a maximum extraction rate of 99.7% at 8.0 min with a plateau within 8–20 min for V(V). In the case of chromium, the extraction rate increases slowly to reach a maximum of 2.9% at 20 min.

Fig. 1 Effect of extraction time on extraction rate of V(V) and Cr(III) by 0.4 mol/L Aliquat 336 from 6.0 mol/L H2SO4 leach liquor
3.1.2 Effect of sulfate concentration
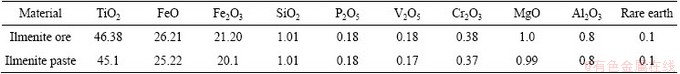
The effect of sulfate concentration on the extraction of V(V) and Cr(III) was studied using 0.4 mol/L Aliquat 336 in kerosene from 6.0 mol/L H2SO4 containing 0.01-0.6 mol/L sulfate (as sodium sulfate was regarded as an electrolyte) and plotted as shown in Fig. 2. The results obtained show that, as the sulfate concentration increases, the extraction rate of V(V) increases to 99.7% with 0.3 mol/L sulfate with slope of about 1.0. This means that 1 mol sulfate is associated in extraction of 1 mol V(V) by Aliquat 336. While the Cr(III) extraction increases slowly. This means that the extraction of Cr(III) is independent on the concentration of sulfate.
This behavior can be attributed to the fact that the electrolyte is one of the main factors governing extraction process of Cr(III) [31] and V(V). Therefore, at pH≤2, the extraction of V(V) increases with the increase in sulfate anion concentration, because sulfate species act as salting out agents with respect to the extractant and extracted complex while the extraction behavior of Cr(III) is independent on this type of electrolytes under these conditions.
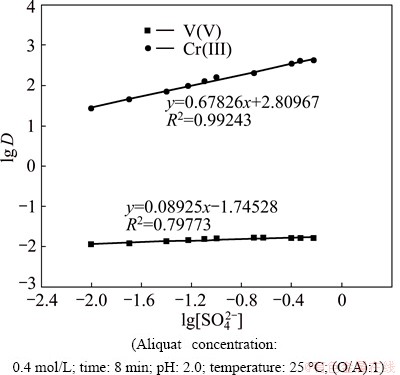
Fig. 2 Plot of lg D vs lg[SO42-]
3.1.3 Effect of Aliquat 336 concentration
The extraction of V(V) and Cr(III) was carried out by using different concentrations of Aliquat 336 (0.1-0.5 mol/L) diluted by kerosene from 6.0 mol/L H2SO4 leach liquor containing 0.3 mol/L Na2SO4 with O/A ratio of unity for 8.0 min and pH≈2.0 for V(V) and for 20.0 min at pH≈12 for Cr(III) at 25 °C. The relationship between the distribution ratio (D) and the concentration of extractant is shown in Fig. 3. Aliquat 336 forms oil soluble salts of anionic species at low pH. Figure 3 illustrates the slopes of unity for both V(V) and Cr(III) under these reported conditions. This indicates that 1.0 mol Aliquat 336 is associated in extraction of 1.0 mol V(V) and 1.0 mol Cr(III) into the organic phase. Some of these results agree with those reported in Refs. [2,22].

Fig. 3 Plot of lg D vs lg[Aliq 336] in kerosene
3.1.4 Effect of equilibrium pH of leach liquor solution
The extraction of V(V) and Cr(III) was studied from 6.0 mol/L H2SO4 leach liquor containing 0.3 mol/L Na2SO4 by 0.4 mol/L Aliquat 336 in kerosene after 8 min mixing at a 1:1 phase ratio and different pH values ranging from 0 to 13 at 25 °C. The extraction efficiency mainly depends on the pH of aqueous medium. Ion association complexes are known to be more stable in acidic media for V(V) and in alkaline media for Cr(III).
Figure 4 shows that, at lower values of pH (0 to 5.0), the extraction rate of V(V) was higher than that of Cr(III). Extraction rate of V(V) increases from 9.1% to 99.7% as pH increases from 0 to 2.0. Then, the extraction decreases slowly as the pH increases from 2.0 to 5.0. These results agree with that reported by TANGRI et al [43]. Then, with gradual increase in pH to 13, the extraction rate of V(V) decreases to about 9.0 % at pH 1.0. This can be explained in terms of the chemistry of vanadium in its aqueous solutions where vanadium exhibits different oxyanion complex speciations with different oxidation states. In leach liquor solutions from the processing of ores, spent catalysts, and residues, V(V) exists in a series of polyanions such as decavanadate V10O286- or metavanadate V4O124- between pH 2 and 12, which are partially protonated according to the pH value [44] and the cationic species (VO2+) is predominant at pH<2 [2,45]. In acidic sulfate media, V(V) exists in the form of VO2SO4-. Therefore, VO2SO4- species can be resulted from the reaction between VO2+ and H2SO4 as
(VO2)++H2SO4 (VO2SO4-)+2H+ (4)
(VO2SO4-)+2H+ (4)
This can successfully explain the V(V) extraction by anionic extractant under these conditions [45] (Fig. (5)).
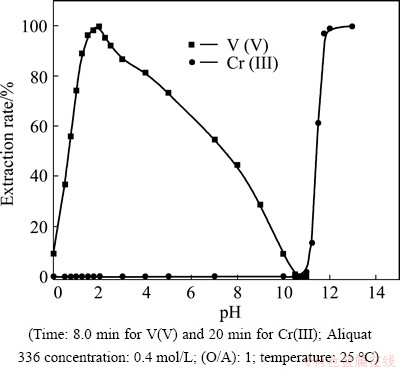
Fig. 4 Effect of pH on extraction rate of V(V) and Cr(III) by 0.4 mol/L Aliquat 336 in kerosene from 6.0 mol/L H2SO4 leach liquor
The extraction rate of Cr(III) increases from 0.001% to 1.7% in the pH range from 2.0 to 11.0. As the pH increases above 11.0, the extraction of Cr(III) ions starts to increase rapidly with gradual increases in pH to reach about 99.8% at pH 12. The previous studies [4] reported that Cr(III) is extracted with the highest yield from the alkaline aqueous solutions by Aliquat 336 as hydroxochromates(III), [Crn(OH)(3n+x)]x-. Cr(OH)4- became dominant in solutions at pH about 12.0 and the complexes formed in the organic phase containing ammonium cations with Cr(OH)4- [31]. These results can be explained based on the data in Fig. 6 [46].

Fig. 5 Activity–pH diagram for V(V)–water–sulfur–water system at 298.15 K [45]
Fig. 6 φh-pH diagram for chromium [46]
In this concern, chromium ion forms soluble Cr(III) in the pH range from 0 to 8.0. At pH 4.0-7.5, Cr3+ was hydrolyzed to form soluble CrOH2+ and Cr(OH)2+. The simple hydrolysis reaction can be written as follows [5]:
Cr3++H2O Cr(OH)2++H+ (5)
Cr(OH)2++H+ (5)
At higher pH, chromium ion forms soluble Cr(III) hydroxyl anion, Cr(OH)4- [47] and the hydrolysis reaction can be assumed as follows:
Cr3++4H2O Cr(OH)4-+4H+ (6)
Cr(OH)4-+4H+ (6)
The difference in extraction values, as shown in Fig. 4, states that it is possible to separate V(V) from Cr(III) at a suitable pH range with 0.4 mol/L Aliquat 336 in kerosene. The relationship between lg D versus pH for extraction of V(V) and Cr(III) gives straight lines with a slope of nearly 2.0 in pH range of 0-2.0 and 4.0 in pH range of 10-12, respectively. This indicates the release of 2 and 4 mol of H+ from the aqueous phase, which is responsible for extraction of V(V) and Cr(III) from H2SO4 solution, respectively, as shown in Fig. 7.
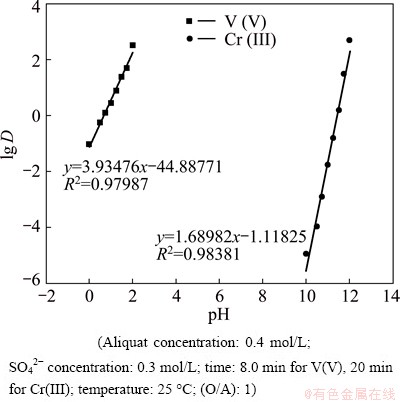
Fig. 7 Plot of lg D vs pH
The calculated separation factor, SV/Cr, increases with the increase in pH to reach a maximum value of 332×107 at pH 2. This value is higher than that reported in the literature for extraction and separation of V(V) from Cr(III) in sulfate solution.
In addition to the above factors, ionic strength of initial aqueous solution is considered as a vital parameter affecting the extraction process of V(V) and Cr(III). In this study, the calculated values of ionic strength (I) are very large (>>5.0 mol/L) in both acidic and alkaline solutions. The data obtained show that, at pH≤2, the extraction rate of V(V) increases with the increase of both ionic strength and sulfate anions concentration. While, at pH≥12, the extraction rate of Cr(III) increases with the increase of both ionic strength and hydroxide anions concentration. This is explained by the fact that these species are working as very weak complexing anions and acting as salting out agents [48].
3.1.5 Extraction mechanism
In high relative molecular mass amines such as Aliquat 336 chloride (R4N-Cl), when equilibrated with an aqueous phase containing complex anions, the exchange of anions between the phases occurs. R4N+Cl- can be dissociated to R4N+ and Cl-.
Based on the preceding results and Eq. (4), the mechanism of V(V) extraction can be represented by
[(VO2)+]aq+[H2SO4]aq+[R4N+Cl-]org [(VO2SO4)R4N]org+2H++Cl- (7)
[(VO2SO4)R4N]org+2H++Cl- (7)
where aq and org denote aqueous and organic phases, respectively.
Therefore, the extraction equilibrium constant, Kex, of the reaction can be written as
Kex=D[H+]2[Cl-]/[H2SO4]aq[R4N+Cl-]org (8)
where D represents the distribution ratio.
By taking the logarithm of Eq. (8) and rearranging, where lg[H+]=-pH, it can be obtained
lg D=lg Kex+lg[R4N+Cl-]org+lg[H2SO4]aq+2pH-lg[Cl-]aq (9)
Slope analysis from Figs. 3 and 7 supports the mechanism.
For Cr(III), previous work was proved that Cr(III) is effectively extracted with Aliquat 336 from alkaline aqueous solutions as tetrahydroxo-chromate(III) [Cr(OH)4-] anions, where at higher pH, Cr forms soluble Cr(III) hydroxy anion, Cr(OH)4- [31,49]. Therefore, based on the preceding results and Eq. (6), the equilibrium of Cr(IIII) extraction can be described by
[Cr(OH)4-]aq+[R4N+Cl-]org [R4N-Cr(OH)4]org+[Cl-]aq (10)
[R4N-Cr(OH)4]org+[Cl-]aq (10)
Therefore, from Eqs. (6) and (10), the extraction equilibrium constant, Kex, of the reaction can be written as
Kex=D[H+]4[Cl-]/[R4N+Cl-]org (11)
By taking the logarithm of Eq. (11) and rearranging, where -lg[H+]=pH, it can be obtained
lg D=lg Kex+lg[R4N+Cl-]org+4pH-lg[Cl-]aq (12)
Slope analysis from Figs. 3 and 7 supports these mechanisms.
3.1.6 Effect of extraction temperature
The relationship between the extraction rates of V(V) and Cr(III) and the temperature from 6.0 mol/L H2SO4 leach liquor solution with 0.3 mol/L Na2SO4 was studied by 0.4 mol/L Aliquat 336 in kerosene after 8 min for V(V) and 20 min for Cr(III) mixing at a 1:1 O/A phase ratio at different temperatures ranging from 20 to 45 °C at pH 2.0 and 12.0 for V(V) and Cr(III), respectively. It can be seen from Fig. 8 that the extraction rate of V(V) slightly increases with increasing temperature from 20 to 45 °C. However, the extraction of Cr(III) decreases with increase of temperature above 25 °C. This negative effect can be attributed to the formation of precipitate of Cr(III) hydroxide since heating favors the hydrolysis of tetrahydroxochromate (III) anions [50]. Therefore, the operation extraction temperature was chosen to be 25 °C.
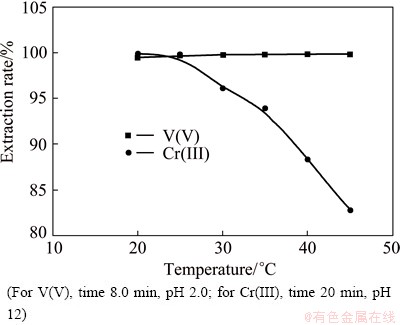
Fig. 8 Effect of temperature on extraction of V(V) and Cr(III) from 6.0 mol/L H2SO4 leach liquor by 0.4 mol/L Aliquat 336

Fig. 9 Plots of lg D vs T-1 for extraction of V(V) and Cr(III) by 0.4 mol/L Aliquat 336 in kerosene
To calculate the thermodynamic parameters, the relation between lg Kex and T-1 is plotted in Fig. 9. From the slopes obtained and applying the Van’t Hoff equations [51], the thermodynamic parameters can be calculated from the following equation:
lg Kex=[-DHex/(2.303RT)]+[DSex/(2.303R)] (13)
where R is the mole gas constant and T is the thermodynamic temperature.
The slopes obtained equal to -DHex/(2.303R) and the intercept equals to DSex/(2.303R). On the basis of Eq. (13) and slopes in Fig. 9, the values of DHex are calculated to be 34.58 kJ/mol for V(V) and -171.27 J/mol for Cr(III) extraction using 0.4 mol/L Aliquat 336, respectively. These indicate that the extraction reactions are endothermic for V(V) and exothermic for Cr(III). The values obtained for DHex are used to calculate the corresponding free energy change (DGex) and entropy change (DSex) for extraction reaction at 298 K, respectively.
DGex=-2.303RTlg Kex (14)
The calculated thermodynamic parameters are presented in Table 2. From these calculated values, the positive value of DGex indicates that the extraction reactions of V(V) and Cr(III) from ilmenite leach liquors by Aliquat 336 extractant proceed as nonspontaneous reaction, while the positive value obtained for DSex shows that the extraction process is more random in nature for V(V).
Table 2 Thermodynamic parameters for extraction process of V(V) and Cr(III) by 0.4 mol/L Alqiuat 336 at 298 K

3.2 Stripping investigations
Different types of stripping agents have been used for the stripping of loaded V(V) and Cr(III) such as H2SO4, HNO3, HCl, NaOH, NaCl, and NH4OH, from various organic extractants. In this study, NH4OH and H2SO4 were used as stripping agents for V(V) and Cr(III) from Aliquat 336 and the results are given in Table 3. The data obtained show that NH4OH and dilute H2SO4 can be considered as good stripping agents for V(V) and Cr(III), respectively, as they could lead to good recovery and separation of these metal ions from the loaded R4N-Cl. It was observed that with the increase of the ammonia concentration from 1.0% to 8.0%, the stripping rates of V(V) and Cr(III) increase from 76.4% to 99.9% and 42.1% to 85.9%, respectively, at O/A phase ratio of 1:2 for 30 min at 25 °C.
Table 3 Stripping of V(V) and Cr(III) at 25 °C with different concentrations of NH4OH (for 20.0 min) and H2SO4 (for 40.0 min) from loaded 0.4 mol/L R4N-Cl solutions at O/A ratio of 1:2
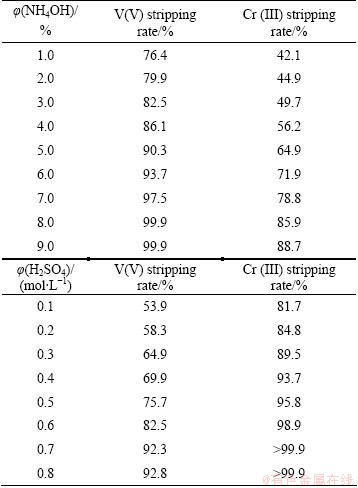
Also, stripping rates of V(V) and Cr(III) increase from 53.9% to 92.3% and from 81.7% to more than 99.9% as the H2SO4 concentration increases from 0.1 to 0.7 mol/L with O/A phase ratio of 1:2 for 40.0 min at 25 °C.
V(V) was precipitated as ammonium metavanadate (red cake) from ammoniacal strip solution at pH of 7.5-8.0 by adding 10%(volume fraction) HNO3. At pH 9.0-10.0, grey green precipitation of chromium hydroxide with excess of ammonia solution was precipitated. Both the two precipitates were then separated and calcined at 500 °C for 2.0 h to produce the corresponding oxide in pure form after rinsing and drying. Chemical analyses of the obtained V2O5 and Cr2O3 by (EDX) spectrometer were found as 97.34% V2O5, Cr2O3 0.59%, Al2O3 0.25% and ≤1.82% of other impurities, and 98.03% Cr2O3, Al2O3 1.29% and ≤0.68% of other impurities, respectively.
4 Conclusions
1) Liquid-liquid extractions of V(V) and Cr(III) from 6.0 mol/L sulfuric acid leach liquor solution obtained from ilmenite hydroxide cake were performed using 0.4 mol/L Aliquat 336 dissolved in kerosene.
2) The data obtained indicate that the extraction of both metals from the acidic media is pH dependent. The extraction of V(V) as well as Cr(III) increases with increasing the pH and with increasing RN4-Cl concentration. V(V) is extracted in preference to Cr(III) at lower pH values (0-2.0) with extraction rate of 99.7% at pH 2.0. Cr(III) is extracted in preference to V(V) at higher pH values (9.0-12.0) with extraction rate of 99.8% at pH 12.0.
3) The extraction process is an endothermic reaction for V(V) and exothermic for Cr(III). The positive values of DG indicate that the extraction reactions proceed non-spontaneously, and the positive values of DS show that the extraction reaction is more random in nature.
4) V(V) is stripped from organic phase with stripping rate of 99.9% by using 8.0% NH4OH and Cr(III) is stripped with stripping rate of more than 99.9% by using 0.7 mol/L H2SO4.
5) V(V) and Cr (III) were precipitated, separated and calcined at 500 °C. Chemical analysis results of the obtained V2O5 and Cr2O3 were found as 97.34% V2O5, Cr2O3 0.59%, Al2O3 0.25% and ≤1.82% of other impurities, and 98.03% Cr2O3, Al2O3 1.29% and ≤0.68% of other impurities, respectively.
References
[1] LI Xing-bin, WEI Chang, WU Jun, LI Cun-xiong, LI Min-ting, DENG Zhi-gan, XU Hong-sheng. Thermodynamics and mechanism of vanadium(IV) extraction from sulphate medium with D2EHPA, EHEHPA and CYANEX 272 in kerosene [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(2): 461-466.
[2] LI W, ZHANG Y, LIU T, HUANG J, WANG Y. Comparison of ion exchange and solvent extraction in recovering vanadium from sulfuric acid leach solutions of stone coal [J]. Hydrometallurgy, 2013, 131-132: 1-7.
[3] KONCZYK J, KOZLOWSKI C, WALKOWIAK W. Removal of chromium (III) from acidic aqueous solution by polymer inclusion membranes with D2EHPA and Aliquat 336 [J]. Desalination, 2010, 263: 211-216.
[4] WIONCZYK W, CIERPISZEWSKI R, MOL A, PROCHASKA K. Studies on the kinetics and equilibrium of the solvent extraction of chromium(III) from alkaline aqueous solutions of different composition in the system with Aliquat 336 [J]. J Hazardous Materials, 2011, 198: 257-268.
[5] LIU Y, GUO L, ZHU L, SUN X, CHEN J. Removal of Cr(III, VI) by quaternary ammonium and quaternary phosphonium ionic liquids functionalized silica materials [J]. Chem Eng J, 2010, 158: 108-114.
[6] CHEMET T. Applied mineralogical studies on Australian sand ilmenite concentrate with special reference to its behavior in the sulphate process [J]. Miner Eng, 1999, 12(5): 485-495.
[7] MACKEY T S. Acid leaching of ilmenite into synthetic rutile [J]. Ind Eng Chem, 1974, 13(1): 9-18.
[8] SASIKUMAR C, RAO D S, SRIKANTH S, RAVIKUMAR B, MUKHOPADHVAY N K, MEHROTRA S P. Effect of mechanical activation on the kinetics of sulfuric acid leaching of beach sand ilmenite from Orissa [J]. India Hydrometallurgy, 2004, 75(1-4): 189-204.
[9] ZHAO J, HU Q, LI Y, LIU H. Efficient separation of vanadium from chromium by a novel ionic liquid-based synergistic extraction strategy [J]. Chem Eng J, 2015, 264: 487-496.
[10] DENG Zhi-gan, WEI Chang, FAN Gang, LI Min-ting, LI Cun-xiong, LI Xing-bin. Extraction vanadium from stone-coal by oxygen pressure acid leaching and solvent extraction [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(S1): s118-s122.
[11] LI M, WEI C, FAN G, LI C, DENG Z, LI X. Extraction of vanadium from black shale using pressure acid leaching [J]. Hydrometallurgy, 2009, 98: 308-313.
[12] NAVARRO R, GUZMAN J, SAUCEDO I, REVILLA J, GUIBAL E. Vanadium recovery from oil fly ash by leaching, precipitation and solvent extraction processes [J]. Waste Management, 2007, 27: 425-438.
[13] NISHIHAMA S, NISHIMURA G, HIRAI T, KOMASAWA I. Separation and recovery of Cr(VI) from simulated plating waste using microcapsules containing quaternary ammonium salt extractant and phosphoric acid extractant [J]. Ind Eng Chem Res, 2004, 43: 751-757.
[14] VITOLO S, SEGGIANI M, FALASCHI F. Recovery of vanadium from a previously burned heavy oil fly ash [J]. Hydrometallurgy, 2001, 62: 145-150.
[15] ZOUHRI A, ERNST B, BURGARD M. Bulk liquid membrane for the recovery of chromium(VI) from a hydrochloric acid medium using Dicyclohexano-18-Crown-6 as extractant-carrier [J]. Separation Science and Technology, 1999, 34: 1891.
[16] LI Xing-bin, WEI Chang, WU Jun, LI Min-ting, DENG Zhi-gan, LI Cu-xiong, XU Hong-sheng. Co-extraction and selective stripping of vanadium (IV) and molybdenum (VI) from sulphuric acid solution using 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester [J]. Separation and Purification Technology, 2012, 86: 64-69.
[17] RIGG T, GAMER J O. Solvent extraction of vanadium from chloride solutions using di-(2-ethylhexyl)-phosphoric acid [J]. J Inorg & Nuclear Chem, 1967, 29(8): 2019-2025.
[18] BISWAS R K, WAKIHARA M, TANIGUCHI M. Recovery of vanadium and molybdenum from heavy oil desulphurization waste catalyst [J]. Hydrometallurgy, 1985, 14: 219-230.
[19] SATO T, NAKAMURA T, KAWAMURA M. The extraction of vanadium(IV) from hydrochloric acid solutions by di-(2-ethylhexyl)- phosphoric acid [J]. Journal of Inorganic and Nuclear Chemistry, 1978, 40(5): 853-856.
[20] HIRAI H, HASHIMOTO T, TSUBOI I, HINO A, KOMOSAWA I. Extraction and separation of molybdenum and vanadium using bis(2-ethylhexyl) monothio phosphoric acid and bis(2-ethylhexyl) phosphoric acid [J]. Journal o Chemical Engineering, Japan, 1995, 28: 85-90.
[21] ISLAM F, BISWAS R K. The solvent-extraction of vanadium(IV) from sulphuric acid solutions with bis-(2-ethyl hexyl)-phosphoric acid in benzene and kerosene [J]. Journal of Inorganic and Nuclear Chemistry, 1980, 42(3): 415-420.
[22] SATO T, TAKEDA T. The extraction of vanadium(IV) from sulphuric acid solutions by di-(2-ethylhexyl)-phosphoric acid [J]. Journal of Inorganic and Nuclear Chemistry, 1970, 32(10): 3387-3396.
[23] ZHANG P W, INOUE K, YOSHIZUKA K, TSUYAMA H. Extraction and selective stripping of molybdenum (VI) and vanadium(IV) from sulfuric acid solution containing aluminum( III), cobalt (II), nickel (II) and iron (III) by LIX 63 in ExxsolD80 [J]. Hydrometallurgy, 1996, 41: 45-53.
[24] EL-NADI Y A, AWWAD N S, NAYL A A. A comparative study of vanadium extraction by Aliquat-336 from acidic and alkaline media with application to spent catalyst [J]. International Journal of Mineral Processing, 2009, 90: 115-120.
[25] BAL Y, BAL K E, COTE G, LALLAM A. Characterization of the solid third phases that precipitate from the organic solutions of Aliquat 336 after extraction of molybdenum (VI) and vanadium (V) [J]. Hydrometallurgy, 2004, 75: 123-134.
[26] LOZANO L J, GODINEZ C. Comparative study of solvent extraction of vanadium from sulphate solutions by Primene 81R and Alamine 336 [J]. Mineral Engineering, 2003, 16: 291–294.
[27] ZHANG P W, INOUE K, YOSHIZUKA K, TSUYAMA H. Solvent extraction of vanadium (IV) from sulfuric acid solution by bis(2,4,4-trimethylpentyl) phosphinic acid in EXXSOL D80 [J]. Journal of Chemical Engineering of Japan, 1996, 29: 82-87.
[28] SZE Y K P, XUE L. Extraction of zinc and chromium(III) and its application to treatment of alloy electroplating wastewater [J]. Separation Science and Technology, 2003, 38: 405-425.
[29] KWAJA A R, SINGH R, TANDON S N. Mono(2-ethylhexyl) phosphoric acid as an extractant for Cr(III) and its application to industrial waste [J]. Separation Science and Technology, 2000, 25: 447-455.
[30] AADIKIN A N, MUIS Z A, SAHA B. Removal of chromium (VI) with Aliquat 336 impregnated in amberlite XAD-16. I: Batch Mode Sorption Studies [J]. Journal Teknologi F, 2007, 46: 1-10.
[31] WIONCZYK B, APOSTOLUK W. Equilibria of extraction of chromium (III) from alkaline solutions with trioctyl methylammonium chloride (Aliquat 336) [J]. Hydrometallurgy, 2005, 78: 116-128.
[32] APOSTOLUK W, BARTECKI B. Extraction of chromium(III) from sodium chloride solutions by means of carboxylic acids [J]. Hydrometallurgy, 1985, 15: 191-202.
[33] ISLAM F, BISWAS R K. The solvent extraction of chromium(III) with bis-(2-ethyl hexyl) phosphoric acid in benzene and other solvents [J]. Journal of Inorganic and Nuclear Chemistry, 1979, 41: 229-233.
[34] PANDEY B D, COTE G, BAUER D. Extraction of chromium(III) from spent tanning baths [J]. Hydrometallurgy, 1996, 40: 343-357.
[35] IRVING H M N H, AL-JARRAH R H. The extraction of the chromium(III)–EDTA complex by solutions of Aliquat 336 in various organic solvents [J]. Analytica Chimica Acta, 1973, 63: 79-84.
[36] WIONCZYK B, APOSTOLUK W. Solvent extraction of chromium(III) from alkaline media with quaternary ammonium compounds, Part I [J]. Hydrometallurgy, 2004, 72: 185-193.
[37] WIONCZYK B, APOSTOLUK W. Solvent extraction of chromium(III) from alkaline media with quaternary ammonium compounds, Part II [J]. Hydrometallurgy, 2004, 72: 195-203.
[38] NAYL A A, ALY H F. Acid leaching of ilmenite decomposed by KOH [J]. Hydrometallurgy, 2009, 97: 86–93.
[39] de MENDONC F,  A, MANSUR M B. Liquid–liquid extraction of mercury(II) from hydrochloric acid solutions by Aliquat 336 [J]. Hydrometallurgy, 2007, 87: 83-90.
A, MANSUR M B. Liquid–liquid extraction of mercury(II) from hydrochloric acid solutions by Aliquat 336 [J]. Hydrometallurgy, 2007, 87: 83-90.
[40] SOLE K C. Recovery of titanium from the leach liquors of titaniferous magnetites by solvent extraction. Part 1: Review of the literature and aqueous thermodynamics [J]. Hydrometallurgy, 1999, 51(2): 239-253.
[41] CICERI D, MASON L R, HARVIE D J E, PERERA J M, STEVENS G W. Extraction kinetics of Fe(III) by di-(2-ethylhexyl)phosphoric acid using a Y–Y shaped microfluidicdevice [J]. Chemical Engineering Research and Design, 2013, 92(3): 571-580.
[42] FERNANDEZ-SANJURJO M J A, LVAREZ E, GARCIA-RODEJA E. Speciation and solubility control of aluminium in soils developed from slates of the river Sor watershed (Galicia,NWSpain) [J]. Water Air Soil Pollution, 1998, 103: 35–53.
[43] TANGRI S K, SURI A K, GUPTA C K. Development of solvent extraction processes for production of high purity oxides of molybdenum, tungsten and vanadium [J]. Transactions of the Indian Institute of Metals, 1998, 51(1): 27-39.
[44] HO E M, KYLE J, LALLENEC S, MUIR D M. Recovery of vanadium from spent catalysts and alumina residues [D]. Perth, Western Australia: Murdoch University, 1994.
[45] OLAZABAL M A, ORIVE M M, FERNANDEZ I A, MADARIAGA J M. Selective extraction of vanadium (V) from solutions containing molybdenum (VI) by ammonium salts dissolved in toluene [J]. Solvent Extraction and Ion Exchange, 1992, 10(4): 623-635.
[46] ZHOU X, WEI C, LI M, QIU S, LI X. Thermodynamics of vanadium–sulfur–water systems at 298 K [J]. Hydrometallurgy, 2001, 106: 104–112.
[47] BALL J W, NORDSTROM D K. Critical evaluation and selection of standard state thermodynamic properties for chromium metal and its aqueous ions, hydrolysis species, oxides, and hydroxides [J]. Journal of Chemical Engineering Data, 1998, 43: 895-918.
[48] NAZAL M K, ALBAYYARI M A, KHALILI F I. Effect of high ionic strength on the extraction of uranium(VI) ions [J]. Journal of Saudi Chemical Society, 2014, 18: 59–67.
[49] BEDEMO A. Removal of chromium from waste water using locally available adsorbents [D]. Addis Ababa: Addis Ababa University, 2007.
[50] WIONCZYK B, APOSTOLUK W, CHAREWICZ W A. Solvent extraction of chromium (III) from spent tanning liquors with Aliquat 336 [J]. Hydrometallurgy, 2006, 82: 83–92.
[51] ISLAM F, RAHMAN H, ALI M. Solvent extraction separation study of Ti(IV), Fe(III) and Fe(II) from aqueous solution with di-2-ethylexyl phosphoric acid in benzene [J]. Journal o Inorganic Nuclear Chemistry, 1997, 41: 217-221.
利用甲基三烷基氯化铵从酸性钛铁矿浸取液中萃取V(V)和Cr(III)
A. A. NAYL1,2, H. F. ALY2
1. Chemistry Department, College of Science, Aljouf University, Skaka 2014, Saudi Arabia;
2. Hot Laboratories Centre, Atomic Energy Authority, Cairo 13759, Egypt
摘 要:采用0.4 mol/L煤油稀释的甲基三烷基氯化铵从钛铁矿的酸性硫酸盐浸取液中萃取V(V)和Cr(III)。研究不同参数如平衡时间、硫酸盐浓度、甲基三烷基氯化铵浓度、平衡pH值和萃取温度对萃取过程的影响。利用甲基三烷基氯化铵萃取V(V)和Cr(III)包含了阴离子交换机制,对于V(V),在低平衡pH值时萃取物为[(VO2SO4)R4N]org,而对于Cr(III),在高平衡pH值时萃取物为[R4N-Cr(OH)4]org。计算的热力参数表明,对于V(I),萃取过程是一个吸热反应,而对于Cr(III),萃取过程是一个放热反应。DGex 和DSex的计算值表明,V(V)和Cr(III)的萃取反应为自发随机进行的。V(V)和Cr(III)经剥离、沉淀、分离后,在500 °C煅烧2.0 h,经过冲洗和干燥后,得到相应的纯氧化物。
关键词:萃取;酸性浸取液;V(V);Cr(III)
(Edited by Xiang-qun LI)
Corresponding author: A. A. NAYL; Tel: +20-1116373843; Fax: +20-2-4462-0796; E-mail: aanayl@yahoo.com
DOI: 10.1016/S1003-6326(15)64021-3
Abstract: Extraction of V(V) and Cr(III) from acidic sulfate leach liquors of ilmenite using 0.4 mol/L Aliquat 336 chloride in kerosene was carried out. Different parameters affecting the extraction process such as equilibrium time, sulfate concentration, Aliquat 336 concentration, equilibrium pH and the extraction temperature were investigated. Extraction of V(V) and Cr(III) by Aliquat 336 involved anion exchange mechanism, and the extracted species are [(VO2SO4)R4N]org at low equilibrium pH for V(V) and [R4N-Cr(OH)4]org at high equilibrium pH for Cr(III). Calculated thermodynamic parameters show that the extraction process is endothermic reaction for V(V) and exothermic for Cr(III). Also, calculated values of DGex and DSex indicate that the extraction reactions of V(V) and Cr(III) proceed as non-spontaneous reaction is more random. V(V) and Cr(III) were stripped, precipitated, separated and calcined at 500 °C for 2.0 h to produce the corresponding oxide in pure form after rinsing and drying.


