J. Cent. South Univ. Technol. (2009) 16: 0218-0222
DOI: 10.1007/s11771-009-0037-z
![]()
Bioleaching of chalcocite by mixed microorganisms subjected to mutation
KANG Jian(康 健)1, 2, QIU Guan-zhou(邱冠周)1, GAO Jian(高 健)2,
WANG Hai-hua (王海华) 2, WU Xue-ling(吴学玲)1, DING Jian-nan(丁建南)1
(1. School of Resources Processing and Bioengineering, Central South University,
Changsha 410083, China;
2. School of Life Science, Hunan University of Science and Technology, Xiangtan 411201, China)
Abstract:
Mixed microorganisms with elevated activity of chalcocite-leaching were screened by mutation methods. The original microorganisms collected from acid mine drainage of different sites were mixed and then treated with mutagens NO2-, diethyl sulfate (DES), UV and their combinations, respectively. Five groups of mixed microorganisms with much stronger ore-leaching ability were obtained by screening on the leaching media. Among them, group E of mixed microorganisms (treated with 1% DES for 60 min) with the best performance on chalcocite-leaching, increases the content of Cu2+ by 101.4% in 20 d of leaching compared with the control culture. In addition, group E is more tolerant to Cu2+ in media than the control without mutation treatment. Analysis for the diversity of microbial clones indicates that half of operational taxonomic units (OTUs) in group E are Acidithiobacillus ferrooxidans. These observations suggest that group E might have potentials for industrial application.
Key words:
chalcocite; mixed microorganisms; bioleaching; mutation;
1 Introduction
It is widely accepted that certain microorganisms play a major role in the leaching of basal and precious metals from various mineral resources, especially sulfide minerals. Bioleaching processes are commercially applied to dump and heap leachings of copper from low-grade sulfide minerals. A mass of low-grade sulfide ores exist all over the world. They are identified as complex sulfides or complex pyrites containing profitable amounts of valuable metals such as copper and zinc. Thus, there is a growing interest in the study of processes that lead to the increase in the production of metals from minerals. Particularly, the bioleaching technology for recovering metals becomes a focus of attention in metallurgy. Classically, these kinds of processes have been studied by controlling the dissolution rate of the metals [1].
A number of microbial genera have been shown to be very important in the process of bioleaching, including Acidothiobacillus (previously Thiobacillus), Leptospirillum, Acidiphilium, Sulfobacillus, Ferroplasma, Sulfolobus, Metallosphaera, and Acidianus [2-7]. The key factors in hindering biohydrometallurgy from large-scale application are low microbial activity and long leaching time in comparison with chemical metallurgy. Presently, there are two strategies to improve the bioleaching efficiency of microorganisms. One is the adapted method, which is a very effective way and widely used, the other is the mutation method of single microbial species. Nevertheless, bioleaching is a complicated process involving many microbial species. So, mutation of mixed microbial cultures might be an alternative strategy for improving efficiency of bioleaching [8-9]. In this work, in order to obtain mixed microbial cultures with higher Cu2+-leaching efficiency, several mutation agents and their combinations were employed to mutagenize microbial cultures.
2 Experimental
2.1 Materials
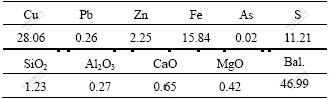
The ore used was the concentrate of chalcocite from Yunnan Province, China. It was pulverized to 75 μm for microorganisms ore leaching. Chemical analysis shows that chalcocite is of higher grade (Table 1).
Table 1 Composition of chalcoite (mass fraction, %)
2.2 Cultivation and mutagenesis of mixed cultures
Microorganisms were cultured in 9K liquid medium [10-11] supplemented with Fe2+ at 30 ℃ for 48 h, collected by centrifugation at 8 000 r/min for 10 min and suspended in sterile physiological saline. The suspension was adjusted to the concentration of 1×108 /mL for mutation.
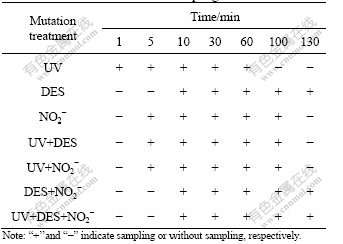
Mutation treatments and sampling time are shown in Table 2. The mutagenesis was performed as follows. For UV radiation, the suspended cells were radiated with two UV lamps, which placed 30 cm above the samples. For diethyl sulfate (DES) treatment, the suspended cells were treated with 1% DES. The reaction was terminated with 25% sodium hyposulfite. For NO2- mutagenizing, the cells were treated with 0.1 mol/L NaNO2 in acetic acid buffer (pH=4.6), and the reaction was terminated with 0.07 mol/L Na2HPO4 (pH=8.6). The combinations of mutagens, UV+DES, UV+NaNO2, UV+DES+NaNO2 and DES+NaNO2 were also used.
Table 2 Mutation treatments and sampling time
The mixed ore-leaching microorganisms were collected from acid mine drainage of more than 30 copper mines around the country, mixed, and enriched.
2.3 Bioleaching tests
After mutation, the cell suspensions were applied to bioleaching. For preliminary screening, 1 mL mixed culture was inoculated in 100 mL 9K medium containing 1 g of chalcocite and was incubated under shaking (200 r/min) at 30 ℃ for 10 d. Cell suspensions with larger amounts of biomass were selected for the second bioleaching test, which was conducted in 100 mL 9K medium containing 10 g of chalcocite for 20 d.
For rescreening, the selected cultures that were positively mutated were cultured for 40 d in fresh Leathen or 9K medium containing 10 g of chalcocite.
Then, the group of mixed cultures with higher Cu2+- leaching yield was used for continuous leaching of lower-concentration chalcocite in 9K medium for 20 d. The ore added to the 9K medium was from the second bioleaching test. The microbial culture for bioleaching test was under the same conditions as the first one in the preliminary screening.
2.4 Cu2+-tolerance test
The mixed cultures with the highest Cu2+-leaching yield in the rescreening were cultured in 9K medium supplemented with S0, Fe2+, S0+Fe2+ and chalcocite, containing 1, 2, 4, 8, 16 and 32 g/L Cu2+, respectively. The cells of the mixed culture were determined using a counting chamber 15 d after leaching. The soluble Cu2+, Fe2+ and Fe3+ were determined according to standard GB437—80.
2.5 Diversity of microbial clones
Analysis for restriction fragment length polymorphism (RFLP) was adopted [12-16].
3 Results and discussion
3.1 Mutation and preliminary screening
In the primary culture after mutation, we selected five groups of microorganisms with high turbidity growing in chalcocite, to investigate preliminarily the species of these groups of mixed microorganisms.
The results of the second bioleaching test indicate that all of the five mutated cultures achieve higher levels of Cu2+-leaching yields than cultures without mutation. The Cu2+-leaching yields with cultures A, B, C, D and E are increased by 95.4%, 81.2%, 70.6%, 58.7%, and 101.4% (Fig.1), respectively, demonstrating that the mutation of the mixed microorganisms with a traditional mutation strategy leads to an increase in Cu2+ chalcocite- leaching.
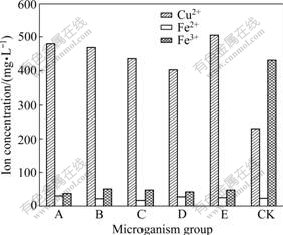
Fig.1 Metal dissolution from chalcocite with mixed cultures after leaching for 20 d (A: DES+NO2- with 60 min of mutation; B: DES with 10 min of mutation; C: UV with 10 min of mutation, D: UV+ DES+NO2- with 100 min of mutation; E: DES with 60 min of mutation; CK: Mixed microorganisms without mutation treatment)
3.2 Rescreening
To investigate whether the bioleaching performances of the five positively mutated mixed cultures are stable, the third bioleaching experiments with two kinds of media 9K and Leathen were conducted. The Cu2+- leaching yields in all of the selected five positively mutated mixed cultures are larger than those of the untreated control in 9K (KCK) and Leathen (LCK) media (Figs.2-3). The mixed culture E has the highest Cu2+ leaching yield. When the samples are leached with mixed culture E in 9K and Leathen media for 40 d, the Cu2+-leaching yields are increased by 131.1% and 187.2%, respectively, compared with those of the control culture. The results demonstrate that the five positively mutated cultures are stable in bioleaching process, suggesting that they might be adaptive to industrial applications.
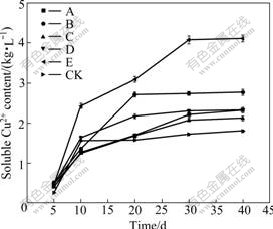
Fig.2 Time-course of Cu2+ leaching by mutated mixed micro- organisms in 9K medium
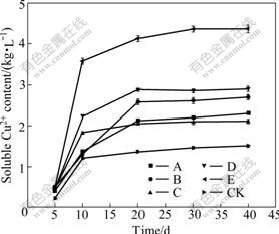
Fig.3 Time-course of Cu2+ leaching by mutated mixed micro- organisms in Leathen medium
3.3 Cu2+-tolerance and continuous leaching
Cu2+ leached from chalcocite may be inhibitory to microbial growth, which is disadvantageous for bioleaching. The Cu2+ tolerance of culture E was studied using different Cu2+ concentrations in 9K medium supplemented with S0, Fe2+, Fe2++S0, and chalcocite. The numbers of microorganisms were determined by counting chamber 15 d after leaching. As shown in Fig.4, the mixed culture E is tolerant to 32 g/L Cu2+. However, for the control culture without mutation, living cells are not observed in the S0, Fe2+ and Fe2++S0 supplemented media with the addition of 16 g/L Cu2+ and in chalcocite- medium with the addition of 8 g/L Cu2+ after 15 d. These results show that mixed culture E is more tolerant to Cu2+ than the control culture.
Feedback mechanisms may be operated in the process of bioleaching. As products of bioleaching, metal ions bring restrictions for further dissolution at high concentrations. Therefore, the tolerance to metal ions of microorganisms is an important characteristic for practical bioleaching. In the present study, group E of the mixed microorganisms acquired a high capacity of Cu2+ tolerance. This may contribute, at least partly, to its high performance of Cu2+-dissolution in the leaching tests. Group E of the mixed microorganisms could be applied to practical industrial production, the production efficiency might be improved, and the production costs might be reduced to certain degree. Our continuous leaching experiments demonstrate that the leaching yield of group E culture is also greatly enhanced (data are not shown).
3.4 Diversity of microbial clones
The mixed microorganisms were cultivated in 9K medium containing 1% chalcocite pulp, and the diversity of microbial clones was analyzed by restriction fragment length polymorphism. Six operational taxonomic units (OTUs) were isolated from the culture and identified. 2#, 3# and 4# OTUs belong to Acidithiobacillus ferrooxidans, constituting 50% of identified OTUs. 5# and 6# OTUs belong to Acidithiobacillus caldus, and 7# OTU belongs to Leptospirillum ferriphilum. Their phylogenetic tree of mixed microorganisms based on 16S rDNA sequences is shown in Fig.5.
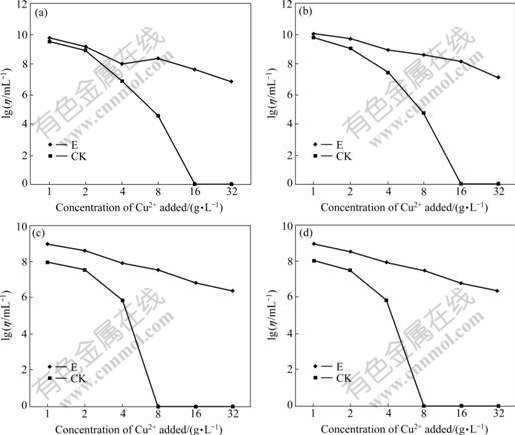
Fig.4 Tolerance of mixed microorganisms group E to Cu2+ on different Leathen media supplemented with S0 (a), Fe2+ (b), S0+Fe2+ (c) and chalcocite (d), respectively (η stands for concentration of bacteria, mL-1; E represents mixed microorganisms group E; CK represents mixed microorganisms without mutation)
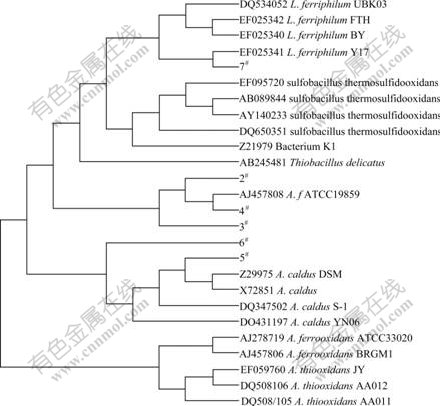
Fig.5 Phylogenetic tree of mixed microorganisms based on 16S rDNA sequences
4 Conclusions
(1) The Cu2+-leaching yields using mixed cultures A, B, C, D and E are significantly increased, compared with those of the unmutated control culture, suggesting that the mutation of mixed cultures with a mutation strategy has an excellent effect on Cu2+ chalcocite-leaching.
(2) Cu2+-tolerance of the mixed culture E is greatly enhanced after mutation.
(3) 50% OTUs in the mixed culture E are identified as Acidithiobacillus ferrooxidans.
References
[1] YASUHIRO K, SATORU A, MASAHIKO T, TORU S. Kinetics of the bioleaching of chalcopyrite concentrate by acidophilic thermophile Acidianus brierleyi [J]. Biotechnology Progress, 1999, 15(4): 681-688.
[2] HARRISON A P. Genomic and physiological diversity amongst strains of Thiobacillus ferrooxidans, and genomic comparison with Thiobacillus thiooxidans [J]. Archives of Microbiology, 1982, 131(1): 68-76.
[3] IZAMA H M, SUZUKI I J. Bacterial leaching of a sulphide ore by Thiobacillus ferrooxidans and Thiobacillus thiooxidans shake flask studies [J]. Biotechology and Bioengineering, 1988, 22: 110-116.
[4] LI H X, WANG D Z. Review of investigation on microorganism behaviors in ore bioleaching [J]. Nonferrous Metals, 2003, 55(2): 59-63.
[5] NORRIS P R, MARSH R M, LINSTROM E B. Growth of mesophilic and thermophilic acidophilic baeteria on sulfur and tetrathionate biotechnology [J]. Applied Biochemistry, 1986, 8: 318-329.
[6] NORRIS P R, BARR D B. Growth and iron oxidation by moderate thermophiles [J]. FEMS Microbiology Letters, 1985, 28(3): 221-224.
[7] KONIG H, SKORKO R J. Zillig W, Reiter W. D. Glycogen in Thermoacidophilic archaebacteria of the genera sulfolobus, thermoproteus, desulfurococcus and thermococcus [J]. Achives of Microbiology, 1982, 132(4): 297-303.
[8] KANG Jian, GAO Jian, WU Xue-lin, DING Jian-nan, QIU Guan-zhou. Mutagenesis of mixed bacteria and influence on bioleaching of sphalerite with the mutagenized bacterial admixture [J]. Journal of Central South University: Science and Technology, 2007, 38(3): 439-444. (in Chinese)
[9] KANG Jian, DING Jian-nan, GAO Jian, WU Xue-lin, QIU Guan-zhou. Study on growth of mixed bacteria on zinc ore after mutation [J]. Progress in Modern Biomedicine, 2007, 7(4): 489-493. (in Chinese)
[10] SILVERMAN M P, LUNDGREN D J. Studies on the chemoautotrophic iron bacterium Thiobacillus ferrooxidans (I): An improved medium and a harvesting procedure for securing high cellular yields [J]. Journal of Bacteriology, 1959, 77(5): 642-647.
[11] KARAVAIKO G I, KARAVAIKO G I, ROSSI G, AGATE A D, Groudev S, Avakyan Z. Biogeotechnology of metals-manual [M]. Moscow: Centre for International Projects GKNT, 1988: 59-61.
[12] HORTON T R, BRUNS T D. The molecular revolution in ectomycorrhizal ecology: Peeking into the black-box [J]. Molecular Ecology, 2001, 10(8): 1855-1871.
[13] OGRAM A. Discussion soil molecular microbial ecology at age 20: Methodological challenges for the future [J]. Soil Biology and Biochemistry, 2000, 32(11/12): l499-l504.
[14] BRIDGE P, BRIAN S. Soil fungi: Diversity and detection [J]. Plant and Soil, 2001, 232(1/2): 147-154.
[15] DUNBAR J, TICKNOR L O, KUSKE C R I. Assessment of microbial diversity in four Southwestern United States soils by 16S rRNA gene terminal restriction fragment analysis [J]. Applied and Environmental Microbiology, 2000, 66(7): 2943-2950.
[16] MA W L, JOSQUIN T, MARK A. A new method for research on soil microbial diversity [J]. Acta Pedologica Sinica, 2004, 41(1): 103-107.
Foundation item: Project(50321402) supported by the National Natural Science Foundation of China; Project(2004CB619201) supported by the Major State Basic Research and Development Program of China
Received date: 2008-08-22; Accepted date: 2008-10-12
Corresponding author: QIU Guan-zhou, Professor, PhD; Tel: +86-731-8879212; E-mail: qgzfblw@163.com
(Edited by CHEN Wei-ping)
Abstract: Mixed microorganisms with elevated activity of chalcocite-leaching were screened by mutation methods. The original microorganisms collected from acid mine drainage of different sites were mixed and then treated with mutagens NO2-, diethyl sulfate (DES), UV and their combinations, respectively. Five groups of mixed microorganisms with much stronger ore-leaching ability were obtained by screening on the leaching media. Among them, group E of mixed microorganisms (treated with 1% DES for 60 min) with the best performance on chalcocite-leaching, increases the content of Cu2+ by 101.4% in 20 d of leaching compared with the control culture. In addition, group E is more tolerant to Cu2+ in media than the control without mutation treatment. Analysis for the diversity of microbial clones indicates that half of operational taxonomic units (OTUs) in group E are Acidithiobacillus ferrooxidans. These observations suggest that group E might have potentials for industrial application.


