Trans. Nonferrous Met. Soc. China 23(2013) 804-811
Comparison of Fe2+ oxidation by Acidithiobacillus ferrooxidans in rotating-drum and stirred-tank reactors
Jian JIN1, Shao-yuan SHI1,2, Guo-liang LIU1, Qing-hua ZHANG 1, Wei CONG1
1. National Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China;
2. State Key Laboratory of Bioreactor Engineering, East China University of Science and Technology, Shanghai 200237, China
Received 10 January 2012; accepted 15 May 2012
Abstract:
Fe2+ oxidation by Acidithiobacillus ferrooxidans (At. ferrooxidans) under different solid contents by adding inert Al2O3 powder was examined in rotating-drum and stirred-tank reactors. The results show that the bioactivity of At. ferrooxidans in the stirred-tank is higher than that in the rotating-drum in the absence of Al2O3 powder, but the biooxidation rate of Fe2+ decreases markedly from 0.23 g/(L·h) to 0.025 g/(L·h) with increasing the content of Al2O3 powder from 0 to 50% (mass fraction) in the stirred- tank probably due to the deactivation of At. ferrooxidans resulting from the collision and friction of solid particles. The increase in Al2O3 content has a little adverse effect on the bioactivity of At. ferrooxidans in the rotating-drum due to different mixing mechanisms of the two reactors. The biooxidation rate of Fe2+ in the rotating-drum is higher than that in the stirred-tank at the same content of Al2O3 powder, especially at high solid content. The higher bioactivity of At. ferrooxidans can be maintained for allowing high solid content in the rotating-drum reactor, but its application potential still needs to be verified further by the sulfide bioleaching for the property differences of Al2O3 powder and sulfide minerals.
Key words:
Fe2+; Acidithiobacillus ferrooxidans; oxidation; bioactivity; solid content; rotating-drum reactor; stirred-tank reactor;
1 Introduction
Acidithiobacillus ferrooxidans (At. ferrooxidans) is one of the most important bacteria used in the bioleaching of sulfide ores [1,2]. The ability of At. ferrooxidans to oxidize ferrous ion and reduce sulfur compounds to ferric ion and sulfate respectively can result in the solubilisation of metals from their sulfides, which is generally referred to as the indirect leaching mechanism [3]. The oxidation rate of Fe2+ is an important indicator of the bioactivity of At. ferrooxidans. The high bioactivity of At. ferrooxidans means fast regeneration of Fe3+, which is favorable to the bioleaching of sulfide minerals.
Some researches related to the oxidation of Fe2+ by At. ferrooxidans were performed in the bioreactors with different support particles such as polyurethane foam [4], activated carbon [5] and chitosan beads [6]. The Fe2+ oxidation was separated from the bioleaching process of sulfide minerals in these investigations. Although Fe2+ biooxidation was also extensively studied in stirred-tank reactors [7] and shake flasks [8], no related investigation was reported on rotating-drum reactors, which can be viewed as a potential alternative to stirred-tank reactors.
The mixing and suspension of ore pulp in a rotating- drum is achieved by the direct lifting of baffle plates, whereas the mixing of ore pulp in a stirred-tank is driven by high-speed impellers. HERRERA et al [9] found that the total concentration of iron in the rotating-drum reactor was 2.3 times higher than that in the stirred-tank at high pulp densities in the bioleaching of refractory gold concentrates, but its gas-liquid mass transfer performance was not satisfactory for the lack of an efficient gas sparger [10]. The numerical simulation reported by LIU et al [11] showed that the increase of aeration rate could increase the volumetric averaged gas holdup in a rotary drum. JIN et al [12] improved the rotating-drum reactor by fitting a modified gas sparger to enhance its gas-liquid mass transfer performance and evaluated it by examining gas-liquid mass transfer, solid particles distribution and power consumption, but its application potential still needs to be verified further by the bioleaching of sulfide minerals.
In this work, the Fe2+ oxidation by At. ferrooxidans was investigated at different solid content of inert Al2O3 powders in rotating-drum and stirred-tank reactors firstly. The effects of Al2O3 content, inoculum concentration and baffle angle on the Fe2+ biooxidation were probed in the rotating-drum, and the results were compared with those obtained in the stirred-tank. The changes of pH, redox potential (φh) and Fe2+ concentration were measured in the biooxidation processes of Fe2+ for indexing the bioactivity of At. ferrooxidans.
2 Experimental
2.1 Al2O3 powder
Al2O3 powder, with the fraction of more than 93% particles below 135 μm, was added as inert material to study Fe2+ oxidation at different solid contents. The Al2O3 powder was soaked in and washed several times with acidified distilled water (pH 1.5), and then dried in an oven at 105 °C before the experiments.
2.2 Microorganism and medium
The bacterial strains of At. ferrooxidans used in the experiments were obtained from Institute of Microbiology, Chinese Academy of Sciences. The 9K medium developed by SILVERMAN and LUNDGREN [13] was used for the cell growth with the composition as follows: 3 g/L (NH4)2SO4, 0.5 g/L MgSO4·7H2O, 0.5 g/L K2HPO4, 0.15 g/L K2SO4, and 0.01 g/L Ca(NO3)2, and the Fe2+ concentration was controlled by adding FeSO4·7H2O according to the experimental schedule. The initial pH of the medium was adjusted to 2.0 with 6 mol/L H2SO4. All the reagents used in the experiments were analytical grade. Before the experiments the bacterial strains of At. ferrooxidans were cultured in the 9K medium for 3 months by subculturing repeatedly. The inoculum of At. ferrooxidans was prepared in an air-lifting reactor with the working volume of 1.8 L, which was then inoculated after culturing for 22 h, respectively, in the rotating-drum and stirred-tank reactors according to the experimental plan.
2.3 Bioreactor configuration
The rotating-drum bioreactor (13 L in working volume) was described by CONG et al [14] and the drum was made of Plexiglas with a thickness of 10 mm. A schematic diagram of the reactor and its dimensions are shown in Fig. 1(a). Eight straight baffles were arranged at equal distances around the circumference of the bioreactor. To improve the gas-liquid mass transfer performance of the rotating-drum bioreactor, three single channel microfiltration ceramic membranes with a pore size of about 1.0 μm were used as sparger [15], and their relative positions are shown in Fig. 1(a). The oxidation of Fe2+ by At. ferrooxidans in the bioreactor was examined to evaluate its application potential for bioleaching.
with a thickness of 10 mm. A schematic diagram of the reactor and its dimensions are shown in Fig. 1(a). Eight straight baffles were arranged at equal distances around the circumference of the bioreactor. To improve the gas-liquid mass transfer performance of the rotating-drum bioreactor, three single channel microfiltration ceramic membranes with a pore size of about 1.0 μm were used as sparger [15], and their relative positions are shown in Fig. 1(a). The oxidation of Fe2+ by At. ferrooxidans in the bioreactor was examined to evaluate its application potential for bioleaching.
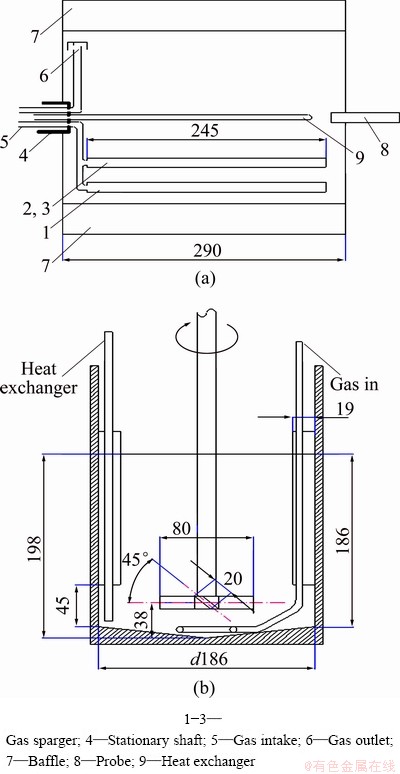
Fig. 1 Schematic diagrams of rotating-drum reactor with fitting gas sparger and stirred-tank reactor (Unit: mm)
For the sake of comparison, a stirred-tank reactor (4.5 L in working volume), which had 4 baffle plates installed on the inner wall of the stirred-tank (Fig. 1(b)), was also used in the oxidation of Fe2+. A 4-bladed 45° downward pitched blade turbine (impeller diameter of 0.1 m) was installed with off-bottom clearance of 38 mm in order to efficiently suspend the particles. Gas sparging was achieved by using a circular stainless sparger (below turbine) with nine 0.8 mm holes (facing downwards). The temperatures of tap water/slurry in both the rotating- drum and stirred-tank bioreactors were controlled at 35 °C with circulating water from a thermostat during all experiments.
2.4 Experimental procedure
The growth of At. ferrooxidans in the rotating-drum and stirred-tank in the absence of Al2O3 powder was investigated. The operating conditions in the reactors were pH value of 1.85, inoculum concentration of 10%, temperature at 35 °C and aeration rate of 1 L/(L·min).
Fe2+ oxidation by At. ferrooxidans was performed at different solid contents by adding different amount of inert Al2O3 powder in two reactors. The values of pH, redox potential (φh) and Fe2+ concentration were monitored during the biooxidation process. The effects of aeration rate and rotational speed of the drum on the distribution of solid particles and gas mass transfer were investigated in the previous work [12]. Unless otherwise specified, the drum rotating speed was 3.33 r/min and the impeller rotating rate in the stirred-tank was 600 r/min with the same aeration rate of 1 L/(L·min) in two reactors. The inoculum concentration was 10% of the total solution volume and the temperature was controlled at about 35 °C.
2.5 Analytical methods
During the oxidation of Fe2+ by At. ferrooxidans, the values of pH and φh were measured by a pH/ORP electrode and the dissolved oxygen (DO) concentration of the systems was monitored by a DO electrode plugged into the reactors. The solution samples were withdrawn at the predetermined intervals. The residual concentration of Fe2+ was analyzed by titrating with a standardized K2Cr2O7 solution using sodium diphenyl- amine sulphonate as an indicator [16].
3 Results and discussion
3.1 Growth of At. ferrooxidans
The growth of At. ferrooxidans was compared in the two reactors. The changes in pH, φh and Fe2+ concentration in the liquid medium in the absence of Al2O3 powder are shown in Fig. 2. Compared with the stirred-tank, the rotating-drum has the lower oxidation rate of Fe2+ and slower changes of pH and φh, which means that the bioactivity of At. ferrooxidans is higher in the stirred-tank in the absence of Al2O3 powder. The results indicate that the better mixing and oxygen mass transfer can be achieved in the stirred-tank at 600 r/min of the impeller rotational speed, compared with the rotating-drum at 3.33 r/min of the drum rotational speed. It is considered that the fluid shear from rotating impellers even at high speed in the stirred-tank does not affect the bioactivity of At. ferrooxidans, which is consistent to the result reported by LIU et al [17].

Fig. 2 Growth of At. ferrooxidans at 3.33 r/min of drum rotational speed in rotating-drum and at 600 r/min of impeller rotational speed in stirred-tank in absence of Al2O3 powder
It is also found from Fig. 2 that the obvious decrease in pH is presented in the latter period of the experiments. The reason for the decrease in pH may be the formation of precipitates such as jarosite or/and iron hydroxide due to the high Fe3+ concentration during the later stage of the oxidation process [18]. The related reaction is shown as follows:
X++3Fe3++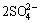 +6H2O→XFe3(SO4)2(OH)6+6H+ (1)
+6H2O→XFe3(SO4)2(OH)6+6H+ (1)
where X=K+, Na+, H3O+ or 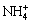 . The precipitates can lead to the passivation of the surface of sulfide ores during the sulfide bioleaching [19]. The decrease in pH value is too little to decrease the oxidation of Fe2+ by At. ferrooxidans in this work.
. The precipitates can lead to the passivation of the surface of sulfide ores during the sulfide bioleaching [19]. The decrease in pH value is too little to decrease the oxidation of Fe2+ by At. ferrooxidans in this work.
3.2 Effect of solid content
The biooxidation of Fe2+ by At. ferrooxidans at the solid content of 15% and 30% (mass fraction) of Al2O3 powder in the two reactors is shown in Fig. 3. The results show that the addition of Al2O3 can reduce the apparent bioactivity of At. ferrooxidans by prolonging the oxidation period of Fe2+ in both the reactors, compared with that in the absence of Al2O3 powder. It is found from Fig. 3 that the changes of pH, φh and Fe2+ concentration in the rotating-drum are larger than those in the stirred-tank, which means that the higher bioactivity of At. ferrooxidans can be maintained in the rotating-drum in the presence of Al2O3.
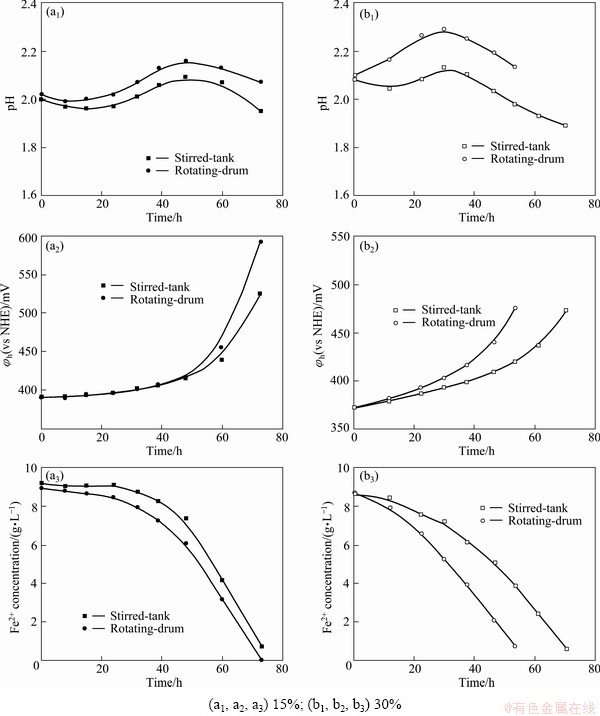
Fig. 3 Oxidation of Fe2+ by At. ferrooxidans in rotating-drum and stirred-tank reactors at different solid contents of Al2O3 powder
However, it is worth noting that the oxidation period of Fe2+ at 30% Al2O3 content is shorter than that at 15% Al2O3 content, and the lag time of Fe2+ oxidation at 30% Al2O3 is less than that at 0 and 15% Al2O3. The possible reasons for the results are that no metal ions are introduced to affect the bioactivity of At. ferrooxidans when only adding Al2O3 powder, which has less effect on Fe2+ oxidation than sulfide minerals. In addition, the state of inoculum can also affect the oxidation of Fe2+ by At. ferrooxidans at different solid contents. Compared with the stirred-tank, the rotating-drum gives higher oxidation rate of Fe2+ by At. ferrooxidans even at higher content of Al2O3, which is very favorable to the industrial bioleaching of sulfide minerals where the high solid content is allowed.
3.3 Inoculum concentration
The effects of inoculum concentrations on the biooxidation of Fe2+ by At. ferrooxidans were examined in two reactors. Under the same content of 30% Al2O3, the changes in pH, φh and Fe2+ concentrations at the inoculum concentrations of 5% and 10% (volume fraction), are shown in Fig. 4. The results indicate that two reactors behave similarly at the same inoculum concentration as pH, φh and Fe2+ concentration are concerned. Moreover, the lag period for the oxidation of Fe2+ increases as the concentration of inoculum decreases from 10% to 5%. The bacterial population available for the oxidation of Fe2+ by At. ferrooxidans is too low at the inoculum concentration of 5% in the two reactors. It is suitable to increase the inoculum concentration of At. ferrooxidans for the Fe2+ biooxidation since the advantages of shortened lag phase and increased oxidation rate of Fe2+ are favorable to the enhancement of bioleaching of sulfide minerals. The similar results were also reported by WANG et al [20].
3.4 Effect of baffle angle
In order to examine the effect of the inner structure of the rotating-drum on the oxidation of Fe2+ by At. ferrooxidans, the deflection angle of all baffles was adjusted to about 20° with the same orientation, which leaned backwards to the rotating direction of the drum. The ways to install the baffles with 0° and 20° in the rotating-drum are shown in Fig. 5. The Fe2+ oxidation by At. ferrooxidans was examined in the rotating-drum after the baffle angle was adjusted to 20° when the Al2O3 content increased from 0 to 50%, and the similar experiments were performed in the stirred-tank by adding different amount of Al2O3.

Fig. 4 Oxidation of Fe2+ by At. ferrooxidans under solid content of 30% Al2O3 at different inoculum concentrations
The changes of φh and Fe2+ concentrations under different solid contents of Al2O3 during Fe2+ biooxidation in the two reactors are shown in Fig. 6. The results from Figs. 6(a1) and (a2) indicate that the Fe2+ oxidation rate decreases gradually with the increase of solid content of Al2O3 and φh presents the similar changes in the stirred- tank. However, the changes of Fe2+ oxidation and φh are not affected markedly by the increase of Al2O3 content in the rotating-drum (shown Figs. 6(b1) and (b2)). LIU et al [17] confirmed that the main factor to decrease the bioactivity of At. ferrooxidans was the collision and friction between solid particles, instead of the fluid shear from the rotating impellers with high speed. It is presumed that the collision and friction between solid particles in the stirred-tank increase rapidly with the increase of Al2O3 content, which results in the obvious decrease of Fe2+ oxidation rate due to the deactivation of At. ferrooxidans. The effect of high Al2O3 content on the bioactivity of At. ferrooxidans in the rotating-drum is lower than that in the stirred-tank due to their different pulp mixing mechanisms, and the rotating-drum reactor is more favorable to the application in sulfide bioleaching for allowing higher solid contents.
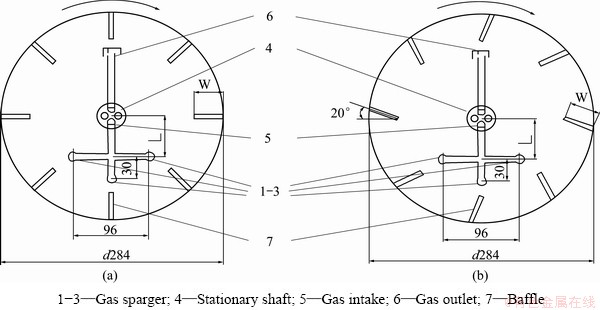
Fig. 5 Cross sections of rotating-drum reactor before (a) and after (b) angle adjustment of baffle plates (Unit: mm)

Fig. 6 Changes of φh and Fe2+ concentrations at different solid contents of Al2O3 in rotating-drum and stirred-tank reactors during biooxidation processes of Fe2+
In the bacterial oxidation of sulfide minerals, the gas-liquid transfer of O2 and CO2 often become the reaction rate determining factor [21]. It is found that the dissolved O2 level is maintained at 1-3 mg/L at different solid contents, indicating that the dissolved O2 seems not to be a limiting factor for the oxidation of Fe2+ by At. ferrooxidans at the aeration rate of 1 L/(L·min) in the two reactors. Although the increase of the solid content can lead to the decrease of dissolved O2 level in the stirred-tank for deteriorating the slurry mixing and gas-liquid transfer [22], the actual solid content suspended in the medium may be less than the amount of Al2O3 added because the particles of Al2O3 are not suspended fully in the stirred-tank at 600 r/min of the impeller rotational speed. The dissolved O2 level in the rotating-drum may be affected slightly by the increase of Al2O3 content due to gas pre-dispersion by the modified gas sparger, which is favorable to maintaining the high dissolved O2 level at high solid content for suppressing the diffusion and coalescence of small bubbles.
The results shown in Fig. 6 also indicate that the oxidation rate of Fe2+ by At. ferrooxidans decreases with increasing the Al2O3 content from 0 to 50%. DEVECI [23,24] showed that the viability of microbes decreased much rapidly when the solid content exceeded 20% in stirred-tank bioreactor. However, the oxidation of Fe2+ seems not to be affected markedly as expected when the content of Al2O3 powder is higher than 20% in the stirred-tank reactor. The possible reason should be that the impeller speed of 600 r/min (about 2.5 m/s) under aeration might be too low to suspend all the solid particles at high solid content (>30%) in the stirred-tank. Thus, the real solid content of the slurry system is lower than the nominal density calculated by the added amount of Al2O3. In contrast, the excellent mixing and gas mass transfer in rotating-drum reactors are easily achieved even at high solid content by optimizing the operating parameters such as the drum rotational speed and aeration rate [12], which do not produce the adverse effect on the bioactivity of microorganisms for different mixing mechanisms of the two reactors.
In order to improve the mixing and gas-liquid mass transfer of the reactor, the deflection angle of all baffles in the rotating-drum was adjusted to about 20° with the same orientation. However, considering the differences of inoculum state and measurement error in batches experiments, it is found that the oxidation of Fe2+ by At. ferrooxidans is not affected markedly by the angle adjustment of baffle plates in the rotating-drum when comparing the results shown in Figs. 3 and 6. It is presumed that the possible reason is the smaller working volume of the rotating-drum; moreover, the small changes in the actual height of the baffle plates after the angle adjustment of 20° do not markedly affect the performance of mixing and gas-liquid mass transfer in the rotating-drum.
The results in this work show that the oxidation rate of Fe2+ by At. ferrooxidans in the rotating-drum is higher than that in the stirred-tank, particularly at high solid content. Compared with the stirred-tank, the rotating- drum can provide the excellent mixing and gas mass transfer even at high solid content for their different mixing mechanisms, which means that the higher solid content is allowed in the rotating- drum reactor used in the bioleaching of sulfide minerals for the lower collision and friction of solid particles. However, the effect of scale-up on solids mixing and gas mass transfer efficiency of rotating-drum reactor needs to be further investigated, and the application performance of the reactor should be verified further for the bioleaching of sulfide minerals because of the differences of inert Al2O3 powder and sulfide minerals in physicochemical properties.
4 Conclusions
1) A higher bioactivity of At. ferrooxidans was observed in the stirred-tank than that in the rotating-drum in the absence of Al2O3 powder , but the oxidation rate of Fe2+ decreased markedly from 0.23 g/(L·h) to 0.025 g/(L·h) with increasing the content of Al2O3 powder from 0 to 50% in the stirred-tank probably due to the deactivation of At. ferrooxidans resulting from collision and friction of solid particles.
2) The high content of Al2O3 has less effect on the bioactivity of At. ferrooxidans in the rotating-drum than that in the stirred-tank due to their different mixing mechanisms. The higher bioactivity of At. ferrooxidans can be maintained in the rotating-drum even at the higher Al2O3 content, which is favorable to the sulfide bioleaching for allowing the higher solid content.
3) The application performance of rotating-drum reactor for the bioleaching of sulfide minerals still needs further verification because of the property differences of inert Al2O3 powder and sulfide minerals.
References
[1] BRIERLEY C L. Biohydrometallurgical prospects [J]. Hydro- metallurgy, 2010, 104(3-4): 324-328.
[2] CLARK M E, BATTY J D, BUUREN C B, DEW D W, EAMON M A. Biotechnology in minerals processing: Technological breakthroughs creating value [J]. Hydrometallurgy, 2006, 83(1-4): 3-9.
[3] SAND W, GEHRKE T, HALLMANN R, SCHIPPERS A. Sulfur chemistry, biofilm, and the (in) direct attack mechanism—A critical evaluation of bacterial leaching [J]. Applied Microbiology and Biotechnology, 1995, 43(6): 961-966.
[4] ARMENTIA H, WEBB C. Ferrous sulphate oxidation using Thiobacillus ferrooxidans cells immobilised in polyurethane foam support particles [J]. Applied Microbiology and Biotechnology, 1991, 36(5): 697-700.
[5] ZHOU H B, LIU X, QIU G Z, LIU J S, CHEN X H. Immobilization of Acidithiobacillus ferrooxidans and ferric iron production [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(4): 931-936.
[6] GIAVENO A, LAVALLE L, GUIBAL E, DONATI E. Biological ferrous sulfate oxidation by A. ferrooxidans immobilized on chitosan beads [J]. Journal of Microbiological Methods, 2008, 72(3): 227-234.
[7]  J M, CANTERO D. Kinetic study of biological ferrous sulphate oxidation by iron-oxidising bacteria in continuous stirred tank and packed bed bioreactors [J]. Process Biochemistry, 2003, 38(6): 867-875.
J M, CANTERO D. Kinetic study of biological ferrous sulphate oxidation by iron-oxidising bacteria in continuous stirred tank and packed bed bioreactors [J]. Process Biochemistry, 2003, 38(6): 867-875.
[8] CABRERA G,  J M, CANTERO D. Influence of heavy metals on growth and ferrous sulphate oxidation by Acidithiobacillus ferrooxidans in pure and mixed cultures [J]. Process Biochemistry, 2005, 40(8): 2683-2687.
J M, CANTERO D. Influence of heavy metals on growth and ferrous sulphate oxidation by Acidithiobacillus ferrooxidans in pure and mixed cultures [J]. Process Biochemistry, 2005, 40(8): 2683-2687.
[9] HERRERA M N, ESCOBAR B, PARRA N,  C, VARGAS T. Bioleaching of refractory gold concentrates at high pulp densities in a nonconventional rotating-drum reactor [J]. Minerals and Metallurgy Processing, 1998, 15: 15-19.
C, VARGAS T. Bioleaching of refractory gold concentrates at high pulp densities in a nonconventional rotating-drum reactor [J]. Minerals and Metallurgy Processing, 1998, 15: 15-19.
[10]  POGGI-VARALDO H M. Oxygen transfer to slurries treated in a rotating drum operated at atmospheric pressure [J]. Bioprocess and Biosystems Engineering, 2006, 29(5-6): 391-398.
POGGI-VARALDO H M. Oxygen transfer to slurries treated in a rotating drum operated at atmospheric pressure [J]. Bioprocess and Biosystems Engineering, 2006, 29(5-6): 391-398.
[11] LIU T, QI X, SU G, JIN J, CONG W. Computational fluid dynamics (CFD) simulation of flow in the rotary drum for pyrite bio-preoxidization [J]. Chemical Engineering and Processing, 2008, 47(5): 971-978.
[12] JIN J, LIU G L, SHI S Y, CONG W. Studies on the performance of a rotating drum bioreactor for bioleaching processes—Oxygen transfer, solids distribution and power consumption [J]. Hydrometallurgy, 2010, 103(1): 30-34.
[13] SILVERMAN M P, LUNDGREN D G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans [J]. Journal of Bacteriology, 1959, 77(5): 642-647.
[14] CONG W, JIN J, LIU G L, SHI S Y, FANG Z H. A rotating drum bioreactor used in bioleaching: CN101191154B [P]. 2010-05-12. (in Chinese)
[15] SHI S Y, CONG W, LIU G L, JIN J, WEN S M, WU Z C. A gas- sparger used in a rotating drum reactor: Chinese Patent, ZL201020274510.5 [P]. 2010-07-27. (in Chinese)
[16] HERRERA L, RUIZ P, AGUILLON J C, FEHRMANN A. A new spectrophotometric method for the determination of ferrous iron in the presence of ferric iron [J]. Journal of Chemical Technology and Biotechnology, 1989, 44(3): 171-181.
[17] LIU G L, YIN J F, CONG W. Effect of fluid shear and particles collision on the oxidation of ferrous iron by Acidithiobacillus ferrooxidans [J]. Minerals Engineering, 2007, 20(13): 1227-1231.
[18] LEAHY M J, SCHWARZ M P. Modelling jarosite precipitation in isothermal chalcopyrite bioleaching columns [J]. Hydrometallurgy, 2009, 98(1-2): 181-191.
[19] HENAO D M O, GODOY M A M. Jarosite pseudomorph formation from arsenopyrite oxidation using Acidithiobacillus ferrooxidans [J]. Hydrometallurgy, 2010, 104(2): 162-168.
[20] WANG S T, ZHANG G J, YUAN Q H, FANG Z H, YANG C. Comparative study of external addition of Fe2+ and inoculum on bioleaching of marmatite flotation concentrate using mesophilic and moderate thermophilic bacteria [J]. Hydrometallurgy, 2008, 93(1-2): 51-56.
[21] BOOM M, HEIJNEN J J. Gas-liquid mass transfer phenomena in bio-oxidation experiments of sulphide minerals: A critical review of literature data [J]. Hydrometallurgy, 1998, 48(2): 187-204.
[22] WITNE J Y, PHILIIIPS C V. Bioleaching of Ok Tedi copper concentrate in oxygen- and carbon dioxide-enriched air [J]. Minerals Engineering, 2001, 14(1): 25-48.
[23] DEVECI H. Effect of solids on viability of acidophilic bacteria [J]. Minerals Engineering, 2002, 15(12): 1181-1189.
[24] DEVECI H. Effect of particle size and shape of solids on the viability of acidophilic bacteria during mixing in stirred tank reactors [J]. Hydrometallurgy, 2004, 71(3-4): 385-396.
转鼓和搅拌槽反应器中氧化亚铁硫杆菌氧化Fe2+的比较
金 建1, 石绍渊1,2, 刘国梁1, 张庆华1, 丛 威1
1. 中国科学院 过程工程研究所 生化工程国家重点实验室,北京 100190;
2. 华东理工大学 生物反应器工程国家重点实验室,上海 200237
摘 要:研究转鼓和搅拌槽反应器中氧化亚铁硫杆菌在不同Al2O3粉末含量下对Fe2+的氧化。结果表明:未添加Al2O3粉末时,氧化亚铁硫杆菌在搅拌槽中的生物活性比在转鼓中的生物活性高。当Al2O3粉末含量从0增加到50% (质量分数)时,Fe2+的生物氧化速率从0.23 g/(L·h) 显著降低到 0.025 g/(L·h),可能是搅拌槽中的固体颗粒碰撞和研磨作用导致氧化亚铁硫杆菌失活。转鼓中Al2O3的含量增加对氧化亚铁硫杆菌的生物活性仅有较小的负面影响,这是由于两个反应器不同的混合机制所致。在相同的Al2O3含量下,Fe2+在转鼓反应器中的生物氧化速率比在搅拌槽中的生物氧化速率更高,尤其在较高的固体含量下,表明转鼓反应器能允许较高的固体含量和维持较高的生物活性。由于Al2O3粉末与真实硫化矿具有不同的物理化学性质,因此转鼓反应器用于硫化矿生物浸出的可行性还需进一步验证。
关键词:Fe2+;氧化亚铁硫杆菌;氧化;生物活性;固体含量;转鼓反应器;搅拌槽反应器
(Edited by Wei-ping CHEN)
Foundation item: Project ( 2010CB630904) supported by the National Basic Research Program of China; Project (5102030) supported by the Beijing Natural Science Foundation, China; Projects (21076214, 21006108) supported by the National Natural Science Foundation of China; Project supported by the Open Funding Project of the State Key Laboratory of Bioreactor Engineering, China
Corresponding author: Shao-yuan SHI; Tel: +86-10-82544844; Fax: +86-10-82544844-816; E-mail: syshi@home.ipe.ac.cn
DOI: 10.1016/S1003-6326(13)62532-7
Abstract: Fe2+ oxidation by Acidithiobacillus ferrooxidans (At. ferrooxidans) under different solid contents by adding inert Al2O3 powder was examined in rotating-drum and stirred-tank reactors. The results show that the bioactivity of At. ferrooxidans in the stirred-tank is higher than that in the rotating-drum in the absence of Al2O3 powder, but the biooxidation rate of Fe2+ decreases markedly from 0.23 g/(L·h) to 0.025 g/(L·h) with increasing the content of Al2O3 powder from 0 to 50% (mass fraction) in the stirred- tank probably due to the deactivation of At. ferrooxidans resulting from the collision and friction of solid particles. The increase in Al2O3 content has a little adverse effect on the bioactivity of At. ferrooxidans in the rotating-drum due to different mixing mechanisms of the two reactors. The biooxidation rate of Fe2+ in the rotating-drum is higher than that in the stirred-tank at the same content of Al2O3 powder, especially at high solid content. The higher bioactivity of At. ferrooxidans can be maintained for allowing high solid content in the rotating-drum reactor, but its application potential still needs to be verified further by the sulfide bioleaching for the property differences of Al2O3 powder and sulfide minerals.


