
Preparation and electrochemical properties of LiFePO4/C composite with network structure for lithium ion batteries
CHEN Han(陈 晗), YU Wen-zhi(于文志), HAN Shao-chang(韩绍昌), XU Zhong-yu(徐仲榆)
College of Materials Science and Engineering, Hunan University, Changsha 410082, China
Received 14 March 2007; accepted 22 June 2007
Abstract:
The bare LiFePO4 and LiFePO4/C composites with network structure were prepared by solid-state reaction. The crystalline structures, morphologies and specific surface areas of the materials were investigated by X-ray diffractometry(XRD), scanning electron microscopy(SEM) and multi-point brunauer emmett and teller(BET) method. The results show that the LiFePO4/C composite with the best network structure is obtained by adding 10% phenolic resin carbon. Its electronic conductivity increases to 2.86×10-2 S/cm. It possesses the highest specific surface area of 115.65 m2/g, which exhibits the highest discharge specific capacity of 164.33 mA?h/g at C/10 rate and 149.12 mA?h/g at 1 C rate. The discharge capacity is completely recovered when C/10 rate is applied again.
Key words:
LiFePO4/C composite; lithium ion batteries; electrochemical properties; network structure;
1 Introduction
LiFePO4 is an interesting alternative cathode material for lithium ion batteries, due to its cheap starting materials, good environmental compatibility, excellent cycling stability and high temperature performance[1-2]. The main disadvantages are very low electronic conductivity and diffusion coefficient of lithium ion, which lead to its poor rate capability and hinder its commercialization as cathode material for lithium ion batteries[3-4]. Some efforts to increase its conductivity have focused on reducing particle size[5-6], coating carbon[7-8] and doping metal cation[9-10].
Carbon coating can not change the intrinsical conductivity of LiFePO4, but it is very effective to enhance the capacity and rate capability[11-12]. Carbon black and sugar are used as main carbon resources. The electronic conductivity of LiFePO4/C composite prepared by BEWLAY et al[13] added with sucrose reached 0.1 S/cm. PROSINI’s research[14] showed that the practical capacity and rate capability of LiFePO4 can be improved by adding 10% carbon black to the starting materials. Carbon can control particles growth and enhance its conductivity. Therefore higher capacity and better rate capability can be obtained. At present, it is necessary to optimize carbon source and prepare LiFePO4/C composite with excellent electrochemical properties, especially rate capability.
Phenolic resin was used as carbon resource by YANG et al[15], the discharge capacity of LiFePO4/C composite is only 65 mA?h/g at C/3 rate and its structure has not been reported in their research. In this study, LiFePO4/C composite with porous network structure was prepared using phenolic resin as carbon resource.
2 Experimental
2.1 Materials preparation
LiFePO4/C composites were prepared by solid-state reaction. Li2CO3 (AR, Taishan Chemical Plant), FeC2O4·2H2O (AR, Fluka), and NH4H2PO4 (AR, Mingfeng Reagent Corporation) were used as the starting materials. Phenolic resin was added with different mass fractions. The starting materials were weighed in stoichio- metric ratio and homogenously mixed by ball grinding in anhydrous ethanol. The mixture was solidified at 150 ℃ for 5 h. To decompose the oxalate and the phosphate, the mixture was placed in a tubular furnace and treated at 350 ℃ for 5 h in argon flow. The resultant powders were cooled down to room temperature and reground, then returned to the tubular furnace and calcined at 650 ℃ for 18 h in argon flow. The last powders were cooled down to room temperature. Thus the LiFePO4/C composites containing various carbon amount were obtained.
2.2 Materials characterization
The crystalline structure was confirmed by a powder X-ray diffractometer (D5000) with Cu Kα radia- tion. The XRD data were obtained over an angular 2θ range from 15? to 45? with the step size of 0.02? and constant counting time of 0.2 s per step. The crystallite size of samples was calculated by the Scherrer equation D=Kλ/(β×cosθ) from the full width at half maximum (β) of the (131) diffraction peaks, in which K value is 0.89. The morphology of the composites was observed by scanning electron microscopy (JSM-6700F). The specific surface area of the powders was measured by the multi-point Brunauer Emmett and Teller (NOVA-1000) method. Conductivity measurement using four-probe testing instrument (SX1934) was made on disc-shaped pellet by four-point direct current method at room temperature, the measured value was revised for the thickness of pellet.
2.3 Electrochemical tests
Active material, conductive additive and polytetrafluorethylene(PTFE) were mixed homo- geneously in mass ratio of 75?20?5. The mixture was rolled into a 0.1 mm thin sheet with uniform thickness, from which pellets with 12 mm in diameter were cut. The pellet was used as the cathode and the electrolyte was 1 mol/L LiClO4 in ethylene carbonate and dimethyl carbonate (1?1 in volume). Lithium foil was used for the counter and reference electrodes. The separator was Celgard 2400 microporous membrane. The cell was assembled in an argon glove box. The galvanostalical tests were run by Arbin instrument (BT-2000) between 2.5 V and 4.1 V versus Li/Li+ at various rates.
3 Results and discussions
3.1 XRD analysis
The XRD patterns of LiFePO4/C composites containing various carbon amount and bare LiFePO4 are shown in Fig.1. All peaks of the samples can be indexed as olivine LiFePO4 phase without any observable secondary phase, but the XRD patterns have somewhat difference with the carbon amount increasing. The position of diffraction peaks deviates to lower diffraction angle, which shows that the distance between neighboring crystal planes becomes larger according to the Bragg equation 2dsinθ=λ. The intensity of diffraction peaks turns weaker gradually. The practical temperatures of composites are slightly lower than that of bare LiFePO4, because of adsorption of heat during decomposition of phenolic resin[16]. The lattice parameters of the samples are calculated from the Rietveld refinement on the basis of the Pmnb space group and shown in Table 1. The crystallite size of samples is calculated based on the strongest practical peak (131). With the carbon amount increasing, the lattice parameters of the composites become slightly larger. When cell volume increases, the size (D131) decreases gradually. When cell volume is too small, the atoms around lithium ions will hider their diffusion. On the contrary, when the cell volume is too large, the cell will be destroyed due to extraction and insertion of lithium ions. The lithium ions have to diffuse over larger distances between the boundary and center of crystallite grains due to larger crystallite size[17]. Therefore sample c has a good compromise between cell volume and crystallite size.
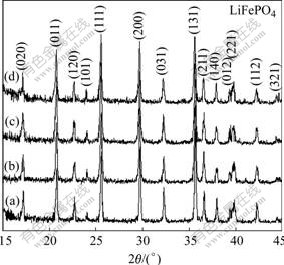
Fig.1 XRD patterns of samples with various carbon amount: (a) 0%; (b) 5%; (c) 10%; (d) 20%
Table 1 Comparison of lattice parameters and crystallite size of samples
3.2 SEM micrographs analysis
The SEM micrographs of the composites are shown in Fig.2. The porous network structure can be clearly observed in Figs.2(b) and (d). When phenolic resin is solidified, lots of polymeric webs ![]() form. Since the starting materials with phenolic resin are uniformly dispersed in anhydrous ethanol during solidification, the starting materials are trapped into the polymeric network. During the heat treatment, phenolic resin carbonizes, the porous network structure forms, and LiFePO4 covers on the carbon framework. This kind of structure characterized in detail in other paper is entirely different from carbon coating. The coexisted network structural composite and LiFePO4 grains can be clearly observed in Figs.2(a) and (b), which is due to inadequate phenolic resin portion to the starting materials of LiFePO4. As shown in Fig.2(c), the perfect network structure without any LiFePO4 grains because of the enough carbon framework adhering LiFePO4 grains is observed. As shown in Figs.2(e) and (f), it is obvious that the porous network structure disappears and the smooth grains pile together because the neighboring network incorporates one another during the teat treatment, when the 20% carbon is applied.
form. Since the starting materials with phenolic resin are uniformly dispersed in anhydrous ethanol during solidification, the starting materials are trapped into the polymeric network. During the heat treatment, phenolic resin carbonizes, the porous network structure forms, and LiFePO4 covers on the carbon framework. This kind of structure characterized in detail in other paper is entirely different from carbon coating. The coexisted network structural composite and LiFePO4 grains can be clearly observed in Figs.2(a) and (b), which is due to inadequate phenolic resin portion to the starting materials of LiFePO4. As shown in Fig.2(c), the perfect network structure without any LiFePO4 grains because of the enough carbon framework adhering LiFePO4 grains is observed. As shown in Figs.2(e) and (f), it is obvious that the porous network structure disappears and the smooth grains pile together because the neighboring network incorporates one another during the teat treatment, when the 20% carbon is applied.

Fig.2 SEM micrographs of composites with various carbon amount: (a), (b) 5%; (c), (d) 10%; (e), (f) 20%
3.3 BET tests
Fig.3 shows the specific surface areas of the samples with various carbon amount. As shown in Fig.3, the specific areas of materials increase rapidly with increasing the carbon amount. When 10% carbon is applied, the sample exhibits the highest specific surface area of 115.65 m2/g, nearly twenty times greater than that of bare LiFePO4. The high specific surface area is mainly attributed to porous network structure. When 20% carbon is applied, the specific surface area decreases abruptly because of the disappearance of porous network structure and the formation of smooth grains.

Fig.3 Specific surface areas of samples with various carbon amount
3.4 Conductivity measurement
The electronic conductivity of LiFePO4/C composites compared with bare LiFePO4 is shown in Table 2. Phenolic resin carbon plays a significantly important role to enhance electronic conductivity. The electronic conductivities of LiFePO4/C composites are much higher than that of bare LiFePO4. They increase with increasing carbon amount. The lowest limit of conductivity testing instrument (SX1934) is 10-6 S/cm, and the electronic conductivity of bare LiFePO4 is cited to compare with that of LiFePO4/C composite.
Table 2 Electronic conductivity of samples with various carbon amount

3.5 Electrochemical properties analysis
Fig.4 shows the charge and discharge curves of LiFePO4/C composites and bare LiFePO4 at C/10 rate. The calculation of specific capacity of LiFePO4/C composites is based on LiFePO4. The potential difference between the charge and discharge plateau of LiFePO4/C composites is only 0.05 V, which shows the weaker potential hystereresis compared with that of bare LiFePO4 because of the formation of porous network structure. The initial discharge specific capacity of LiFePO4/C composites is much larger than that of the bare LiFePO4. Especially, the LiFePO4/C composite containing 10% carbon has the largest initial discharge specific capacity (164.33 mA?h/g). The perfect porous network structure increases the contact area between carbon and LiFePO4, providing multi-dimension channels for diffusion of lithium ions.

Fig.4 Charge and discharge curves of samples with various carbon amount: (a) 0%; (b) 5%; (c) 10%; (d) 20%
The extended line of the potential plateau intersects the tangents of discharge curves at points A and B, shown in Fig.5. The capacity between points A and B is defined as plateau capacity. The ratio of the plateau capacity to the full discharge capacity is defined as plateau ratio. The materials with higher plateau capacity and plateau ratio can work longer. The comparison on the plateau performance of bare LiFePO4 and LiFePO4/C composites is listed in Table 3. When 10% carbon is applied, sample c possesses the highest plateau capacity and considerably high plateau ratio. But the plateau capacity of sample d decreases due to the hindrance of excessive carbon to the diffusion of lithium ions and the disappearance of porous network structure.
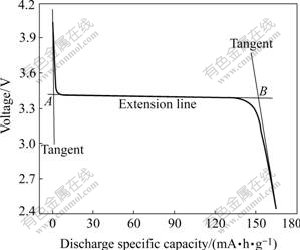
Fig.5 Sketch graph of plateau capacity
Table 3 Plateau capacity and ratio of samples with various carbon amount
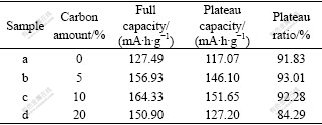
Fig.6 shows the curves of rate and cycling capability of all samples. To investigate the rate capability, various current densities corresponding to C/10, C/2 and 1 C are applied. All of them show excellent cycling stability. The discharge specific capacity of bare LiFePO4 at 1 C rate is only half as much as that at C/10 rate. When the rate increases, the capacity loss of LiFePO4/C composites containing 5% and 20% carbon is much larger than that of the composite containing 10% carbon. The composite containing 5% carbon has fewer network structure and more bare LiFePO4 grains. Likewise, when 20% carbon is applied, the network structure disappears, the smooth grains forms because of incorporation of neighboring network, and the excessive carbon hiders the diffusion of lithium ions, so the discharge specific capacity of LiFePO4/C composite containing 5% and 20% carbon is 100 mA?h/g at 1 C rate. The discharge specific capacity of LiFePO4/C composite containing 10% carbon is 149.12 mA?h/g at 1 C rate, and its capacity loss is only 9.5%. The good rate performance is attributed to perfect porous network structure that provides multi-dimension channels for diffusion of lithium ion and reduces the resistance for diffusion of lithium ion. Moreover, the composite with porous network structure can suck up electrolyte to shorten enormously the diffusive distance of lithium ion. When C/10 is applied again, the discharge specific capacity of all samples recovers completely, which shows that their structure has not been broken, and the capacity loss is due to stronger polarization at high rate. Therefore the porous network structure plays a significantly important role in improving the rate capability of LiFePO4.
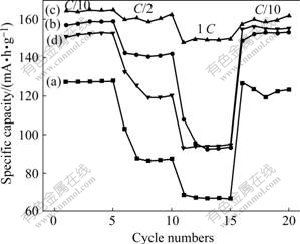
Fig.6 Curves of rate and cyclic capability of samples with various carbon amount: (a): 0%; (b) 5%; (c) 10%; (d) 20%
4 Conclusions
1) LiFePO4/C composites containing various amount of carbon have well-crystallized olivine structure.
2) The LiFePO4/C composite with excellent porous network structure and the highest specific surface area is obtained by adding 10% phenolic resin carbon, which exhibits excellent cycling and rate capability.
3) The LiFePO4/C composite with porous network structure is a promising cathode material for lithium ion batteries.
References
[1] QU Tao, TIAN Yan-wen, DING Yang, ZHONG Can-yun, ZHAI Yu-chun. Optimized synthesis technology of LiFePO4 for Li-ion battery [J]. Trans Nonferrous Met Soc China, 2005, 15(3): 583-588.
[2] PADHI A K, NANJJUNDASWAMY K S, GOODENOUGH J B. Phospho-olivines as positive electrode materials for rechargeable lithium batteries [J]. J Electrochem Soc, 1997, 144(4): 1188-1194.
[3] SLVSIN F, FREDEREC L C, CAROLE B, HELENE R. Comparison between different LiFePO4 synthesis routes and their influence on its physico-chemical properties [J]. J Power Source, 2003, 119/121: 252-257.
[4] CHIHIRO Y, YASUTOSHI I, JEONG S K, TAKESHI A , MINORU I, ZEMPACHI O. Electrochemical properties of LiFePO4 thin films prepared by pulsed laser deposition [J]. J Power Sources, 2005, 146: 559-564.
[5] PARK K S, KANG K T, LEE S B, KIM G Y, PARK Y J, KIM H G. Synthesis of LiFePO4 with fine particle by Co-precipitation method [J]. Mater Res Bull, 2004, 39(12): 1803-1810.
[6] PROSINI P P, CAREWSKA M, SCACCIA S, PAWEL W, MAURO P. Long-term cyclebility of nanostructured LiFePO4 [J]. Electrochim Acta, 2003, 48(28): 4205-4211.
[7] MYUNG S T, OMABA S K, TAKAGAI R. Emulsion drying preparation of LiFePO4/C composite and its enhanced high-rate performance at 50 ℃ [J]. Chem Lett, 2003, 32(7): 566-567.
[8] DELACOURT C, WURM C, LAFFONT L, LERICHE J B, MASQUELIER C. Electrochemical and electrical properties of Nb-and/or C-containing LiFePO4 composites [J]. Solid State Ionics, 2006, 177: 333-341.
[9] CHUNG S Y, BLOKING J T, CHIANG Y M. Electronically conductive phospho-olivines as lithium storage electrodes [J]. Nat Mater, 2002, 1(1): 123-128.
[10] WANG S Y, LI H, SHI S, HUANG X J, CHEN L Q. Improving the rate performance of LiFePO4 by Fe-site doping [J]. Electreochim Acta, 2005, 50: 2955-2958.
[11] XEI H, ZHOR Z. Physical and electrochemical properties of mix-doped lithium iron phosphate as cathoce material for lithium ion battery [J]. Electrochim Acta, 2006, 51: 2063-2067.
[12] ANDERSSON A S, THOMAS J O. The source of first-cycle capacity loss in LiFePO4 [J]. J Power Sources, 2001, 97/98: 498-502.
[13] BEWLAY S L, KONSTANTINOV K, WANG G X, DOU S X, LIU H K. Conductivity improvements to spray-produced LiFePO4 by addition of a carbon source [J]. Mater Lett, 2004, 58(11): 1788-1791.
[14] PROSINI P P, ZANE D, PASQUALI M. Improved electrochemical performance of a LiFePO4-based composite cathode [J]. Electrochim Acta, 2001, 46(23): 3517-3523.
[15] YANG S T, ZHAO N H, DONG H Y, YANG J X, YUE H Y. Synthesis and characterization of LiFePO4 cathode material dispersed with nano-structured carbon [J]. Electrochim Acta, 2005, 51(1): 166-171.
[16] CHEN H, HAN S C, YU W Z, FAN C L, XU Z Y. Preparation and electrochemical properties of LiFePO4/C composite cathodes for lithium-ion batteries [J]. Bull Mater Sci, 2006, 29(7): 689-692.
[17] MASYA T, SHINICHE T, KOJI T, YOJI S. Characterization of LiFePO4 as the cathode material for rechargeable lithium batteries [J]. J Power Sources, 2001, 97/98: 508-511.
[18] KWON S J, KIM W C, JEONG W T, KYUNG S L. Synthesis and electrochemical properties of olivine LiFePO4 as a cathode material prepared by mechanical alloying [J]. J Power Sources, 2004, 137(1): 93-99.
Foundation item: Project(50672024) supported by the National Natural Science Foundation of China; Project(06FJ2006) supported by the Applied Basic Research of Hunan Province, China
Corresponding author: HAN Shao-chang; Tel: +86-731-8822967; E-mail: hansc@hnu.cn


