Trans. Nonferrous Met. Soc. China 27(2017) 1374-1384
Relatedness between catalytic effect of activated carbon and passivation phenomenon during chalcopyrite bioleaching by mixed thermophilic Archaea culture at 65 °C
Ya-long MA1, Hong-chang LIU1, Jin-lan XIA1,2, Zhen-yuan NIE1,2, Hong-rui ZHU1, Yi-dong ZHAO3, Chen-yan MA3, Lei ZHENG3, Cai-hao HONG3, Wen WEN4
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Biometallurgy, Ministry of Education, Central South University, Changsha 410083, China;
3. Beijing Synchrotron Radiation Facility, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, China;
4. Shanghai Synchrotron Radiation Facility, Shanghai Institute of Applied Physics, Chinese Academy of Sciences, Shanghai 201800, China
Received 23 March 2016; accepted 21 February 2017
Abstract:
The relatedness between catalytic effect of activated carbon and passivation phenomenon during chalcopyrite bioleaching by mixed thermophilic Archaea culture (Acidianus brierleyi, Metallosphaera sedula, Acidianus manzaensis and Sulfolobus metallicus) at 65 °C was studied. Leaching experiments showed that the addition of activated carbon could significantly promote the dissolution of chalcopyrite for both bioleaching and chemical leaching. The results of synchrotron-based X-ray diffraction, iron L-edge and sulfur K-edge X-ray absorption near edge structure spectroscopy indicated that activated carbon could change the transition path of electrons through galvanic interactions to form more readily dissolved secondary mineral chalcocite at a low redox potential (<400 mV) and then enhanced the copper dissolution. Jarosite accumulated immediately in the initial stage of bioleaching with activated carbon but copper dissolution was not hindered. However, much jarosite precipitated on the surface of chalcopyrite in the late stage of bioleaching, which might account for the decrease of copper dissolution rate. More elemental sulfur (S0) was also detected with additional activated carbon but the mixed thermophilic Archaea culture had a great sulfur oxidation activity, thus S0 was eliminated and seemed to have no significant influence on the dissolution of chalcopyrite.
Key words:
chalcopyrite; bioleaching; activated carbon; passivation phenomenon; mixed thermophilic Archaea culture;
1 Introduction
As the most abundant but refractory copper sulfide, chalcopyrite has still not been successfully bioleached on a commercial scale [1]. However, with the increasing consumption of global copper resources and the decreasing reserves of high grade copper ores, it is necessary to extend the application of bioleaching, which is eco-friendly for the treatment of low grade copper sulfide. Commercial application of chalcopyrite bioleaching has been restricted by its extremely slow dissolution kinetics and low copper recovery because of the formation of a passivation layer on the mineral surface [2].
Over the past decades, although comprehensive investigations employing surface analytical methods (such as X-ray photoelectron spectroscopy, X-ray absorption spectroscopy, and Raman spectroscopy) have been conducted to evaluate the passivation layer, researchers have not yet reached a consensus about the chemical compositions of the passivation layer, for example, ferric precipitates (jarosite) [3], elemental sulfur (S0) [4] and polysulfide  [5] were reported.
[5] were reported.
On the other hand, in order to enhance the leaching rate and overcome the inhibitory effect of the passivation layer, several strategies have been proved to be beneficial to increasing the copper recovery rate, for instance, bioleaching of chalcopyrite with thermophilic Archaea strains, adding modifier (surfactants, activated carbon and silver) and controlling the redox potential. WAN et al [6] exhibited for the first time that the formation of chalcopyrite/carbon aggregates enhanced the dissolution rate of chalcopyrite in the ferric sulfate leaching. After that, many researchers paid much attention to the positive effects of activated carbon on chalcopyrite bioleaching [7-11], which indicated that the catalytic effect was attributed to the galvanic interaction between chalcopyrite and activated carbon. Galvanic interaction is based on the contact between chalcopyrite and activated carbon with different rest potentials. The activated carbon with higher rest potential acts as cathode (Eq. (1)) and chalcopyrite with a lower rest potential serves as anode and is oxidized (Eq. (2)) [7].
O2+4H++4e→2H2O (1)
CuFeS2→Cu2++Fe3++2S0+5e (2)
Chalcopyrite dissolution is a complex process including the chemical speciation transformation and evolution of three elements (S, Fe and Cu) on chalcopyrite surface [12-14]. When introducing activated carbon, the transition path of electrons could be changed and the composition and properties of chalcopyrite surface could be modified, finally affecting the sulfur oxidation activity of leaching microorganisms and the further dissolution of chalcopyrite [2]. It is still unclear how the passivation layer and the catalytic effect of additional activated carbon interact with each other to influence the dissolution of chalcopyrite.
The intermediate compounds formed during bioleaching are the keys to clarify the decomposition mechanism of chalcopyrite; however, usually their amounts are too small to be detected. Up to date, synchrotron radiation X-ray diffraction (SR-XRD) and X-ray absorption near edge structure (XANES) spectroscopy analysis have shown efficiency to study the composition on chalcopyrite surface with high spatial resolution and high sensitivity [15]. Therefore, by combining SR-XRD and XANES spectroscopy analysis, more details can be provided during chalcopyrite bioleaching with additional activated carbon.
In the present study, the relatedness between catalytic effect of activated carbon and passivation phenomenon during chalcopyrite bioleaching by mixed thermophilic Archaea culture (Acidianus brierleyi, Metallosphaera sedula, Acidianus manzaensis and Sulfolobus metallicus) and chemical leaching was investigated by combining iron L-edge and sulfur K-edge XANES spectroscopy as well as SR-XRD. It could be valuable for better understanding the dissolution mechanism of chalcopyrite during bioleaching by mixed thermophilic Archaea culture with additional activated carbon.
2 Experimental
2.1 Metal sulfide sample and activated carbon
The standard mineral samples including chalcopyrite (CuFeS2), chalcocite (Cu2S), covellite (CuS), bornite (Cu5FeS4), elemental sulfur (S0) and jarosite (KFe3(SO4)2(OH)6) used in this study were provided by the School of Minerals Processing and Bioengineering, Central South University, China. The X-ray fluorescence analysis (Axios mAX, PANalytical, the Netherlands) showed that the original chalcopyrite contained 33.69% Cu, 29.62% Fe, 34.34% S, 1.91% O, 0.31% Si, 0.04% Al, 0.02% Ca, 0.01% Se and 0.02% P. XRD analysis (DX-2700, Haoyuan Instruments, Danyang, China) showed that the original chalcopyrite mineral is basically pure. Before the experiment, original chalcopyrite and activated carbon (spectrum grade) were first milled to powder and passed through a sieve of 37-74 μm.
2.2 Microorganisms and leaching experiments
It was reported that mixed culture of thermophilic Archaea could significantly promote the leaching rate of chalcopyrite due to a higher sulfur oxidation activity than that of the pure culture [16]. Consequently, a mixed culture of thermophilic Archaea containing Acidianus brierleyi, Metallosphaera sedula, Acidianus manzaensis and Sulfolobus metallicus was used as inoculum in the bioleaching experiment. The detailed description of these four thermophilic Archaea strains was as referred to Ref. [17]. The basal medium used for cell cultivation contained the following components: 3.0 g/L (NH4)2SO4, 0.5 g/L MgSO4·7H2O, 0.5 g/L K2HPO4, 0.1 g/L KCl, 0.01 g/L Ca(NO3)2, and 0.2 g/L yeast extracts. The initial pH of the basal medium was adjusted to 1.5 with dilute sulfuric acid.
Bioleaching experiments were carried out in 250 mL of Erlenmeyer flasks containing 100 mL sterilized medium and 1 g chalcopyrite. According to previous research [11], the optimum concentration of activated carbon enhanced copper dissolution was 2 g/L during chalcopyrite bioleached by Acidianus manzaensis and the same concentration was applied in the present study. Activated carbon was mixed with chalcopyrite concentrate in a porcelain mortar and pestle for 5 min before they were added to the bioleaching system. The mixed thermophilic Archaea in the presence of 2 g/L or absence of activated carbon was incubated at 65 °C and 170 r/min in a high-temperature water-bath rotary shaker. The initial cell density was 1×107 cell/mL. The two chemical leaching experiments (one contained 2 g/L activated carbon, the other was free of activated carbon) were carried out as sterile controls. All experiments were performed in triplicate under the same conditions.
In the process of cultivation, 0.2 mL of samples was taken out every 24 h to monitor cell densities, pH values, total [Fe], [Fe3+] and [Cu2+]. The loss due to sampling every time was compensated by adding equal amount of sterilized basal medium. In addition, the loss of the water due to evaporation at 65 °C was also compensated with adding sterilized ultra-pure water based on mass loss during cultivation. The cell densities were determined by direct counting with a blood corpuscle counter (XB-K-25). The pH values of the culture solution were measured with a pH meter (PHS-3C). The redox potential values were measured with a platinum (Pt) electrode, using a calomel electrode (Hg/Hg2Cl2) as reference. The total [Fe], [Fe3+] and [Cu2+] were determined by atomic absorption spectrophotometer.
2.3 Mineral surface morphology
The surface morphology of chalcopyrite was observed by the scanning electron microscopy (SEM) (NovaTM NanoSEM 230, FEI, USA). The samples of mineral for morphology observation were collected into a 1.5 mL tube at the end of experiments. Before SEM analysis, mineral samples were thoroughly washed with ultra-pure water and dried in vacuum. The samples for SEM observation were first fixed in a 2.5% phosphate buffered glutaraldehyde solution for 2 h, post-fixed in 1% osmium tetroxide solution, washed in 0.1 mol/L phosphate buffer solution (pH 7.2), dehydrated using graded ethanol, immersed in tert-butyl alcohol for about 2 h, and freeze-dried, then coated with gold by a sputter (JEOL, JFC-1600, Japan) and introduced into the SEM chamber for observation.
2.4 SR-XRD
Before SR-XRD analysis, the original mineral sample and leaching residues at the end of day were thoroughly washed with ultra-pure water and dried in vacuum. All the samples were stored at 4 °C in nitrogen atmosphere until analysis. The composition changes of the samples were characterized by SR-XRD recorded at beam-line BL14B1 in Shanghai Synchrotron Radiation Facility (SSRF), China. The energy and the spot size of the X-ray were 10 keV and 0.5 mm × 0.5 mm, respectively.
2.5 Iron L-edge and sulfur K-edge XANES
In order to investigate the transformation of Fe/S speciation on chalcopyrite surfaces, both the original mineral samples and leaching residues at different leaching time were characterized by iron L-edge and sulfur K-edge XANES spectroscopy, respectively. Iron L-edge and sulfur K-edge XANES spectroscopy analyses were carried out at 4B7B beam-line and 4B7A beam-line, respectively, at Beijing Synchrotron Radiation Facility, China. The operational approaches, data acquisition and analysis were performed as described previously [15,18,19].
3 Results and discussion
3.1 Leaching parameters
The leaching behaviors by mixed thermophilic Archaea culture at 65 °C in the presence of 0 and 2 g/L activated carbon and during two chemical leaching experiments are shown in Figs. 1(a)-(f), respectively. It can be seen from Fig. 1(f) that after 8 d, the final copper ion concentration of chalcopyrite leached by mixed thermophilic Archaea in the presence of 2 g/L of activated carbon was (1.89±0.05) g/L, but it was (1.56±0.02) g/L in the absence of activated carbon. Alternatively, the copper ion concentration in the presence of 2 g/L activated carbon was (1.12±0.05) g/L, but it was (0.53±0.01) g/L when activated carbon was absent. Figure 1(a) showed that cells number in the presence of 2 g/L activated carbon decreased from initial (11±1.4)×106 to (0.52±0.1)×106 cell/mL after 1 d, which indicated the attachment of Archaea cells to activated carbon because activated carbon had strong ability of adsorbing microorganisms. According to the previous study [20], adsorption by activated carbon would probably decrease the possibility of attachment of cells onto the mineral surface. It is generally accepted that the attachment of cells onto the mineral surface is essential for the dissolution of chalcopyrite in the initial leaching process [21]. However, such negative effect was insignificant because the catalytic effect of activated carbon played a dominant role. Figure 1(f) showed that it was during the initial 3 d the dissolution kinetics of bioleaching with 2 g/L activated carbon was the highest. After that, cells number increased to the similar number to that in the absence of activated carbon and showed little difference in the rest of leaching time.
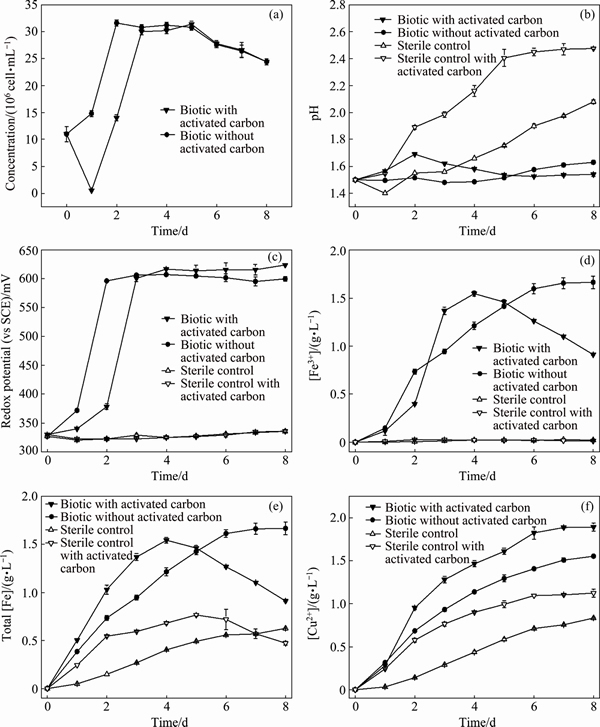
Fig. 1 Changes of cell concentration (a), pH value (b), redox potential (c), [Fe3+] (d), total [Fe] (e), and [Cu2+] (f) during chalcopyrite bioleaching at 65 °C and chemical leaching with 2 g/L and without activated carbon, respectively
The dissolution of chalcopyrite during leaching can be represented by Eqs. (3)-(6) [21]. According to these equations, chalcopyrite could be dissolved by protons and ferric ions in acidic solution. The main role of microorganisms in chalcopyrite bioleaching was regeneration of protons and ferric ions. As shown in Fig. 1(b), the pH demonstrated an increasing trend during chemical leaching in the two sterile controls throughout the whole leaching process. This could be explained by the consumption of protons, leading to the increase of pH. Especially, the pH of chemical leaching with activated carbon increased from the beginning until day 5 and reached the maximum of pH (2.5±0.1). More consumption of protons indicated that the addition of activated carbon could promote the dissolution of chalcopyrite during chemical leaching. While in bioleaching with activated carbon, the pH rose a little in the initial stage, and then stayed around the initial pH 1.5. And in bioleaching without activated carbon, the pH stayed around the initial pH 1.5. Moreover, the steady of pH during bioleaching could be explained by the oxidation of S0 formed as the intermediate sulfur compounds, which regenerated the protons consumed at the beginning.
CuFeS2+4H++O2 Cu2++2S0+Fe2++2H2O (3)
Cu2++2S0+Fe2++2H2O (3)
CuFeS2+4Fe3+ Cu2++5Fe2++2S0 (4)
Cu2++5Fe2++2S0 (4)
4Fe2++O2+4H+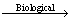 4Fe3++2H2O (5)
4Fe3++2H2O (5)
2S0+3O2+2H2O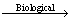
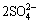 +4H+ (6)
+4H+ (6)
Redox potential of leaching solution was an important factor influencing the dissolution of chalcopyrite [2, 22]. Besides the pH, redox potential of leaching solution showed obvious differences between chemical leaching and bioleaching. It can be seen from Fig. 1(c) that the redox potential of two chemical leaching experiments stayed around the initial value through whole leaching process. In contrast, the redox potential in bioleaching experiments with/without activated carbon increased rapidly in initial 3 d and then basically unchanged. It has been confirmed the copper extraction rate in bioleaching experiment was higher than that in chemical leaching experiment, partially due to higher redox potential and [Fe3+] in bioleaching than that in chemical leaching (Eq. (4)). By comparing the two bioleaching groups, it was interesting to find that during the initial 3 d, the dissolution kinetics of bioleaching without activated carbon was lower than that with activated carbon although the former had higher redox potential and [Fe3+] (Figs. 1(c) and (d)). This phenomenon indicated that the additional activated carbon might change the transition path of electrons through galvanic interactions in contact with chalcopyrite to form more easily dissolved intermediates, thus the analysis of mineral surface components during leaching process was necessary.
It should be noted that with the increase of ferric ion concentration, the formation of jarosite could be also accelerated (Eq. (7)) [23], which was corresponding to the gradual decrease of [Fe3+] from day 4 (Fig. 1(d)) in bioleaching experiment with 2 g/L activated carbon.
M++3Fe3++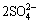 +6H2O→MFe3(SO4)2(OH)6+6H+ (7)
+6H2O→MFe3(SO4)2(OH)6+6H+ (7)
where M+ is a monovalent cation, such as H3O+, K+, Na+ and NH4+.
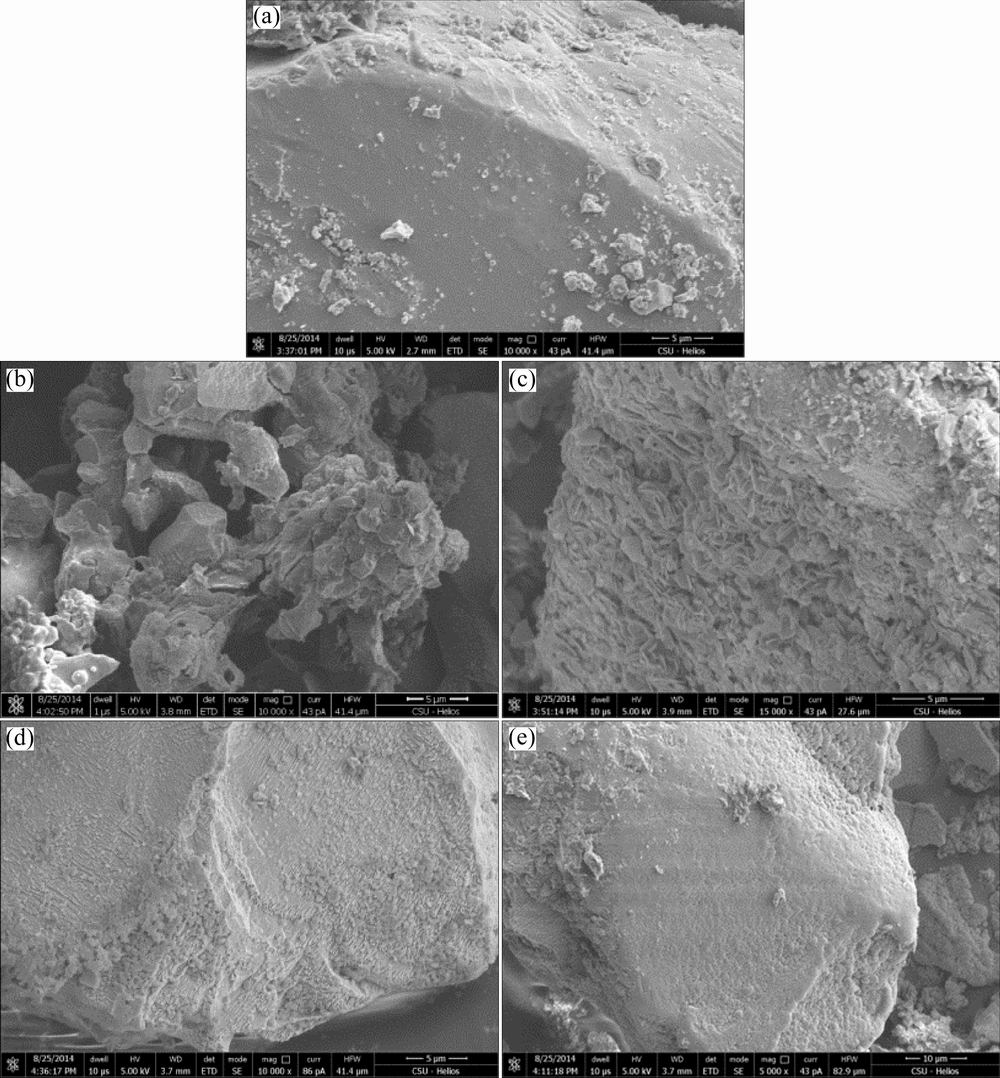
Fig. 2 SEM images of original chalcopyrite (a), chalcopyrite residue after bioleaching at 65 °C with 2 g/L activated carbon (b) and without activated carbon (c), and chalcopyrite residue after chemical leaching with 2 g/L activated carbon (d) and without activated carbon (e) after 8 d
3.2 Surface morphology
As shown in Fig. 2, the surface morphologies of the chalcopyrite residues leached by mixed Archaea culture at 65 °C and in chemical leaching experiments with/without activated carbon respectively were observed by SEM. Obviously, the surface morphology of bioleached chalcopyrite residue without activated carbon (Fig. 2(c)) was corroded seriously and much rougher than original chalcopyrite (Fig. 2(a)). By contrast, the residue grain size of chalcopyrite bioleached with 2 g/L activated carbon (Fig. 2(b)) became smaller than that without activated carbon (Fig. 2(c)), which indicated that the addition of activated carbon caused the corrosion of chalcopyrite much more serious. Similarly, chemical leaching residue with 2 g/L activated carbon (Fig. 2(d)) was rougher than that without activated carbon (Fig. 2(e)). According to the previous study [7], the addition of activated carbon can promote the dissolution of chalcopyrite through galvanic interaction between activated carbon and chalcopyrite, during which activated carbon acted as the cathode and accelerated the anodic dissolution of chalcopyrite.
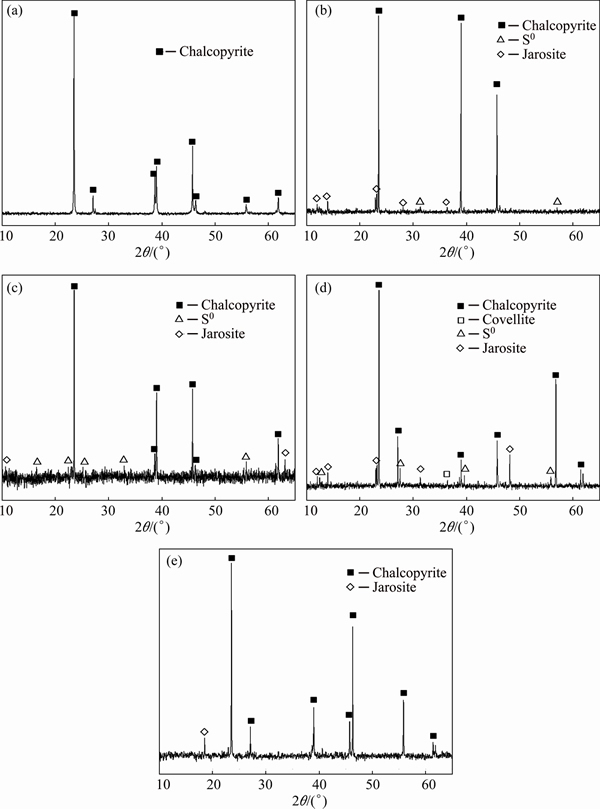
Fig. 3 XRD patterns of original chalcopyrite (a), chalcopyrite residue after bioleaching at 65 °C with 2 g/L activated carbon (b) and without activated carbon (c), and chalcopyrite residue after chemical leaching with 2 g/L activated carbon (d) and without activated carbon (e) after 8 d
3.3 SR-XRD analysis
The surface chemical components of original chalcopyrite, leaching residues by mixed thermophilic Archaea culture and chemical leaching residues after 8 d were studied by SR-XRD (Fig. 3). Results showed that both the residues after bioleaching with activated carbon and without activated carbon contained the diffraction signals of S0 and jarosite but the signal intensities were different (Figs. 3(b) and (c)). The chemical leaching residue with 2 g/L activated carbon showed diffraction signals corresponding to covellite, S0 and jarosite (Fig. 3(d)). By contrast, only a little jarosite was detected after chemical leaching without activated carbon (Fig. 3(e)). The SR-XRD analysis indicated that in bioleaching additional activated carbon did not change the species of final leaching products, which was consistent with previous study [11]. While in chemical leaching with activated carbon, covellite and S0 were detected, which indicated that through galvanic interaction between activated carbon and chalcopyrite, the transition path of electrons was changed. Further analysis by XANES spectroscopy at different time during leaching process could provide more details on the chemical speciation transformation on the mineral surface.
3.4 Iron L-edge and sulfur K-edge XANES analyses
The iron L-edge XANES spectra of the standard samples (chalcopyrite, ferrous sulfate, bornite and jarosite) and chalcopyrite during bioleaching in the presence of 2 g/L and absence of activated carbon are shown in Fig. 4. It can be seen from Fig. 4(a) that the differences in the shape of the L3-edge and the L2-edge among standard samples were significant. Briefly, for iron L3-edge, chalcopyrite had only one obvious peak (peak a). The intensity of peak a for ferrous sulfate was significantly higher than that of peak b. However, the intensities of peak b for bornite and jarosite were much higher than those of peak a, but with different absorption energy positions. The iron L-edge XANES spectra of chalcopyrite during bioleaching with activated carbon (Fig. 4(b)) changed dramatically from day 1 to day 2, in which peak a decreased from high intensity to a low level accompanying with the appearance of peak b instantly. Instead, results in Fig. 4(c) showed that during bioleaching without activated carbon, the intensities of peaks a and c gradually decreased with prolonging time, while the intensity of peak b gradually increased. By comparing with the standard spectra, the transformation of these peaks could be assigned to the formation of jarosite. In addition, chemical leaching residue in the presence of 2 g/L activated carbon (Fig. 4(b)) showed a similar spectra pattern to jarosite. While the iron spectra of chemical leaching without activated carbon (Fig. 4(c)) showed the similar features to the bioleaching without activated carbon at day 4 during which the intensity of peak a decreased and those of peaks b and c increased.
The iron K-edge spectra patterns indicated that during initial process of bioleaching jarosite formed immediately with the catalytic effect of activated carbon. But the leaching kinetics still remained higher than that in the absence of activated carbon, which indicated that copper dissolution was not hindered. In the late stage of bioleaching with activated carbon, a large amount of jarosite precipitated on the chalcopyrite surface, which could partially account for the decrease of copper dissolution rate (Fig. 1(f)).
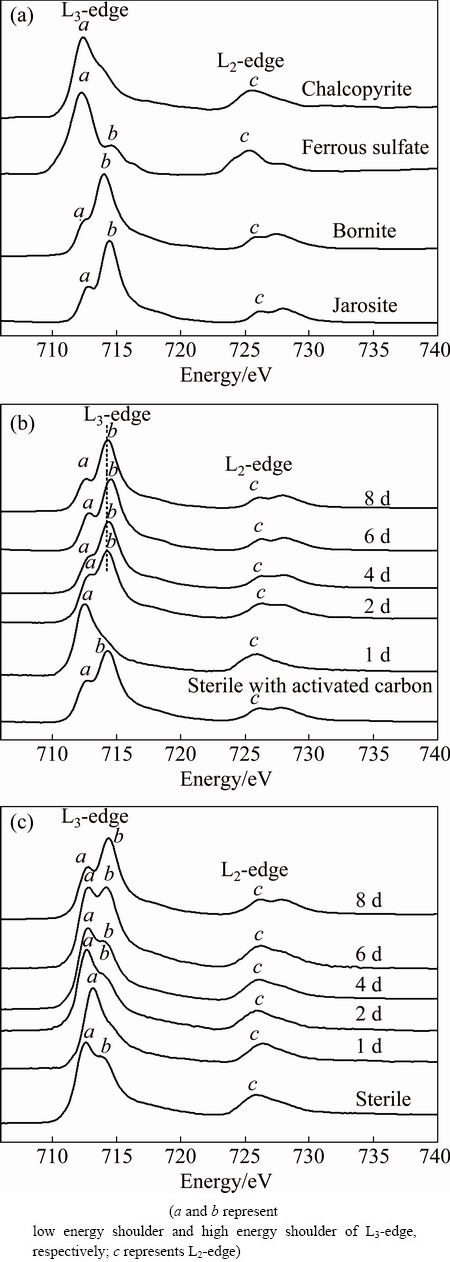
Fig. 4 Normalized iron L-edge XANES spectra of reference compounds (a), normalized spectra of chalcopyrite during bioleaching at 65 °C with 2 g/L activated carbon (b) and without activated carbon (c) at different time
The normalized sulfur K-edge XANES spectra of reference samples and chalcopyrite during bioleaching at 65 °C in the presence of 2 g/L and absence of activated carbon are shown in Fig. 5. The spectra of standard samples (chalcopyrite, bornite, chalcocite, covellite, elemental sulfur, jarosite) showed significant differences in the position intensity and width of the absorption peaks (Fig. 5(a)), which were in accordance with previous study [24]. Figure 5(b) showed that during bioleaching with 2 g/L activated carbon, the peak at 2.470 keV became weaker and weaker, and the new peak at 2.472 keV appeared at day 1 and then gradually increased until day 4, but decreased to a low intensity at day 6. And the peak at 2.480 keV gradually became the main peak. By contrast, the peak at 2.470 keV decreased gradually and the peak at 2.480 keV increased to the main peak overtime in the spectra of chalcopyrite bioleached without activated carbon (Fig. 5(c)). It is worthy to note that a new peak appeared at 2.472 keV in the spectra of chemical leaching with activated carbon comparing with that without activated carbon (Figs. 5(b) and (c)).
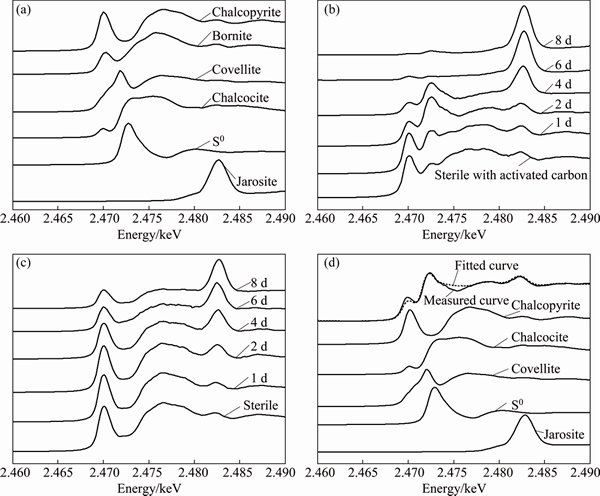
Fig. 5 Normalized sulfur K-edge XANES spectra of reference compounds (a), normalized spectra of chalcopyrite during bioleaching with 2 g/L activated carbon (b) and without activated carbon (c) at different time, and fitted spectra of chalcopyrite bioleaching with 2 g/L activated carbon for 2 d (d)
Table 1 Fitted results of sulfur K-edge XANES spectra of chalcopyrite bioleached in the presence of 2 g/L activated carbon using reference spectra
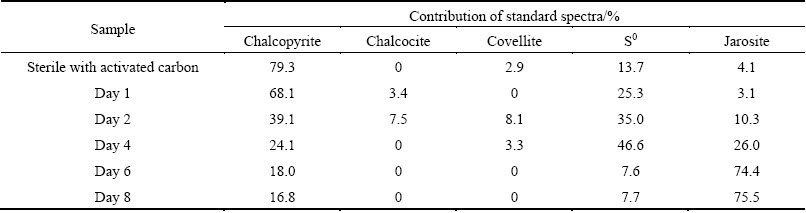
Table 2 Fitted results of sulfur K-edge XANES spectra of chalcopyrite bioleached in the absence of activated carbon using reference spectra
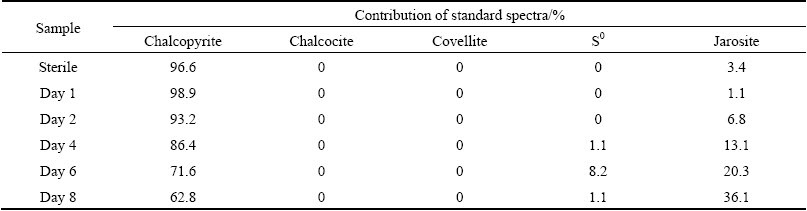
In order to quantify sulfur speciation on the mineral surface, the sulfur K-edge XANES spectra of these unknown samples (Figs. 5(b) and (c)) were fitted for their linear composition using the reference spectra of sulfur-compounds (Fig. 5(a)). The fitted spectrum of chalcopyrite in bioleaching with activated carbon at day 2 was taken as an example to show the quality of the fitting results (Fig. 5(d)). The fitted results (Fig. 5(d)) showed sulfur species on the mineral surface of bioleaching with activated carbon after 2 d were composed of 39.1% chalcopyrite, 7.5% chalcocite, 8.1% covellite, 28.7% S0 and 10.3% jarosite. The fitting results of XANES spectra of chalcopyrite during bioleaching at different time are summarized in Tables 1 and 2. According to the results, chalcocite was detected at day 1, and both chalcocite and covellite were detected at day 2 when the redox potential was below 400 mV during bioleaching with activated carbon. By contrast, neither chalcocite nor covellite was detected during bioleaching without activated carbon. Similarly, covellite was just detected in the residue of chemical leaching with activated carbon.
Activated carbon was beneficial at low redox potential because the dissolution process went through chalcocite oxidation which was more efficient [11]. In this study, chalcocite was detected on the mineral surface at day 1 and day 2 at a low redox potential (<400 mV) during bioleaching with activated carbon (Table 1). In the presence of activated carbon, the more readily dissolved secondary mineral chalcocite was formed by reduction of the chalcopyrite with Cu2+ and Fe2+ at low redox potential (Eqs. (8) and (9)) [2]. Then, the chalcocite was quickly oxidized by Fe3+ and dissolved O2 to release Cu2+. Accompanying with the formation of chalcocite, S0 was also formed (Eq. (8)). The formation of S0 could explain the detection of more S0 during bioleaching with activated carbon as well as the S0 in chemical leaching residue with activated carbon. Covellite was also detected during bioleaching with activated carbon at day 2 and day 4, which indicated that covellite was the transient intermediate compound during bioleaching of chalcopyrite. ZHU et al [16] and HE et al [24] also found covellite using Acidianus manzaensis and mixed thermophilic Archaea respectively to bioleach chalcopyrite. Because covellite could be formed through the oxidation of chalcocite by ferric sulphate, we speculated that the covellite at day 2 and day 4 was the oxidation product of chalcocite formed when the redox potential was below 400 mV. And the covellite found in chemical leaching residue with activated carbon at day 8 which was consistent with the result of SR-XRD could be the oxidation product of chalcocite. In addition, if we check the dissolution kinetics of chalcopyrite bioleached by mixed thermophilic Archaea (Fig. 1(f)), we can find that during the first 3 d, the dissolution kinetics with activated carbon was much higher than that without activated carbon. This indicated that activated carbon could change the transition path of electrons to form more readily dissolved chalcocite through galvanic interaction and finally enhanced the copper dissolution.
CuFeS2+Cu2+→Cu2S+Fe2++S0 (8)
CuFeS2+3Cu2++3Fe2+→2Cu2S+4Fe3+ (9)
It is noteworthy that more S0 and jarosite were detected in the bioleaching residues with activated carbon. Previous studies [15,25] indicated that the extreme thermophile Archaea possessed strong capacity of sulfur elimination. Thus, the by-product S0 could be reduced gradually and seemed to have no influence on the dissolution of chalcopyrite. However, as jarosite accumulated overtime, the leaching rate of copper with activated carbon slowed down and the passivation phenomenon was observed from Fig. 1(f) in which copper concentration ceased to increase from day 6 to the last day. Jarosite is a common leaching by-product and has been reported as a passivation factor previously [26,27]. And at elevated temperatures, jarosite readily formed and deposited onto the surface of chalcopyrite, thus preventing the contact of the microorganisms with chalcopyrite and hindering the bioleaching process [16]. In this study, more jarosite was found in the late stage of chalcopyrite bioleaching with activated carbon (Table 1). More jarosite on the mineral surface might account for the decrease of copper release rate in the late stage.
4 Conclusions
1) The addition of 2 g/L activated carbon could significantly promote the dissolution of chalcopyrite for both bioleaching and chemical leaching at 65 °C.
2) The results of SR-XRD and XANES spectra analysis indicated that activated carbon could change transition path of electrons through galvanic interaction at a low redox potential (<400 mV) to form more readily dissolved secondary mineral chalcocite and finally enhanced the copper dissolution.
3) Jarosite accumulated immediately in the early stage of bioleaching with activated carbon, but the copper dissolution was not hindered. While more jarosite precipitated on the surface of chalcopyrite in the late stage of bioleaching, which could partially account for the decrease of copper dissolution rate.
4) More S0 was also detected with additional activated carbon. However, the extreme thermophile Archaea had a strong S0 oxidation activity and S0 was gradually eliminated by them, thus the S0 seemed to have no significant influence on the dissolution of chalcopyrite.
References
[1] PRADHAN N, NATHSARMA K C, SRINIVASA R K, SUKLA L B, MISHRA B K. Heap bioleaching of chalcopyrite: A review [J]. Minerals Engineering, 2008, 21(5): 355-365.
[2] LI Y, KAWASHIMA N, LI J, CHANDRA A P, GERSON A R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite [J]. Advances in Colloid and Interface Science, 2013, 197-198: 1-32.
[3]  SHCHUKAREV A, PAUL J. XPS characterisation of chalcopyrite chemically and bio-leached at high and low redox potential [J]. Minerals Engineering, 2005, 18(5): 505-515.
SHCHUKAREV A, PAUL J. XPS characterisation of chalcopyrite chemically and bio-leached at high and low redox potential [J]. Minerals Engineering, 2005, 18(5): 505-515.
[4] KINNUNEN P H M, HEIMALA S, RIEKKOLA-VANHANEN M L, PUHAKKA J A. Chalcopyrite concentrate leaching with biologically produced ferric sulphate [J]. Bioresource Technology, 2006, 97(14): 1727-1734.
[5] KLAUBER C, PARKER A, van BRONSWIJK W, WATLING H. Sulphur speciation of leached chalcopyrite surfaces as determined by X-ray photoelectron spectroscopy [J]. International Journal of Mineral Processing, 2001, 62(1-4): 65-94.
[6] WAN R Y, MILLER J D, FOLEY J, PONS S. Electrochemical features of the ferric sulfate leaching of CuFeS2/C aggregates [C]//Electrochemistry in Mineral and Metal Processing. Pennington: Electrochemical Society, 1984: 391-416.
[7] NAKAZAWA H, FUJISAWA H, SATO H. Effect of activated carbon on the bioleaching of chalcopyrite concentrate [J]. International Journal of Mineral Processing, 1998, 55(2): 87-94.
[8] LI Hong-xu, QIU Guan-zhou, HU Yue-hua, WANG Dian-zuo. Galvanic effect on mixed sulfide bioleaching [J]. The Chinese Journal of Nonferrous Metals, 2003, 13(5): 1283-1287. (in Chinese)
[9] ZHANG Wei-min, GU Shi-fei. Catalytic effect of activated carbon on bioleaching of low-grade primary copper sulflde ores [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(5): 1123-1127.
[10] AHMADI A, RANJBAR M, SCHAFFIE M. Effect of activated carbon addition on the conventional and electrochemical bioleaching of chalcopyrite concentrates [J]. Geomicrobiology Journal, 2013, 30(3): 237-244.
[11] LIANG Chang-li, XIA Jin-lan, ZHAO Xiao-jun, YANG Yi, GONG San-qiang, NIE Zhen-yuan, MA Chen-yan, ZHENG Lei, ZHAO Yi-dong, QIU Guan-zhou. Effect of activated carbon on chalcopyrite bioleaching with extreme thermophile Acidianus manzaensis [J]. Hydrometallurgy, 2010, 105(1-2): 179-185.
[12] HARMER S L, THOMAS J E, FORNASIERO D, GERSON A R. The evolution of surface layers formed during chalcopyrite leaching [J]. Geochimica et Cosmochimica Acta, 2006, 70(17): 4392-4402.
[13] NAVA D,  I. Electrochemical characterization of chemical species formed during the electrochemical treatment of chalcopyrite in sulfuric acid [J]. Electrochimica Acta, 2006, 51(25): 5295-5303.
I. Electrochemical characterization of chemical species formed during the electrochemical treatment of chalcopyrite in sulfuric acid [J]. Electrochimica Acta, 2006, 51(25): 5295-5303.
[14] YANG Yi, LIU Wei-hua, CHEN Miao. A copper and iron K-edge XANES study on chalcopyrite leached by mesophiles and moderate thermophiles [J]. Minerals Engineering, 2013, 48: 31-35.
[15] LIU Hong-chang, XIA Jin-lan, NIE Zhen-yuan. Relatedness of Cu and Fe speciation to chalcopyrite bioleaching by Acidithiobacillus ferrooxidans [J]. Hydrometallurgy, 2015, 156: 40-46.
[16] ZHU Wei, XIA Jin-lan, YANG Yi, NIE Zhen-yuan, ZHENG Lei, MA Chen-yan, ZHANG Rui-yong, PENG An-an, TANG Lu, QIU Guan-zhou. Sulfur oxidation activities of pure and mixed thermophiles and sulfur speciation in bioleaching of chalcopyrite [J]. Bioresource Technology, 2011, 102(4): 3877-3882.
[17] ZHU Wei, XIA Jin-lan, YANG Yi, NIE Zhen-yuan, PENG An-an, LIU Hong-chang, QIU Guan-zhou. Thermophilic archaeal community succession and function change associated with the leaching rate in bioleaching of chalcopyrite [J]. Bioresource Technology, 2013, 133: 405-413.
[18] HE Huan, XIA Jin-lan, HUANG Guan-hua, JIANG Hong-cheng, TAO Xiu-xiang, ZHAO Yi-dong, HE Wei. Analysis of the elemental sulfur bio-oxidation by Acidithiobacillus ferrooxidans with sulfur K-edge XANES [J]. World Journal of Microbiology and Biotechnology, 2011, 27(8): 1927-1931.
[19] LIU Hong-chang, XIA Jin-lan, NIE Zhen-yuan, MA Ya-long, MA Chen-Yan, ZHENG Lei, HONG Cai-hao, ZHAO Yi-dong. Iron L-edge and sulfur K-edge XANES spectroscopy analysis of pyrite leached by Acidianus manzaensis [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(7): 2407-2414.
[20] LIU Wei, YANG Hong-ying, SONG Yan, TONG Lin-lin. Catalytic effects of activated carbon and surfactants on bioleaching of cobalt ore [J]. Hydrometallurgy, 2014, 152: 69-75.
[21] SAND W, GEHRKE T, JOZSA P G, SCHIPPERS A. (Bio)chemistry of bacterial leaching—Direct vs. indirect bioleaching [J]. Hydrometallurgy, 2001, 59(2-3): 159-175.
[22] LOTFALIAN M, RANJBAR M, FAZAELIPOOR M H, SCHAFFIE M, MANAFI Z. The effect of redox control on the continuous bioleaching of chalcopyrite concentrate [J]. Minerals Engineering, 2015, 81: 52-57.
[23] NAZARI B, JORJANI E, HHANI, MANAFI Z, RIAHI A. Formation of jarosite and its effect on important ions for Acidithiobacillus ferrooxidans bacteria [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(4): 1152-1160.
[24] HE Huan, XIA Jin-lan, HONG Fen-fen, TAO Xiu-xiang, LENG Yun-wei, ZHAO Yi-dong. Analysis of sulfur speciation on chalcopyrite surface bioleached with Acidithiobacillus ferrooxidans [J]. Minerals Engineering, 2012, 27-28: 60-64.
[25] HE Huan, YANG Yi, XIA Jin-lan, DING Jian-nan, ZHAO Xiao-juan, NIE Zhen-yuan. Growth and surface properties of new thermoacidophilic Archaea strain Acidianus manzaensis YN-25 grown on different substrates [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(6): 1374-1378.
[26] ZENG Wei-min, QIU Guan-zhou, ZHOU Hong-bo, CHEN Miao. Electrochemical behaviour of massive chalcopyrite electrodes bioleached by moderately thermophilic microorganisms at 48 °C [J]. Hydrometallurgy, 2011, 105(S3-S4): s259-s263.
[27] DEBERNARDI G, CARLESI C. Chemical-electrochemical approaches to the study passivation of chalcopyrite [J]. Mineral Processing and Extractive Metallurgy Review, 2013, 34(1): 10-41.
混合嗜热古菌在65 °C生物浸出黄铜矿过程中活性炭的催化作用和钝化现象的相关性
马亚龙1,刘红昌1,夏金兰1, 2,聂珍媛1, 2,朱泓睿1,赵屹东3,马陈燕3,郑 雷3,洪才浩3,文 闻4
1. 中南大学 资源加工与生物工程学院,长沙 410083;
2. 中南大学 生物冶金教育部重点实验室,长沙 410083;
3. 中国科学院 高能物理研究所 北京同步辐射装置,北京 100049;
4. 中国科学院 上海应用物理研究所 上海同步辐射装置,上海 201800
摘 要:研究活性炭对四株典型嗜热古菌混合培养物(Acidianus brierleyi, Metallosphaera sedula, Acidianus manzaensis和Sulfolobus metallicus)在 65 °C时浸出纯黄铜矿过程中活性炭的催化作用和钝化现象的相关性。浸出实验表明,活性炭能够有效地促进黄铜矿的生物浸出和化学浸出。基于同步辐射技术的X射线衍射、铁的L-边和硫的K-边X射线吸收近边结构光谱学分析表明,在生物浸出过程中当氧化还原电位较低(<400 mV)时,活性炭能通过原电池反应改变电子传递途径,生成更易溶解的次生矿物辉铜矿,从而增强黄铜矿的浸出。在添加活性炭的生物浸出过程的前期,黄钾铁矾迅速累积但铜离子的浸出速率未受到抑制,然而在生物浸出的后期,大量黄钾铁矾沉淀在矿物表面,从而抑制黄铜矿的进一步溶解。在添加活性炭时检测到了更多的单质硫,但由于嗜热古菌混合培养物具有很强的硫氧化活性,所以生成的单质硫被其消解,因此,未检测到其对黄铜矿浸出有显著影响。
关键词:黄铜矿;生物浸出;活性炭;钝化现象;混合嗜热古菌
(Edited by Wei-ping CHEN)
Foundation item: Project (51274257) supported by the National Natural Science Foundation of China; Project (U1232103) supported by the Joint Funds of National Natural Science Foundation of China and Large Scientific Facility Foundation of Chinese Academy of Sciences; Project (VR-12419) supported by the Beijing Synchrotron Radiation Facility Public User Program, China; Project (15ssrf00924) supported by the Shanghai Institute of Applied Physics Open Fund of Shanghai Synchrotron Radiation Facility, China
Corresponding author: Jin-lan XIA; Tel: +86-731-88836944; E-mail: jlxia@csu.edu.cn
DOI: 10.1016/S1003-6326(17)60158-4
Abstract: The relatedness between catalytic effect of activated carbon and passivation phenomenon during chalcopyrite bioleaching by mixed thermophilic Archaea culture (Acidianus brierleyi, Metallosphaera sedula, Acidianus manzaensis and Sulfolobus metallicus) at 65 °C was studied. Leaching experiments showed that the addition of activated carbon could significantly promote the dissolution of chalcopyrite for both bioleaching and chemical leaching. The results of synchrotron-based X-ray diffraction, iron L-edge and sulfur K-edge X-ray absorption near edge structure spectroscopy indicated that activated carbon could change the transition path of electrons through galvanic interactions to form more readily dissolved secondary mineral chalcocite at a low redox potential (<400 mV) and then enhanced the copper dissolution. Jarosite accumulated immediately in the initial stage of bioleaching with activated carbon but copper dissolution was not hindered. However, much jarosite precipitated on the surface of chalcopyrite in the late stage of bioleaching, which might account for the decrease of copper dissolution rate. More elemental sulfur (S0) was also detected with additional activated carbon but the mixed thermophilic Archaea culture had a great sulfur oxidation activity, thus S0 was eliminated and seemed to have no significant influence on the dissolution of chalcopyrite.


